摘要/Abstract
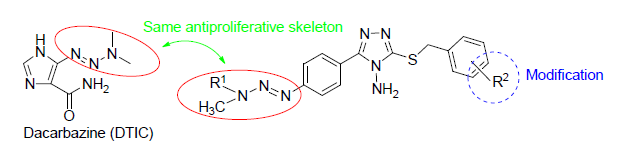
以对氨基苯甲酸为原料,经多步反应合成了16个未见文献报道的1,2,4-三唑三氮烯衍生物.利用1H NMR,13C NMR和HRMS对标题化合的结构进行了表征.衍生物的细胞毒活性测试结果表明,2-[4-(3,3-二甲基三氮烯-1-基)苯基]-3-氨基-4-S-(4-氯基苄基)-1,2,4-三唑(6g)、2-[4-(3,3-二甲基三氮烯-1-基)苯基]-3-氨基-4-S-(2,4-二氯基苄基)-1,2,4-三唑(6h)、2-[4-(3,3-甲基苯甲基三氮烯-1-基)苯基]-3-氨基-4-S-苄基-1,2,4-三唑(6i)、2-[4-(3,3-甲基苯甲基三氮烯-1-基)苯基]-3-氨基-4-S-(4-甲基苄基)-1,2,4-三唑(6j)、2-[4-(3,3-甲基苯甲基三氮烯-1-基)苯基]-3-氨基-4-S-(4-甲氧基苄基)-1,2,4-三唑(6l)、2-[4-(3,3-甲基苯甲基三氮烯-1-基)苯基]-3-氨基-4-S-(2,4-二氯基苄基)-1,2,4-三唑(6p)对膀胱癌细胞具有较好的抑制作用,其IC50值分别为23.883,5.512,8.731,8.077,5.590和12.195 μmol/L,化合物6h,6i,6j,6l对前列腺癌细胞具有较好的抑制作用,其IC50值分别为13.690,21.908,10.772和4.827 μmol/L.
关键词: 三氮烯, 1,2,4-三唑, 合成, 抗肿瘤
Sixteen novel 1,2,4-triazole triazene derivatives were synthesized in multiple steps from the p-aminobenzoic acid. Their structures were confirmed by 1H NMR, 13C NMR and HRMS. All the target compounds were evaluated for their anticancer activity. Among them, 2-[4-(3,3-dimethyltriazol-1-yl)phenyl]-3-amino-4-S-(4-chlorobenzyl)-1,2,4-triazole (6g), 2-[4-(3,3-dimethyltriazol-1-yl)phenyl]-3-amino-4-S-(2,4-dichlorobenzyl)-1,2,4-triazole (6h), 2-[4-(3,3-methylbenzyltriazen-1-yl)-phenyl]-3-amino-4-S-benzyl-1,2,4-trioxazole (6i), 2-[4-(3,3-methylbenzyltriazen-1-yl)phenyl]-3-amino-4-S-(4-methylbenzyl)-1,2,4-triazole (6j), 2-[4-(3,3-methylbenzyltriazen-1-yl)phenyl]-3-amino-4-S-(4-methoxybenzyl)-1,2,4-triazole (6l), 2-[4-(3,3-methylbenzyltriazen-1-yl)phenyl]-3-amino-4-S-(2,4-di-chlorobenzyl)-1,2,4-triazole (6p) exhibited good anti-cancer activity against J82 cells with the IC50 values of 23.883, 5.512, 8.731, 8.077, 5.590 and 12.195 μmol/L, respectively. And compounds 6h, 6i, 6j, 6l exhibited good anti-cancer activity against DU145 cells with the IC50 values of 13.690, 21.908, 10.772 and 4.827 μmol/L, respectively.
Key words: triazene, 1,2,4-triazole, synthesis, antitumor
PDF全文下载地址:
点我下载PDF
