摘要/Abstract
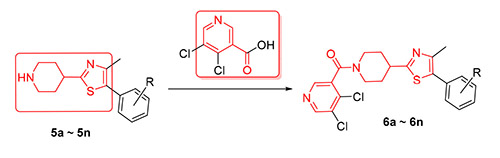
噻唑联哌啶结构能够影响生物体内胆固醇类化合物的代谢活动,是氟噻唑吡乙酮抑制病原菌的氧胆固醇结合蛋白(OSBP)的关键药效基团.为了寻找具有生物活性的新型含噻唑联哌啶结构的化合物,设计并合成了14个未见文献报道的新颖含噻唑联哌啶烟酰胺类化生物,其结构经1H NMR、13C NMR和HRMS确证,生测结果显示:在100 μg/mL浓度下,目标化合物普遍具有抑菌活性,其中1个化合物对小麦赤霉病的抑菌活性为60%;3个化合物对黄瓜灰霉病的抑菌活性为60%;6个化合物对苹果褐斑病的抑菌效果为70%;(4-(5-(2,3-二氯苯基)-4-甲基噻唑-2-基)哌啶-1-基)(5,6-二氯吡啶-3-基)甲酮(6k)对马铃薯晚疫病的抑菌活性为75%.
关键词: 结构, 噻唑联哌啶, 烟酰胺, 抑菌活性
The structure of thiazolidine piperidine could affect the metabolic activitives of cholesterol compounds in vivo, and it is the key pharmacophore of oxythiazolidine to inhibit the oxygen cholesterol-binding protein (OSBP) of pathogenic bacteria. 14 novel thiazolidine piperidine nicotinamide derivatives were designed and synthesized in search of new bioactive compounds containing thiazolidine piperidine structure. The structures of target compounds were characterized by 1H NMR, 13C NMR and HRMS spectra. The preliminary bioassay showed that the target compounds generally had antibacterial activities. At the concentration of 100 μg/mL, the antibacterial activity of one compound against Fusarium graminearum was 60%, the antibacterial activities of three compounds against Botrytis cinerea were 60%, the bacteriostatical activities of six compounds aganist Diplocarpon mali were 70%, and the antibacterial activity of (4-(5-(2,3-dichlorophenyl)-4-methylthiazol-2- yl)piperidin-1-yl)(5,6-dichloropyridin-3-yl)methanone (6k) against Phytophthora infestans (Mont.) de Bary was 75%. There- fore it was worth for further research about structural optimization.
Key words: structure, thiazolidine piperidine, nicotinamide, antibacterial activity
PDF全文下载地址:
点我下载PDF
