

1. 江南大学 粮食发酵工艺与技术国家工程实验室,江苏 无锡 214122;
2. 江南大学 生物工程学院,江苏 无锡 214122
收稿日期:2020-11-26;接收日期:2021-03-03;网络出版时间:2021-04-29
基金项目:国家重点研发计划(No. 2019YFA0905500) 资助
摘要:酪氨酸是重要的芳香族氨基酸,自身不仅具有重要的营养价值,也是合成香豆素类化合物和黄酮类化合物的重要前体。文中以实验室前期构建的一株解除了酪氨酸反馈抑制的酿酒酵母Saccharomyces cerevisiae LTH0 (ARO4K229L,ARO7G141S,Δaro10,Δzwf1,Δura3) 为出发菌株,异源表达甜菜黄素合成基因DOD和CYP76AD1,使酿酒酵母产生黄色荧光。然后利用紫外诱变和常压室温等离子体(Atmospheric and room temperature plasma,ARTP) 诱变相结合的方法对上述菌株进行随机诱变,并通过流式细胞仪筛选荧光强度显著提高的突变株。其中突变株LTH2-5-DOD-CYP76AD1在激发波长485 nm、发射波长505 nm处荧光强度为(5 941±435) AU/OD,比诱变前提高了8.37倍。对诱变后荧光强度提高较多的14株突变株进行发酵生产酪氨酸,胞外酪氨酸产量最高为26.8 mg/L,比出发菌株提高了3.96倍。进一步异源表达约翰逊黄杆菌Flavobacterium johnsoniae来源的酪氨酸解氨酶FjTAL,对香豆酸产量达到119.8 mg/L,比出发菌株LTH0-FjTAL提高了1.02倍。
关键词:酪氨酸对香豆酸甜菜黄素高通量筛选
High-throughput screening of Saccharomyces cerevisiae efficiently producing tyrosine
Tanghao Liu1,2, Youran Li1,2, Liang Zhang1,2, Zhongyang Ding1,2, Zhenghua Gu1,2, Guiyang Shi1,2, Sha Xu1,2


1. National Engineering Laboratory for Cereal Fermentation Technology, Jiangnan University, Wuxi 214122, Jiangsu, China;
2. School of Biotechnology, Jiangnan University, Wuxi 214122, Jiangsu, China
Received: November 26, 2020; Accepted: March 3, 2021; Published: April 29, 2021
Supported by: National Key Research and Development Program of China (No. 2019YFA0905500)
Corresponding author: Sha Xu. Tel/Fax: +86-510-85914371; E-mail: xusha1984@jiangnan.edu.cn.
Abstract: Tyrosine is an important aromatic amino acid. Besides its nutritional value, tyrosine is also an important precursor for the synthesis of coumarins and flavonoids. Previously, our laboratory constructed a Saccharomyces cerevisiae strain LTH0 (ARO4K229L, ARO7G141S, Δaro10, Δzwf1, Δura3) where tyrosine feedback inhibition was released. In the present study, heterologous expression of betaxanthins synthesis genes DOD (from Mirabilis jalapa) and CYP76AD1 (from sugar beet B. vulgaris) in strain LTH0 enabled production of yellow fluorescence. The engineered strain LTH0-DOD-CYP76AD1 was subjected to UV combined with ARTP mutagenesis, followed by flow cytometry screening. Among the mutants screened, the fluorescence intensity of the mutant strain LTH2-5-DOD-CYP76AD1 at the excitation wavelength of 485 nm and emission wavelength of 505 nm was (5 941±435) AU/OD, which was 8.37 times higher than that of strain LTH0-DOD-CYP76AD1. Fourteen mutant strains were subjected to fermentation to evaluate their tyrosine producing ability. The highest extracellular tyrosine titer reached 26.8 mg/L, which was 3.96 times higher than that of strain LTH0-DOD-CYP76AD1. Heterologous expression of the tyrosine ammonia lyase FjTAL derived from Flavobacterium johnsoniae further increased the titer of coumaric acid to 119.8 mg/L, which was 1.02 times higher than that of the original strain LTH0-FjTAL.
