摘要/Abstract
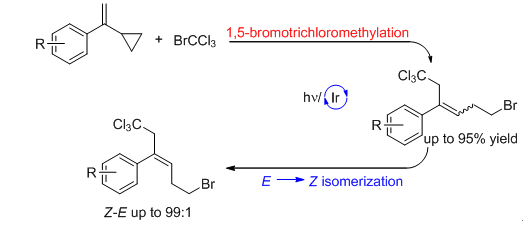
在可见光照射下,以Ir[dF(CF3) ppy]2(dtbbpy) PF6为光催化剂,α-环丙烷苯乙烯通过自由基链式反应机理进行1,5-溴三氯甲基化反应,生成三取代的苯乙烯类化合物,起始的Z/E比例为30∶70.当反应化合物进一步进行光照的时候,产物的Z/E比例最高可达99∶1.利用这一方法,成功合成了一系列的含溴三氯甲基的三取代苯乙烯化合物,产率从良好到优秀,都以Z构型为主.通过量子产率实验和荧光淬灭实验,提出了一个串联的反应历程,包含可见光引发的自由基链式反应及光催化剂催化的E-Z异构化反应.在此基础上,直接对容易制备的E构型的三取代苯乙烯类化合物进行可见光条件下的构型翻转,获得Z构型产物.
关键词: 光化学, 自由基, 烯烃, 异构
1,5-Bromotrichloromethylation of α-cyclopropylstyrenes via a radical chain pathway was achieved with Ir[dF(CF3)ppy]2(dtbbpy)PF6 as a photoinitiator under visible-light irradiation to give trisubstituted styrenes with Z/E ratio of 30:70. When the reaction mixture was further irradiated, the Z/E ratio could be reversed and increased to 99:1, probably via an energy-transfer pathway involving the Ir photocatalyst. This visible-light-induced catalytic isomerization protocol could also be applied to trisubstituted styrenes to obtain products with Z/E ratios up to 99:1.
Key words: photochemistry, radical, alkene, isomerization
PDF全文下载地址:
点我下载PDF
