摘要/Abstract
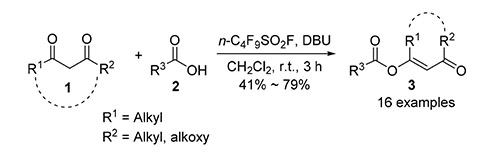
碱性介质中,羧酸在全氟烷基磺酰氟活化下与1,3-二酮化合物和β-酮酸酯能发生一步O-酰基化反应,以中等到良好的产率生成相应的烯醇酯产物.
关键词: 全氟烷基磺酰氟, 1,3-二羰基化合物, 羧酸, 烯醇酯
O-Acylation of 1,3-dicarbonyl compounds provides enol esters which act as precursors for the synthesis of chiral alcohols, natural products, heterocycles and functional materials. Perfluoroalkanosulfonyl fluoride (RfSO2F) is a class of excellent hydroxyl-activating reagent, and has been extensively developed and used in the formation of C-F, C-O, C-N and C-S bonds in organic synthesis. In this work one-step O-acylation of 1,3-dicarbonyl compounds (1,3-diketones and β-ketonic esters) with carboxylic acids activated by RfSO2F in alkaline media was disclosed, and the corresponding O-acylation products (enol esters) were generated in moderate to good yields. The optimized reaction conditions are as follows:1,8-diazabicyclo-[5.4.0]undec-7-ene (DBU) as base, CH2Cl2 as solvent, n-C4F9SO2F as activating reagent, room temperature for 30 min and the molar ratio of n(1,3-dicarbonyls):n(RCOOH):n(RfSO2F):n(DBU) being 1.0:1.0:1.0:4.0. A novel reagent for one-step O-acylation of 1,3-dicarbonyl compounds with carboxylic acids was developed. The application of RfSO2F in organic synthesis was further expanded.
Key words: perfluoroalkanosulfonyl fluoride, 1,3-dicarbonyl compound, carboxylic acids, enol ester
PDF全文下载地址:
点我下载PDF