Keywords: tyrosinep-coumaric acidbetaxanthinshigh-throughput screening
酪氨酸是重要的半必需氨基酸,是所有活生物体中蛋白质合成过程中的重要芳香族氨基酸[1],作为氨基酸类药物或营养添加剂直接添加在食品中[2]。酪氨酸作为重要前体物质[3],在解氨酶的作用下形成对香豆酸,作为重要的初级代谢产物,能够代谢产生各种黄酮类物质[4],对于人体内自由基的清除以及心脑血管疾病的治疗有显著功效。目前,L-酪氨酸的生产方法主要有蛋白质水解法、化学合成法、酶法和微生物发酵法[5]。蛋白质水解法已经逐渐淘汰。化学合成法由于其生产工艺复杂、副产物较多等原因不适于大规模生产。工业上生产L-酪氨酸主要依靠酶法,但天然酶活性低和稳定性差等缺点限制了其应用[6]。微生物发酵法可以利用生物质原料实现酪氨酸的从头合成,可以降低生产成本[7]。
酿酒酵母中芳香族氨基酸和下游黄酮类物质代谢途径以及部分调控机理尚未完全解析(图 1)。目前已知的芳香族氨基酸从头合成分为10步反应[8],存在多处负反馈调控[9],5-磷酸葡萄糖脱氢酶可以使6-磷酸葡萄糖进入磷酸戊糖途径(Pentose phosphate pathway,PPP),将5-磷酸葡萄糖脱氢酶基因(ZWF1) 敲除后可以调节糖酵解途径和磷酸戊糖途径的代谢流,增加赤藓糖-4-磷酸进入莽草酸途径的通量[10]。在莽草酸途径中,突变2-酮-3-脱氧-D-阿拉伯庚酮糖酸-7-磷酸(DAHP) 合酶同工酶ARO4K229L和ARO7G141S,可减弱目的产物L-酪氨酸对ARO4和ARO7的反馈抑制作用以积累酪氨酸[11]。表达约翰逊黄杆菌Flavobacterium johnsoniae来源的酪氨酸解氨酶基因FjTAL,可以进一步合成对香豆酸。
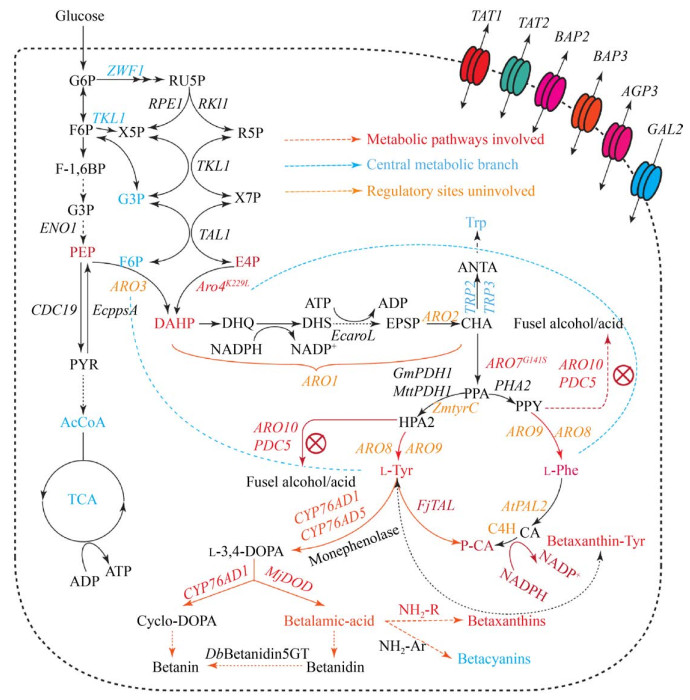 |
| 图 1 酿酒酵母芳香族氨基酸代谢途径 Fig. 1 Metabolic pathways of aromatic amino acids in S. cerevisiae. |
| 图选项 |
目前已有一些通过工业微生物发酵生产酪氨酸以及下游对香豆酸的策略。在大肠杆菌中,过表达编码分支酸突变酶/苯甲酸酯脱氢酶基因TYRA,在200 L发酵罐上进行发酵优化后,48 h得到55 g/L的酪氨酸[12];Santos等通过易错PCR优化与芳香族氨基酸代谢相关的代谢簇并结合高通量筛选手段,对芳香族氨基酸合成途径进行合理修饰,36 h补料发酵得到13.8 g/L酪氨酸[13]。在酿酒酵母中,Rodriguez等以酿酒酵母CEN.PK102-5B为出发菌株,通过敲除丙酮酸脱羧酶基因ARO10和PDC5,突变ARO4K229L与ARO7G141S以减弱反馈抑制,过表达莽草酸途径五官能团酶基因ARO1和分支酸合酶基因ARO2以增强前体物质供应,并过表达约翰逊黄杆菌来源的FjTAL基因,得到(1.93±0.26) g/L对香豆酸[14];Gu等在解酯耶式酵母Yarrowia lipolytica改造DAHP合成酶减轻反馈抑制,并通过异源表达磷酸酮醇酶和敲除丙酮酸激酶积累分支酸,得到(593.53±28.75) mg/L的对香豆酸[15];Borja等以木糖作为碳源发酵得到242 mg/L对香豆酸[16];张思琪等通过敲除酪氨酸合成竞争途径基因ARO10和PDC5,过表达芳香族氨基酸合成调控蛋白ARO4K229L与ARO7G141S解除芳香族氨基酸负反馈抑制,增强前体酪氨酸供应的同时表达对香豆酸合成基因FjTAL,并引入苯丙氨酸为底物的对香豆酸合成途径,经由发酵优化,在摇瓶中得到1 146.48 mg/L对香豆酸,在5 L发酵罐上优化得到3.56 g/L对香豆酸[17]。
本研究利用以酪氨酸作为前体的甜菜黄素能够在激发波长485 nm、发射波长505 nm处出现特异性吸收峰为检测手段,以甜菜黄素荧光强度[18]变化作为筛选条件,建立一种高通量筛选高产酪氨酸酿酒酵母的方法[19-20]。甜菜黄素常被用作染料中的一种有效成分[21]。酪氨酸在甜菜来源的基因CYP76AD1作用下生成左旋多巴,继而在开花植物紫茉莉来源的DOD[22]基因编码的多巴4, 5-双加氧酶作用下生成甜菜黄素[23]。本研究以解除了酪氨酸反馈抑制的突变株LTH0[24]作为出发菌株,经诱变筛选后获得了几株酪氨酸和对香豆酸产量显著提高的突变株。
1 材料与方法1.1 菌株、质粒与所用引物研究过程中所用的菌株及质粒如表 1所示,所用引物如表 2所示,实验所用引物均由苏州金唯智生物科技有限公司合成。
表 1 本实验使用的菌株和质粒Table 1 Strains and plasmids used in this study
| Strains and plasmids | Genotype | Source |
| JM109 | E. coli used for gene cloning | This lab |
| S288c | MATα SUC2 gal2 mal mel flo1 flo8-1 hap1 ho bio1 bio6 | This lab |
| LTH0 | As S288c; Δura3, Δaro10, Δzwf1, ARO4K229L, ARO7G141S | [23] |
| LTH0-DOD-CYP76AD1 | As LTH0; pY26-DOD-CYP76AD1 | This study |
| LTH1-1(2/3/4/5/6) | As LTH0; UV mutated; high tyrosine production | This study |
| LTH2-1(2/3/4/5/6/7/8/9) | As LTH0; UV and ARTP mutated; high tyrosine production | This study |
