摘要/Abstract
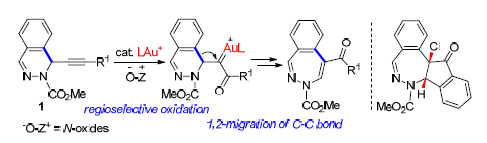
研究了金催化的基于1-炔基-1,2-二氢-2,3-二氮杂萘类底物的氧化/扩环反应,在以PPh3AuNTf2为催化剂8-甲基喹啉氮氧化物为氧化剂的条件下高效地合成了2,3-苯并二氮杂(艹卓)类化合物.该反应可能经历了α-羰基金卡宾的生成以及选择性的1,2-苯基迁移反应,而1,2-H和1,2-N迁移反应未观测到.我们还研究了2,3-苯并二氮杂(艹卓)类产物在FeCl3存在下的转化反应,发现随着FeCl3的用量的不同,可以分别生成吡唑及多并环类产物.
关键词: 金催化, 氧化, 扩环反应, 2,3-苯并二氮杂(艹卓)
A gold-catalyzed oxidative ring expansion of 1-alkynyl-1,2-dihydrophthalazines has been developed. The reaction was catalyzed by PPh3AuNTf2in the presence of 8-methylquinoline N-oxide as the oxidant, leading to 2,3-benzodiazepine derivatives with high efficiency. The reaction likely proceeds through the formation of α-carbonyl gold carbene and 1,2-migration of a phenyl group, while no 1,2-H and 1,2-N migration take place. Further transformation of 2,3-benzodiazepine products in the presence of FeCl3 was also carried out, pyrazole and polyfused heterocycle were formed, respectively, through variation of the amounts of FeCl3.
Key words: gold catalysis, oxidation, ring expansion, 2,3-benzodiazepine
PDF全文下载地址:
点我下载PDF
