摘要/Abstract
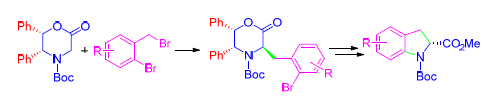
手性吲哚啉衍生物作为众多具有生物活性的天然产物和新型药物的特征结构片段,越来越引起化学家的关注.尽管目前已经存在很多合成方法,发展一种新的、简捷高效的吲哚啉衍生物的不对称合成方法仍然非常必要.以Williams中间体为手性控制试剂,经过亲核取代、分子内Buchwald-Hartwig偶联等关键步骤以非常高的收率和对映体选择性成功地合成得到了(R)-N-叔丁氧羰基吲哚啉-2-羧酸甲酯.
关键词: 吲哚啉-2-羧酸甲酯, Williams手性辅基, Buchwald-Hartwig偶联, 不对称合成
As a characteristic structural motif of numerous biologically active natural products and new drugs, chiral indoline derivatives have attracted much attention of chemists. Although many methods are available, there is still great need to develop a new, simple and highly efficient asymmetric synthetic method of indoline derivatives. Starting from Williams chiral auxiliary, a variety of methyl (R)-N-(tert-butoxycarbonyl)indoline-2-carboxylates were obtained with high overall yields and enantioselectivity through nucleophilic substitution, intramolecular Buchwald-Hartwig coupling reaction, etc.
Key words: indoline-2-carboxylate, Williams chiral auxiliary, Buchwald-Hartig coupling, asymmetric synthesis
PDF全文下载地址:
点我下载PDF