| LTH1-1(2/3/4/5/6)-DOD-CYP76AD1 | As LTH0; UV mutated; high betaxanthin production; pY26-DOD-CYP76AD1 | This study |
| LTH2-1(2/3/4/5/6/7/8/9)-DOD-CYP76AD1 | As LTH0; UV and ARTP mutated; high betaxanthin production; pY26-DOD-CYP76AD1 | This study |
| LTH1-1(2/3/4/5/6)-FjTAL | As LTH0; UV mutated; high p-coumaric acid production; pY26-FjTAL | This study |
| LTH2-1(2/3/4/5/6/7/8/9)-FjTAL | As LTH0; UV and ARTP mutated; high p-courmaic acid production; pY26-FjTAL | This study |
| pY26 | Shuttle plasmid with TEF and GPD promoters, Ampr, Δura3 | This lab |
| pHCas9-Nours | Expressing Cas9 protein, Noursr | This lab |
| pYES2-sgRNA-Hyg | Guiding Cas9 protein to cuttarget segment, Ampr, Hygr | This lab |
| pY26-DOD-CYP76AD1 | Shuttle plasmid with gene CYP76AD1 and DOD | This study |
| pY26-FjTAL | Shuttle plasmid with gene FjTAL | This study |
表选项
表 2 本实验使用的引物Table 2 Primers used in this study
| Primer name | Primer sequence (5′-3′) |
| CYP76AD1-F | TTAAGAACCGTATCTTGGGATTGG |
| CYP76AD1-R | ATGGACCACGCTACTTTG |
| DOD-F | ATGAAGGGTACTTACTACATCAACC |
| DOD-R | TTAAGAACCGTCAGTCTTTTGAGTAG |
| pY26-F | CACCAGAACTTAGTTTCGACGG |
| pY26-R | CTTTTCGGTTAGAGCGGATGTG |
| FjTAL-F | ATGAACACCATTAATGAATACTTGAGT |
| FjTAL-R | TTAATTGTTAATCAAATGATCCTTAACCTTTTG |
| FjTAL-RF | CAGGAATTCGATATCAAGCTTATGAACACCATTAATGAATACTTGAGT(EcoRⅠ) |
| FjTAL-RR | CAAAAGCTTAAGGATCATTTGATTAACAATTAAAAGCTTATCGATACCGTCGAC(EcoRⅠ) |
| URA3-sgRNA-F | TTGGTATATATACGCATATGTGGGTTTTAGAGCTAGAAATAGCAAG |
| URA3-sgRNA-R | AATTATATCAGTTATTACCCGGGGATCATTTATCTTTCACTGCGGAG |
| URA3-v-F | GATTCCGGTTTCTTTGAAATTTTTTTG |
| URA3-v-R | CGCCAGAACCAAGTAACAGTA |
| sgRNA-v-F | GCTAAATGTACGGGCGACAGTCAC |
| sgRNA-v-R | CGCGTTGGCCGATTCATTAATGCAG |
| Note: generated restriction enzyme sites are underlined and indicated in the table. | |
表选项
1.2 培养基大肠杆菌培养基LB (g/L):胰蛋白胨10,酵母粉5,氯化钠10,若需固体培养基添加琼脂粉20,121 ℃、20 min灭菌。
酵母种子培养基YPD (g/L):胰蛋白胨20,酵母粉10,葡萄糖20,若需固体培养基添加琼脂粉20,115 ℃、20 min灭菌。
发酵培养基(g/L):酵母氮基1.7,硫酸铵5,葡萄糖30,甲硫氨酸0.05,115 ℃、20 min灭菌。
筛选培养基(g/L):酵母氮基1.7,硫酸铵5,葡萄糖30,尿嘧啶0.03,5-FOA 1,115 ℃、20 min灭菌。
大肠杆菌在添加100 μg/mL氨苄青霉素的LB培养基中生长,37℃、200 r/min培养。诱变后的细胞洗涤后置于添加尿嘧啶的筛选培养基中培养,荧光定性完成后将高荧光菌株进行传代验证荧光稳定性,将菌株按0.5%接种量接种至液体筛选培养基中培养3-5代,涂布至筛选平板挑取单菌落得到色素合成途径缺失的酪氨酸生产菌株。酿酒酵母经两级活化后,将对数后期细胞接种至装有50 mL发酵培养基的250 mL摇瓶中发酵,接种量为5%,30 ℃、220 r/min培养,24 h补加20 g/L葡萄糖。
1.3 实验材料2×Taq PCR Master Mix、2×Pfu PCR Master Mix,杭州宝赛公司;Fast DigestedTM快速限制性内切酶,购自美国Thermo公司;T4 DNA连接酶、pY26质粒,购自大连TaKaRa公司;氨苄青霉素、遗传霉素G418、诺尔丝菌素、潮霉素,购自Sigma-Aldrich公司;质粒提取试剂盒、DNA纯化试剂盒、DNA胶回收试剂盒、Phanta高保真DNA聚合酶,南京诺唯赞生物有限公司;氨基酸标准品、OPA衍生试剂,购自Agilent公司;对香豆酸标准品、5-氟乳清酸、PI染色试剂盒,购自生工生物工程(上海) 股份有限公司;酵母氮基,购自索莱宝科技有限公司。
