摘要/Abstract
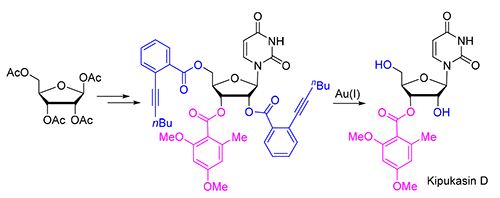
以廉价易得的β-D-四乙酰核糖为起始原料,经过9步反应,以15.7%的总产率完成了海洋天然核苷Kipukasin D的全合成.邻碘苯甲酸酯在Vorbrüggen糖基化反应中作为邻基导向基团,立体选择性地得到β型核苷.合成的关键步骤为Ph3PAuOTFA选择性催化脱除核苷2'和5'位邻炔基苯甲酸酯基团,该方法为温和的中性反应条件,完全避免了核苷2'位和3'位的酯交换副反应.
关键词: 核苷, 邻基效应, 酯交换, 催化, 全合成
The first total synthesis of marine natural nucleoside kipukasin D was disclosed in 9 steps and 15.7% overall yield using commercially available tetra-O-acetyl-β-D-ribose as starting material. In Vorbrüggen glycosylation, ortho-iodinebenzoate acted as neighboring participating group leading to β-nucleoside. The key step was selectively deprotection of 2'-O and 5'-O ortho-alkynylbenzoates by freshing prepared Ph3PAuOTFA in the presence of ethanol (6 equiv.) and H2O (1 equiv.) in dichloromethane (DCM). The reaction condition of our newly developed approach is very mild and neutral, which effectively avoids the transesterification between 2'-OH and 3'-OH. The result further demonstrat that ortho-alkynylbenzoate is an orthogonal protecting group compatible with other ester groups, which could find wide applications in nucleoside and carbohydrate chemistry.
Key words: nucleoside, neighboring-group, transesterification, catalysis, total synthesis
PDF全文下载地址:
点我下载PDF
