摘要/Abstract
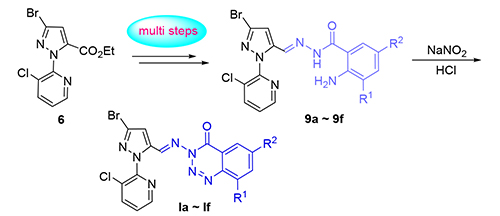
以N-吡啶基吡唑甲酸乙酯、取代邻氨基苯甲酸为原料,经由还原、氧化、亲核加成、缩合、重氮化等多步反应,合成了一系列3-(((3-溴-1-(3-氯吡啶-2-基)-1H-吡唑-5-基)亚甲基)氨基)取代苯并[d][1,2,3]三嗪-4(3H)-酮类目标化合物.初步生物活性测试结果表明,该系列化合物大多具有一定的杀虫活性,其中一个化合物在200 mg·L-1浓度下对东方粘虫(Mythimna separata Walker)具有70%的致死率;部分化合物表现出显著的抑菌活性,有两个化合物在50 mg·L-1浓度下对苹果轮纹病菌(Physalospora piricola)具有92.3%的抑制率,可作为新型抑菌先导结构,为后续的深入研究提供重要参考.
关键词: N-吡啶基吡唑衍生物, 苯并三嗪酮, 杀虫活性, 抑菌活性, 构效关系
A series of 3-(((3-bromo-1-(3-chloropyridin-2-yl) -1H-pyrazol-5-yl) methylene) amino) substituted-benzo[d] [1,2,3]-triazin-4(3H) -ones were synthesized successfully with ethyl N-pyridylpyrazole carboxylate and substituted aminobenzoic acids as starting materials, via multi-step reactions of reduction, oxidation, nucleophilic addition, condensation and diazotization. The preliminary bioassay tests indicated that most of these compounds have certain insecticidal activities, among which one compound showed a mortality rate of 70% towards Mythimna separata Walker at the test concentration of 200 mg·L-1, some compounds exhibited favorable fungicidal activities at 50 mg·L-1, particularly two compounds which possessed 92.3% growth inhibitory rate against Physalospora piricola, could be used as new fungicidal leading structures for further investigations on this type of compounds.
Key words: N-pyridylpyrazole derivatives, benzotriazinone, insecticidal activity, fungicidal activity, structure-activity relationship
PDF全文下载地址:
点我下载PDF