1.4 酿酒酵母中甜菜黄素合成途径的构建Not Ⅰ和Sac Ⅱ双酶切甜菜来源的细胞色素P450多巴氧化酶编码基因CYP76AD1,连接至线性化pY26质粒PTEF表达盒上,BamH Ⅰ和Sal Ⅰ双酶切开花植物紫茉莉来源多巴双加氧酶的编码基因DOD并连接至线性化质粒pY26的PGPD表达盒上,构建重组质粒pY26-DOD-CYP76AD1,利用醋酸锂转化法转化LTH0,得到一株能够积累甜菜黄素的LTH0-DOD-CYP76AD1菌株。
1.5 紫外诱变与ARTP诱变种子液活化后按照生长曲线选取16-18 h的对数生长后期细胞7-8 mL,离心弃上清,用超纯水洗涤后,再用0.01 mol/L PBS缓冲液(pH 7.4) 洗涤,数次后加入与培养基等量的PBS缓冲液悬浮菌体;注入9 mm无菌平板中使菌液平铺为0.5 cm左右的液层,紫外灯254 nm间隔20 cm照射60-90 s,严格避光减轻光复活。诱变后将菌液按梯度稀释至4×103-6×103个细胞/mL,取100 μL涂布酵母基础培养基(SC) 平板,锡箔纸避光30 ℃培养48-72 h。ARTP诱变取10 μL菌悬液平铺于ARTP载片上,气量10 L/min,功率100 W,等离子诱变45-55 s,诱变后将载片上的菌液于1 mL SC培养基中30 ℃、220 r/min培养72 h,采取先紫外诱变再进行ARTP诱变的策略。
1.6 高通量筛选甜菜黄素高产菌株将培养好的菌液离心弃上清,用0.01 mol/L PBS缓冲液洗涤两次并悬浮菌体,控制OD600为0.3-0.5,PI染料染色,利用BD FACSAriaⅢ流式细胞仪分选,采用双向计光器,纵向计光器运用激发波长536 nm、发射波长617 nm特异性识别PI染色,死细胞被染色呈阳性,横向计光器利用485 nm激发、515 nm发射识别甜菜黄素自身的黄色荧光,挑取存活且具有高黄色荧光的细胞分选至96孔浅孔板中,在台面振荡器上30 ℃培养48 h,按5%接种量接种至盛有新鲜SC培养基的96孔深孔板中,30 ℃培养72 h。孔板离心机4 ℃离心菌悬液,弃上清并用0.01 mol/L PBS洗涤两次,使用Synergy H4 Hybrid Microplate Reader测定黄色荧光,激发波长为485 nm,发射波长为505 nm,波宽为9 nm,增益值为120。
1.7 酪氨酸和对香豆酸含量鉴定将筛选出的高荧光菌株接种至筛选培养基中,5-氟乳清酸将含有URA3基因的质粒的菌株杀死,得到失去甜菜黄素合成能力的酪氨酸生产菌株。发酵培养基发酵120 h,取上清液加10%三氯乙酸混合后4 ℃过夜放置沉降杂蛋白,0.22 μm尼龙膜过滤,高效液相色谱测定胞外酪氨酸含量;提取胞内酪氨酸时,将菌体和等体积的PBS缓冲液和0.5 mm玻璃珠混合,振荡30 min,取上清液加10%三氯乙酸混合后4 ℃过夜放置,0.22 μm尼龙膜过滤。使用岛津高效液相色谱仪LC-20A,配置PDA检测器和氨基酸柱前自动衍生程序,Thermo Hypersil ODS C18 Column (250 mm×4.6 mm,5 μm),流动相为水相(25 mmol/L十二水磷酸氢二钠,25 mmol/L十水合四硼酸钠,pH 8.2)、有机相(甲醇:乙腈:水=4.5:4.5:1),流速1.6 mL/min,柱温50 ℃。梯度洗脱程序为:0 min,5% B;6 min,10% B;10 min;16% B;23 min,40% B;30 min,50% B;31 min,100% B;35 min,5% B。
取1 mL发酵液测量对香豆酸含量,加入1 mL色谱级甲醇过夜放置,离心后取上清氮吹浓缩,上清液过0.22 μm尼龙滤膜。使用岛津高效液相色谱仪LC-20A,配置UV检测器,Thermo Hypersil ODS C18 Column (250 mm×4.6 mm,5 μm),进样量为10 μL,柱温40 ℃,检测波长290 nm,流速1 mL/min。流动相A (0.1%三氟乙酸+水),B (乙腈)。梯度洗脱程序为:0 min,10% B;0.1 min-9 min,10%-40% B;9 min-15 min,40%-60% B;15 min-18 min,60%-10% B;18 min-20 min,10% B[17]。
1.8 胞外代谢物含量测定使用岛津高效液相色谱仪LC-20A,配置视差检测器,Dikma CarboPac H+色谱柱(300 mm×8.0 mm,6 μm) 测定胞外代谢物乙醇和葡萄糖的含量变化,流动相为5 mmol/L稀硫酸,流速0.8 mL/min,柱温40 ℃,发酵液使用10%三氯乙酸沉淀过夜沉降杂蛋白。
2 结果与分析2.1 重组菌的构建以酿酒酵母LTH0 (ARO4K229L,ARO7G141S,Δaro10,Δzwf1,Δura3) 为出发菌株,转化重组质粒pY26-DOD-CYP76AD1得到LTH0-DOD-CYP76AD1,利用一步克隆法将FjTAL片段构建至pY26质粒的PGPD表达盒上,得到pY26-FjTAL,转化得到LTH0-FjTAL,质粒图如图 2所示。表达甜菜黄素后酵母菌落(左侧划线区域) 显示黄色(图 3)。
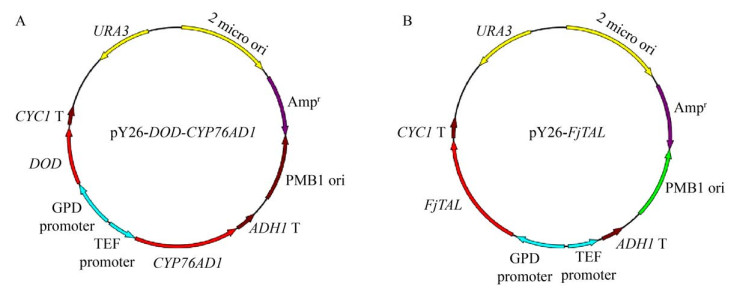 |
| 图 2 重组质粒pY26-DOD-CYP76AD1和pY26-FjTAL的构建 Fig. 2 Construction of recombinant plasmids pY26-DOD-CYP76AD1 (A) and pY26-FjTAL (B). |
| 图选项 |
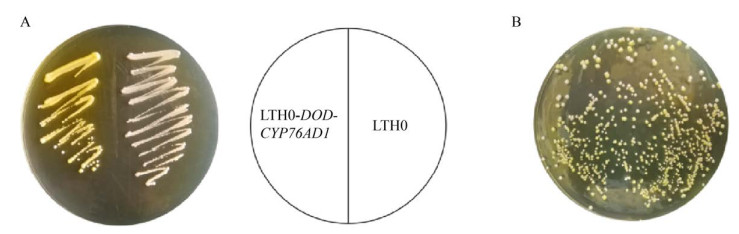 |
| 图 3 甜菜黄素的合成 Fig. 3 Synthesis of betaxanthin. (A) Expression of DOD and CYP76AD1. (B) Different fluorescence in mutant strains. |
