摘要/Abstract
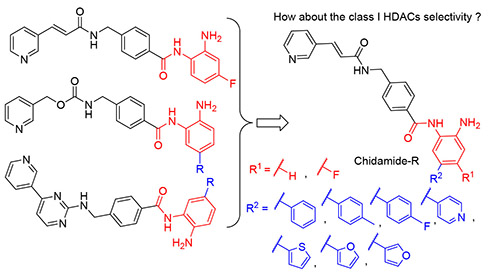
以西达本胺为基础设计合成了一系列新型组蛋白去乙酰化酶(HDACs)抑制剂,以提高与Zn2+的螯合作用和亚型选择性.大部分化合物表现出一定的抗肿瘤增殖活性.其中,(E)-N-(4-氨基-6-氟-[1,1'联苯]3-基)-4-((3-(吡啶-3-基)丙烯酰氨基)甲基)苯甲酰胺(7i)和(E)-N-(2-氨基-4-氟-5-(噻吩-2-基)苯基)-4-((3-(吡啶-3-基)丙烯酰胺基)甲基)苯甲酰胺(7j)抗肿瘤增殖活性最佳,对Jurkat细胞的IC50分别为3.29和12.59 μmol/L,并且这两个化合物表现出一定的HDAC抑制活性,为更有潜力的西达本胺衍生物的设计合成提供了新思路.
关键词: 西达本胺, HDACs, 抗肿瘤活性, 苯甲酰胺
A series of novel chidamide based histone deacetylases (HDACs) inhibitors were rationally designed and synthesized to increase the Zn2+ chelating and selectivity. Biological characterization established that most of the compounds showed moderate antiproliferative activitites in cancer cell lines. Among the tested analogs, (E)-N-(4-amino-6- fluoro-[1,1'-biphenyl]-3-yl)-4-((3-(pyridin-3-yl)acrylamido)methyl)benzamide (7i) and (E)-N-(2-amino-4-fluoro-5-(thiophen- 2-yl)phenyl)-4-((3-(pyridin-3-yl)acrylamido)methyl)benzamide (7j) exhibit the most potent antiproliferative activity with IC50 of 3.29 and 2.59 μmol/L in Jurkat cells, respectively. Furthermore, these two compounds have a certain HDAC inhibitory activity. Collectively, the results partly encourage further development of more potential analogs based on chidamide.
Key words: chidamide, HDACs, anti-tumor activity, benzamide
PDF全文下载地址:
点我下载PDF
