摘要/Abstract
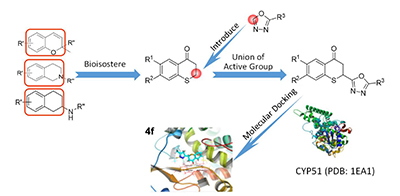
利用活性亚结构拼接原理,将硫色满酮与1,3,4-噁二唑杂环拼合,设计合成了12个2-(1,3,4-噁二唑)硫色满酮衍生物.目标化合物均经核磁共振氢谱(1H NMR)和高分辨质谱(HRMS)进行了结构确证.初步的抗真菌活性实验表明,目标化合物对4种动物病原真菌和4种植物病原真菌表现出一定程度的抑制作用.其中,化合物4f对白色念珠菌Canidia albicans的最小抑菌浓度达到了4 μg·mL-1,4d对花生冠腐病菌Aspergillusnigervan tiegh的最小抑菌浓度达到了8 μg·mL-1,均高于阳性对照.化合物分子对接研究表明,4f与白色念珠菌CYP51有很强的结合能力,可能为潜在的CYP51抑制剂.
关键词: 硫色满酮, 1,3,4-噁二唑, 抗真菌活性, 分子对接
Following the principle of union of active group, the thiochromanone was combined with 1,3,4-oxadiazole, and 12 compounds were designed and synthesized. The target compounds were confirmed by 1H NMR and HRMS. The preliminary antifungal activity assay showed that most of the target compounds exhibited significant inhibition activity against four animal pathogenic fungi and four plant pathogenic fungi. Among them, the minimum inhibitory concentration (MIC) value of compound 4f against Canidia albicans reached 4 μg·mL-1, and the MIC value of 4d against Aspergillusnigervan tiegh reached 8 μg·mL-1, which were higher than the positive controls. And the molecular docking studies have found that 4f has strong binding ability to CYP51 of Canidia albicans, which may be a potential CYP51 inhibitor.
Key words: thiochromanone, 1,3,4-oxadiazole, antifungal, molecular docking
PDF全文下载地址:
点我下载PDF