| 图选项 |
2.2 紫外诱变筛选高产甜菜黄素的突变株LTH0-DOD-CYP76AD1经SC培养基培养72 h,在激发波长485 nm、发射波长505 nm处测定得到相对荧光强度为(634±45) AU/OD。紫外灯于254 nm间隔20 cm以恒定功率照射,致死率与照射时间之间的关系如图 4A所示,照射45 s时致死率达到90%,随着时间进一步提高致死率继续升高,本研究选取45-60 s进行诱变。经过紫外诱变挑选出其中10株荧光强度明显提高的突变株,如图 4C所示,其中LTH1-5-DOD-CYP76AD1生长72 h荧光强度为(3 352±104) AU/OD,比对照组提高了4.29倍,且菌体生长情况良好,因此对LTH1-5-DOD-CYP76AD1进一步诱变。紫外诱变虽然操作简单,但其严重的光复活效应也会产生许多回复突变。进一步遗传稳定性实验发现,紫外诱变产生的高荧光强度突变株随着传代次数的增加,荧光强度会逐渐呈现波动式下降趋势,其中LTH1-1-DOD-CYP76AD1传代7次后荧光降至第一代的73%,紫外诱变作为随机突变方式虽然能提供大量突变可能性,但不宜于优良性状的保持。
 |
| 图 4 紫外诱变后细胞荧光变化情况 Fig. 4 Cell fluorescence changes after UV mutagenesis. (A) The lethal rate varies with UV exposure time. (B) Fluorescence intensity in mutant strains. (C) Changes of fluorescence intensity over generations. |
| 图选项 |
2.3 ARTP诱变及流式分选筛选高产甜菜黄素的突变株利用流式细胞仪进行高通量筛选,流式细胞仪使用双向激光器检测,PE-A通道检测PI染料所激发出的红色荧光,FITC-A检测细胞表面甜菜黄素散发的黄色荧光,利用双向激光器检测出的细胞参数,挑选出处于Q4象限、具有高黄色荧光并具有生物活性的突变株。
如图 5A所示,LTH0未合成甜菜黄素时,细胞分群情况集中在Q3区域,几乎观察不到死细胞以及带有荧光的细胞,细胞密度呈现从内而外逐级降低的趋势,细胞分群情况良好,说明初始菌株不具备黄色荧光。LTH1-5-DOD-CYP76AD1的荧光分布如图 5B所示:单位时间流经通路的25 000个细胞中,Q4区域占比19.1%,即具有高荧光强度的活细胞,最高黄色荧光值约2.5×104 AU,选取P4区域(占比约0.4%) 分选至96孔板培养。LTH2-5-DOD-CYP76AD1的荧光分布如图 5C所示,观察发现荧光分群明显向右移动,黄色荧光进一步增强,单位时间流经通路的29 000个细胞中Q4区域占比29.2%,荧光最高值达到8×104 AU。对比单一紫外诱变,复合诱变能够得到荧光强度更高的突变株。经过SC培养基复苏培养后的细胞群中,复合诱变菌株死亡率为32%,显著高于紫外诱变的16%,原因可能是紫外光复活效应在细胞复苏过程中发挥作用导致大量细胞复苏,从而降低突变效率。
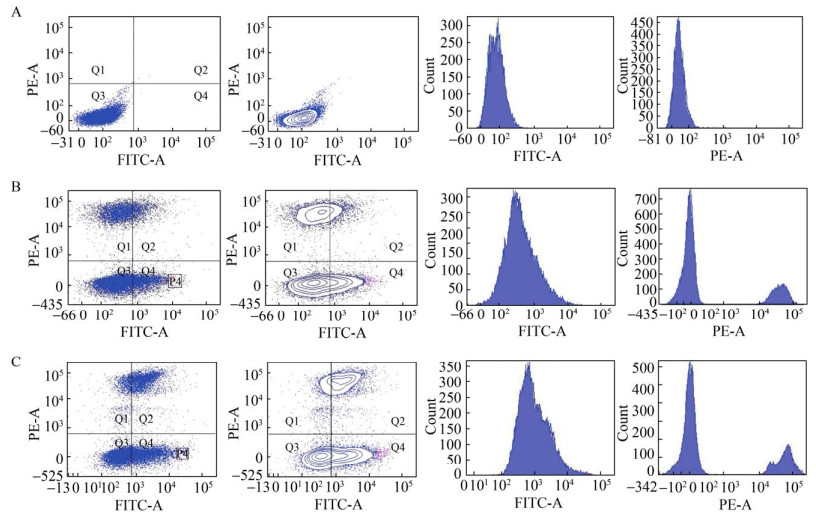 |
| 图 5 诱变株在流式细胞仪中的分群情况 Fig. 5 Sorting cells using flow cytometry, PE-A channel uses 536 nm as excitation light and 610 nm as emission light, FITC-A channel uses 485 nm as excitation light and 515 nm as emission light. The fluorescence grouping and intensity for strain LTH0 (A), strain LTH1-5-DOD-CYP76AD1 (B), and strain LTH2-5-DOD-CYP76AD1 (C). |
| 图选项 |
ARTP诱变致死曲线如图 6A所示,45-55 s诱变时间较为合适,致死率为(90±3)%,能产生较多正向突变且细胞存活数量适中。将已经经过紫外诱变的LTH1-5-DOD-CYP76AD1经过ARTP诱变,通过流式细胞仪分选后选取9株高荧光菌株,相对荧光强度如图 6B所示。其中LTH2-5-DOD-CYP76AD1突变株72 h相对荧光强度达到(5 941±435) AU/OD,比LTH0-DOD-CYP76AD1相对荧光强度增加了8.37倍;比LTH1-5-DOD-CYP76AD1相对荧光强度提高了77%。在复合突变株中随机选取3株LTH2-1-DOD-CYP76AD1、LTH2-2-DOD-CYP76AD1、LTH2-3-DOD-CYP76AD1进行遗传稳定性分析,传代7次后荧光强度仍保持在第1代的93.4%、90.2%、92.3%,荧光稳定性较强。
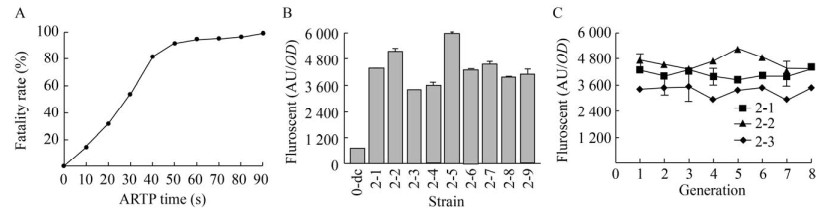 |
| 图 6 复合诱变后细胞荧光变化情况 Fig. 6 Cell fluorescence changes after compound mutation. (A) The lethal rate varies with ARTP processing time. (B) Fluorescence intensity in mutant strains. (C) Changes of fluorescence intensity over generations. |
| 图选项 |
