摘要/Abstract
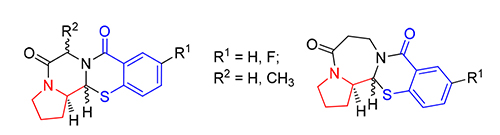
以叔丁氧羰基(Boc)保护的脯氨醛1、氨基酸酯盐酸盐2a~2d和巯基水杨酸3a~3b为原料,三组分一锅法得到苯并噻嗪烷-4-酮中间体.酸性条件下脱除Boc,分子内酰胺缩合制备稠合四环噻嗪烷-4-酮衍生物6~11.新生成手性碳(1-C)的构型通过H-1和H-2的偶合常数及X-ray单晶衍射确定.测试了化合物抗Hela和A549的肿瘤细胞增殖活性.结果表明,部分化合物具有中等的抗Hela细胞活性,其中(13aR,13bR)-1,2,3,13b-四氢苯并[e]吡咯并[2',1':3,4]吡嗪并[2,1-b][1,3]噻嗪-5,8(6H,13aH)-二酮(6b)的IC50值为9.50 μmol/L.所有化合物对A549的细胞没有抑制活性.
关键词: 稠合衍生物, 噻嗪烷-4-酮, 三组分缩合, 酰胺环缩合, 抗肿瘤活性
The benzothiazin-4-one intermediates were prepared by the one-pot three-components condensation from the N-Boc-L-prolinal 1, amino acid ethyl/methyl ester hydrochlorides 2a~2d, and mercaptobenzoic acids 3a~3b. After removal of Boc, the target novel fused tetracyclic thiazinan-4-one derivatives 6~11 were afforded by the intramolecular cyclo-amidation reaction. The absolute configurations of the newly generated chiral carbon (1-C) were determined by the coupling constants of H-1 and H-2 and the X-ray crystallographic structures. The tetracyclic alkaloids were examined for their anti-proliferative activity against Hela and A549 tumor cells. The results showed that some compounds could moderately inhibit the growth of Hela cells, and among them, (13aR,13bR) -1,2,3,13b-tetrahydrobenzo[e]pyrrolo[2',1':3,4]pyrazino[2,1-b] [1,3]thiazine-5,8(6H,13aH) -dione (6b) was the best one with the IC50 value of 9.50 μmol/L. However, all the compounds showed no anti-tumor activity against A549.
Key words: fused heterocyclic derivatives, thiazinan-4-one, three-components condensation, cyclo-amidation, anti-tumor activity
PDF全文下载地址:
点我下载PDF
