摘要/Abstract
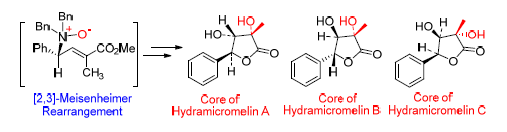
Hydramicromelins A~C是具有独特化学结构和生物活性的香豆素类化合物.以本实验室发展的[2,3]-Meisenheimer重排为关键反应,以L-苯甘氨醇为起始原料,经过Wittig反应,[2,3]-Meisenheimer重排,环氧化,双羟化等7~8步反应,高收率、高对映选择性地制备出Hydramicromelins A,B和C的母核或其对映体.
关键词: Hydramicromelins A,B和C, [2,3]-Meisenheimer重排, 手性三级醇, 环氧化
Hydramicromelins A~C are coumarin compounds with unique chemical structure and biological activity. With[2,3]-Meisenheimer rearrangemen as a key reaction which has been developed in our laboratory, the core structures of Hydramicromelin A, B and C were synthesized from L-phenylglycine. The route included Wittig reaction,[2,3]-Meisenheimer rearrangement, epoxidation and dihydroxylation reaction, and it was high-yield and high-enantioselectivity.
Key words: hydramicromelins A, B and C, [2,3]-Meisenheimer rearrangement, chiral tertiary alcohol, epoxidation
PDF全文下载地址:
点我下载PDF