2.4 突变株生产酪氨酸和对香豆酸选取14株优势突变株摇瓶发酵,紫外诱变得到的高荧光强度突变株生产酪氨酸结果如图 7所示。LTH0 72 h在胞外积累5.4 mg/L的酪氨酸,胞内积累2.7 mg/L酪氨酸。突变株中LTH1-1、LTH1-4、LTH1-5分别胞外积累13.4 mg/L、8.6 mg/L、16.0 mg/L的酪氨酸,与LTH0相比胞外酪氨酸产量分别增加1.48倍、0.59倍以及2.11倍。LTH1-4胞内积累4.2 mg/L酪氨酸,其余突变株胞内酪氨酸积累量相比LTH0有所减少,有可能是酪氨酸转运蛋白发生了相应突变,表达质粒pY26-FjTAL后,LTH0-FjTAL摇瓶发酵120 h时胞外积累了59.2 mg/L的对香豆酸,LTH1-1-FjTAL和LTH1-4-FjTAL分别积累了68.8 mg/L和64.9 mg/L的对香豆酸,比LTH0-FjTAL分别提高了16.2%和11%。
 |
| 图 7 紫外突变株发酵生产酪氨酸和对香豆酸 Fig. 7 Tyrosine and p-coumaric acid production in strains by UV mutation. (A) Intracellular tyrosine production. (B) Extracellular tyrosine production. (C) Intracellcular p-coumaric acid production. (D) Extracellular p-coumaric acid production. |
| 图选项 |
复合诱变得到的突变株能够更加有效地积累胞外酪氨酸和对香豆酸。从图 8中可以看出,LTH2-1摇瓶发酵120 h胞外积累了16.8 mg/L酪氨酸,LTH2-1-FjTAL对香豆酸120 h产量为74.3 mg/L,比对照菌株LTH0胞外酪氨酸积累量提高了2.05倍,比对照菌株LTH0-FjTAL对香豆酸产量提高了24.2%。LTH2-5发酵120 h胞外积累了26.8 mg/L酪氨酸,LTH2-5-FjTAL对香豆酸120 h为119.4 mg/L,相比于对照菌株LTH0胞外酪氨酸产量提高了3.96倍,比LTH0-FjTAL胞外对香豆酸积累量提高了1.02倍。此外,大部分突变株的胞内酪氨酸的积累量下降,胞外酪氨酸积累量增加,可能是因为酪氨酸转运蛋白在突变过程中发生了改变,需要通过基因组测序比对分析的手段进一步确定。对香豆酸胞内浓度较低,可能有利于减轻其产物抑制。
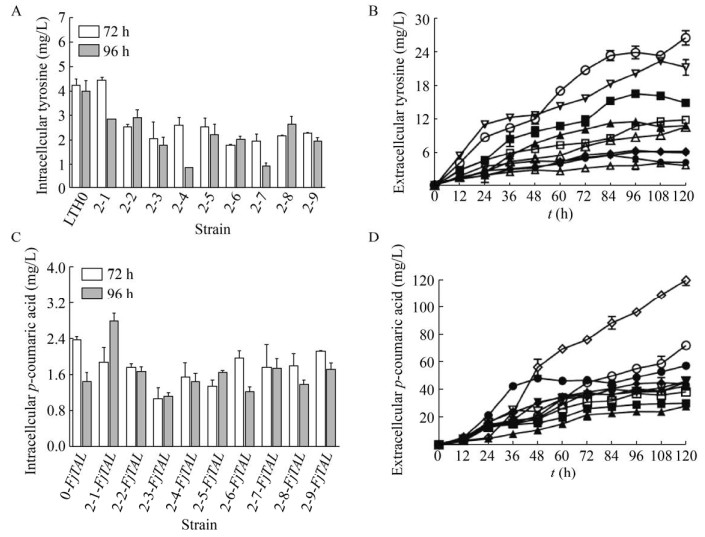 |
| 图 8 复合诱变株对香豆酸与酪氨酸积累情况 Fig. 8 Tyrosine and p-coumaric acid production in strains by compound mutation. ●: LTH0; ○: LTH2-1; ▲: LTH2-2; △: LTH2-3; ◆: LTH2-4; ◇: LTH2-5; ■: LTH2-6; □: LTH2-7; ▼: LTH2-8; ▽: LTH2-9. (A) Intracellular tyrosine production. (B) Extracellular tyrosine production. (C) Intracellular p-coumaric acid production. (D) Extracellcular p-coumaric acid production. |
| 图选项 |
从上述突变株中挑选出酪氨酸和对香豆酸显著提高的LTH1-1、LTH1-4、LTH2-1、LTH2-5四株突变株进一步分析其生长代谢,结果如图 9所示。其中产量最高的LTH2-5发酵过程中乙醇生产量高于其他菌株,乙醇的大量积累可能是其生长情况不佳的重要因素。相较于LTH0,4株突变株的葡萄糖消耗速率有所减缓,在发酵前期突变株生长速度低于LTH0,但是在发酵后期依然能缓慢生长,原因可能是多代突变影响了菌株自身的生长活力。
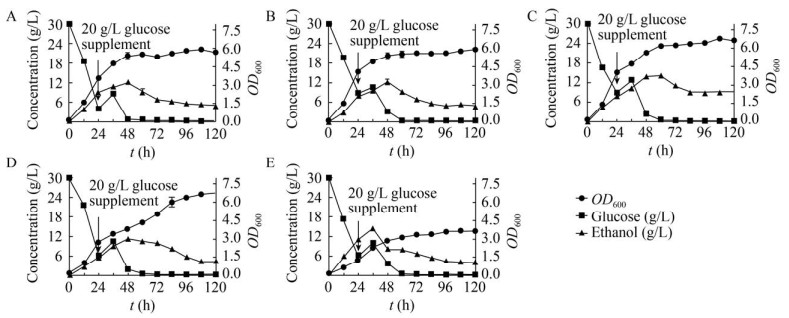 |
| 图 9 高效突变株生长情况及副产物积累 Fig. 9 The growth profile of mutant strains LTH0 (A), LTH1-1 (B), LTH1-4 (C), LTH2-1 (D), LTH2-5 (E) and the accumulation of by-products. |
| 图选项 |
3 讨论笔者在酿酒酵母中异源表达植物中细胞色素P450多巴氧化酶基因CYP76AD1和多巴双加氧酶合成基因DOD,酵母积累甜菜黄素使得细胞带有明显黄色,将荧光强度这一易于检测的指标作为高产酪氨酸的筛选标记,能够大大简化产物测定的过程并实现高产酪氨酸菌株的高通量筛选。
本实验室前期构建了一株能初步积累酪氨酸的酿酒酵母LTH0。通过紫外诱变与ARTP等离子体诱变相结合,经过流式细胞仪荧光分选出14株高产甜菜黄素菌株,对比了紫外诱变与复合诱变的诱变效果后发现复合诱变效果最佳,得到突变株LTH2-5-DOD-CYP76AD1,荧光强度达到(5 941±435) AU/OD,相比于LTH0-DOD-CYP76AD1提高了8.29倍。最终,LTH2-5在胞外积累了26.8 mg/L的酪氨酸和119.8 mg/L的对香豆酸,相比于对照菌株胞外酪氨酸提高了3.96倍,对香豆酸提高了1.02倍。
由于发酵过程均采用SC合成培养基,营养不足,菌体生长比较缓慢。后续研究将继续整合FjTAL到基因组上,并进一步优化发酵条件,改造莽草酸及分支酸途径加强前体供应,大部分突变株胞内酪氨酸含量减少而胞外积累量增加,下一步将对高产突变株进行基因组重测序,从基因组学的角度分析可能的调控位点并采取反向代谢工程策略,对高速生长但低产酪氨酸及对香豆酸的菌株进行定向改造以期得到高速生长的高产菌株。
参考文献
| [1] | Lopez-Nieves S, Pringle A, Maeda HA. Biochemical characterization of TyrA dehydrogenases from Saccharomyces cerevisiae (Ascomycota) and Pleurotus ostreatus (Basidiomycota). Arch Biochem Biophys, 2019, 665: 12-19. DOI:10.1016/j.abb.2019.02.005 |
