摘要/Abstract
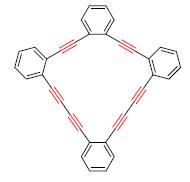
脱二苯膦酰(Ph2P(O))保护基/分子间炔烃偶联环化反应可一锅进行,高效形成环苯基六炔烃.与Haley等的脱硅基/Eglington偶联分步反应的制备方法相比,极性Ph2P(O)保护基使产物与少量剩余原料具有不同极性而易分离,且本反应与产物Rf值相近的分子间偶联副产物量较少,使得产物易分离.且一锅反应可避免脱Ph2P(O)的后处理,减少化合物损失,有效提高了环苯基六炔烃的产率.此外,本方法还具有中间体易制备、反应条件温和等优点.
关键词: 一锅反应, 二苯基膦酰基保护基, 环苯基多炔烃
Ph2P (O)-deprotection/intramolecular Eglington coupling cyclization, proceeding in one-pot manner, can be applied to synthesize cyclic phenyl polyynes. Compared to Micheal M. Haley's stepwise desilylation/Eglington coupling reaction, the polar Ph2P (O) protecting group enabled facile seperation of product from remaining starting compound for their different polarity, and it also led to easy separation of target compound because the amont of by-products which showed similar Rf to target compound decreased. Furthermore, our one-pot reaction, avoiding the workup after Ph2P (O)-deprotection and reducing losses of materials, increased the yield of cyclic aromatic polyynes. This method showed some other outstanding features including easy synthesis of intermediates and mild reaction conditions.
Key words: one-pot reaction, diphenylphosphoryl protecting group, cyclic phenyl polyyne
PDF全文下载地址:
点我下载PDF