| [2] | Tan X, Song W, Chen XL, et al. Recent advances in biocatalytic derivatization of L-tyrosine. Appl Microbiol Biotechnol, 2020, 104(23): 9907-9920. DOI:10.1007/s00253-020-10949-6 |
| [3] | Lynch JH, Dudareva N. Aromatic amino acids: a complex network ripe for future exploration. Trends Plant Sci, 2020, 25(7): 670-681. DOI:10.1016/j.tplants.2020.02.005 |
| [4] | Li YK, Li J, Qian BB, et al. De novo biosynthesis of p-coumaric acid in E. coli with a trans-cinnamic acid 4-hydroxylase from the amaryllidaceae plant Lycoris aurea. Molecules, 2018, 23(12): 3185. DOI:10.3390/molecules23123185 |
| [5] | Shen YP, Niu FX, Yan ZB, et al. Recent advances in metabolically engineered microorganisms for the production of aromatic chemicals derived from aromatic amino acids. Front Bioeng Biotechnol, 2020, 8: 43. DOI:10.3389/fbioe.2020.00043 |
| [6] | Leuchtenberger W, Huthmacher K, Drauz K. Biotechnological production of amino acids and derivatives: current status and prospects. Appl Microbiol Biotechnol, 2005, 69(1): 1-8. DOI:10.1007/s00253-005-0155-y |
| [7] | Huccetogullari D, Luo ZW, Lee SY. Metabolic engineering of microorganisms for production of aromatic compounds. Microb Cell Fact, 2019, 18(1): 41. DOI:10.1186/s12934-019-1090-4 |
| [8] | Averesch NJH, Kr?mer JO. Metabolic engineering of the shikimate pathway for production of aromatics and derived compounds-present and future strain construction strategies. Front Bioeng Biotechnol, 2018, 6: 32. DOI:10.3389/fbioe.2018.00032 |
| [9] | Gold ND, Gowen CM, Lussier FX, et al. Metabolic engineering of a tyrosine-overproducing yeast platform using targeted metabolomics. Microb Cell Fact, 2015, 14: 73. DOI:10.1186/s12934-015-0252-2 |
| [10] | Deaner M, Alper HS. Systematic testing of enzyme perturbation sensitivities via graded dCas9 modulation in Saccharomyces cerevisiae. Metab Eng, 2017, 40: 14-22. DOI:10.1016/j.ymben.2017.01.012 |
| [11] | Luttik MAH, Vuralhan Z, Suir E, et al. Alleviation of feedback inhibition in Saccharomyces cerevisiae aromatic amino acid biosynthesis: quantification of metabolic impact. Metab Eng, 2008, 10(3/4): 141-153. |
| [12] | Patnaik R, Zolandz RR, Green DA, et al. L-tyrosine production by recombinant Escherichia coli: fermentation optimization and recovery. Biotechnol Bioeng, 2008, 99(4): 741-752. DOI:10.1002/bit.21765 |
| [13] | Santos CNS, Xiao WH, Stephanopoulos G. Rational, combinatorial, and genomic approaches for engineering L-tyrosine production in Escherichia coli. Proc Natl Acad Sci USA, 2012, 109(34): 13538-13543. DOI:10.1073/pnas.1206346109 |
| [14] | Rodriguez A, Kildegaard KR, Li MJ, et al. Establishment of a yeast platform strain for production of p-coumaric acid through metabolic engineering of aromatic amino acid biosynthesis. Metab Eng, 2015, 31: 181-188. DOI:10.1016/j.ymben.2015.08.003 |
| [15] | Gu Y, Ma JB, Zhu YL, et al. Engineering Yarrowia lipolytica as a chassis for de novo synthesis of five aromatic-derived natural products and chemicals. ACS Synth Biol, 2020, 9(8): 2096-2106. DOI:10.1021/acssynbio.0c00185 |
| [16] | Borja GM, Rodriguez A, Campbell K, et al. Metabolic engineering and transcriptomic analysis of Saccharomyces cerevisiae producing p-coumaric acid from xylose. Microb Cell Fact, 2019, 18(1): 191. DOI:10.1186/s12934-019-1244-4 |
| [17] | 张思琪, 周景文, 张国强, 等. 产对香豆酸酿酒酵母工程菌株的构建与优化. 生物工程学报, 2020, 36(9): 1838-1848. Zhang SQ, Zhou JW, Zhang GQ, et al. Construction and optimization of p-coumaric acid-producing Saccharomyces cerevisiae. Chin J Biotech, 2020, 36(9): 1838-1848 (in Chinese). |
| [18] | Mao JW, Liu QL, Li YZ, et al. A high-throughput method for screening of L-tyrosine high-yield strains by Saccharomyces cerevisiae. J Gen Appl Microbiol, 2018, 64(4): 198-201. DOI:10.2323/jgam.2017.12.001 |
| [19] | Hou YN, Liu X, Li SL, et al. Metabolic engineering of Escherichia coli for de novo production of betaxanthins. J Agric Food Chem, 2020, 68(31): 8370-8380. DOI:10.1021/acs.jafc.0c02949 |
| [20] | Mao JW, Liu QL, Song XF, et al. Combinatorial analysis of enzymatic bottlenecks of L-tyrosine pathway by p-coumaric acid production in Saccharomyces cerevisiae. Biotechnol Lett, 2017, 39(7): 977-982. DOI:10.1007/s10529-017-2322-5 |
| [21] | Gandia-Herrero F, Garcia-Carmona F, Escribano J. Floral fluorescence effect. Nature, 2005, 437(7057): 334. DOI:10.1038/437334a |
| [22] | Sasaki N, Abe Y, Goda Y, et al. Detection of DOPA 4, 5-dioxygenase (DOD) activity using recombinant protein prepared from Escherichia coli cells harboring cDNA encoding DOD from Mirabilis jalapa. Plant Cell Physiol, 2009, 50(5): 1012-1016. DOI:10.1093/pcp/pcp053 |
| [23] | DeLoache WC, Russ ZN, Narcross L, et al. An enzyme-coupled biosensor enables (S)-reticuline production in yeast from glucose. Nat Chem Biol, 2015, 11(7): 465-471. DOI:10.1038/nchembio.1816 |
| [24] | 冉艳朋, 徐沙, 李由然, 等. 代谢工程改造酿酒酵母促进L-苯丙氨酸的合成. 食品与发酵工业, 2020, 46(9): 1-9. Ran YP, Xu S, Li YR, et al. Metabolically engineered Saccharomyces cerevisiae for L-phenylalanine synthesis. Food Ferment Ind, 2020, 46(9): 1-9 (in Chinese). |
