

中国农业科学院饲料研究所,北京 100081
收稿日期:2020-02-04;接收日期:2020-04-27;网络出版时间:2020-05-09
基金项目:国家自然科学基金(No. 31601976)资助
摘要:甘露聚糖酶和木聚糖酶是主要的半纤维素降解酶,在食品、饲料、纺织、造纸等工业应用广泛且通常搭配使用。文中将蓝状菌Talaromyces leycettanus JCM12802来源的性质优良的甘露聚糖酶编码基因man5A的CBM (Carbohydrate-binding module)编码区去除,留下连接区和催化区,并将木聚糖酶基因Tlxyn11B成熟区编码序列与man5A的连接区进行融合,形成Tlxyn11B-linker-man5A融合基因,并在毕赤酵母中成功表达,获得了融合蛋白Tlxyn11B-Man5A。Tlxyn11B、不含CBM区的Man5A和Tlxyn11B-Man5A的理论分子量分别为21.6 kDa、41.0 kDa、62.6 kDa。对纯化后的融合蛋白进行了性质分析,融合蛋白同时具有高的木聚糖酶和甘露聚糖酶活性。融合后的木聚糖酶的最适温度为70 ℃,较单独表达时提高了5 ℃。甘露聚糖酶的最适温度为90 ℃,与融合前一致。融合后的木聚糖酶热稳定性明显提高,60 ℃处理1 h剩余48%的酶活力,单独表达的木聚糖酶60 ℃处理20 min仅剩余20%的酶活力。融合后的木聚糖酶和甘露聚糖酶的最适pH分别为4.0和5.0,较单独表达时分别提高了0.5和1.0个单位,融合后酶的作用pH范围有所拓宽。融合前后的蛋白均具有较好的pH稳定性。融合后木聚糖酶和甘露聚糖酶的比活分别为1 784.3 U/mg和1 639.6 U/mg,较单独表达时比活(8 300 U/mg和1 979 U/mg)降低,与融合酶分子量增大相关。融合后的木聚糖酶和甘露聚糖酶的Km值分别为1.2 mg/mL和1.7 mg/mL, Vmax分别为2 000.0 μmol/(min·mg)和2 831.6 μmol/(min·mg)。综合其性质特点,融合木聚糖酶和甘露聚糖酶在饲料、食品等工业生产中有较大应用潜力,并为酶的性能改良提供了新的思路。
关键词:甘露聚糖酶木聚糖酶融合表达毕赤酵母性质分析
Fusion expression of β-mannanase Man5A and xylanase Tlxyn11B in Pichia pastoris
Haiyang Kong, Xiao Jiang, Yuan Wang, Huoqing Huang, Huiying Luo


Feed Research Institute, Chinese Academy of Agricultural Sciences, Beijing 100081, China
Received: February 4, 2020; Accepted: April 27, 2020; Published: May 9, 2020
Supported by: National Natural Science Foundation of China (No. 31601976)
Corresponding author: Huiying Luo. Tel: +86-10-82106065; E-mail: luohuiying@caas.cn.
Abstract: Mannanase and xylanase, the main hemicellulolytic enzymes, are widely used in food, feed, textile and papermaking industries, and usually they are used in combination. Mannanase Man5A from Talaromyces leycettanus JCM12802 consist of the carbohydrate binding module (CBM), linker region and catalytic domain. The CBM coding region of Man5A was removed and fused to C-terminal of the xylanase gene Tlxyn11B. The fusion gene Tlxyn11B-linker-man5A was successfully expressed in Pichia pastoris and the fusion protein Tlxyn11B-Man5A was purified and characterized. The theoretical molecular weights of Tlxyn11B, Man5A without CBM region, and Tlxyn11B-Man5A are 21.6 kDa, 41.0 kDa, and 62.6 kDa, respectively. The fusion protein had high xylanase and mannanase activities. The optimal temperature of the fused xylanase is 70 ℃, which is 5 ℃ higher than Tlxyn11B-w (xylanase before fusion). The fused mannanase exhibited maximal activity at 90 ℃, which is similar to Man5A-w (mannanase before fusion). More than 48% of xylanase activity of Tlxyn11B-Man5A was residual after the condition of 60 ℃ with 1 h, which is significantly higher than Tlxyn11B-w (only 20% of activity was left at 60 ℃ for 20 minutes). The optimal pHs of Tlxyn11B-Man5A for xylanase and mannanase activity are 4.0 and 5.0, respectively, which are 0.5 and 1.0 units higher than those of Tlxyn11B-w and Man5A-w. The pH range of fused enzymes got wider and the pH stability is improved. The specific activities of xylanase and mannanase of Tlxyn11B-Man5A are 1 784.3 U/mg and 1 639.6 U/mg, respectively, which is lower than those of Tlxyn11B and Man5A (8 300.0 U/mg and 1 979.0 U/mg). It may be due to of the high molecular weight of fusion enzyme. The Km and Vmax of the fused xylanase and mannanase are 1.2 mg/mL and 1.7 mg/mL, 2 000.0 μmol/(min·mg) and 2 831.6 μmol/(min·mg), respectively. Tlxyn11B and Man5A were successfully fusion expressed in P. pastoris, and the good properties of fusion of xylanase and mannanase make it has great application potential in animal feed, food and other industrial production, and it provided new ideas for the improvement of enzyme performance.
Keywords: β-mannanasexylanasefusion expressionPichia pastorischaracterization
半纤维素这一概念于1891年由Schulze提出,表示植物中能够用2%–20%的NaOH溶出的酸性多糖,通常占植物干重的15%–30%[1],硬质木材和禾本科植物中半纤维素的主要成分为木聚糖[2],而植物的种子和果实中半纤维素主要成分为甘露聚糖[3]。木聚糖酶、甘露聚糖酶在半纤维素的生物转化过程中发挥重要作用[4]。在半纤维素的水解中甘露聚糖酶和木聚糖酶通常联合使用[3],且两种酶在降解半纤维素方面存在协同效果[5]。木聚糖酶(Endo-1, 4-β-xylanase,EC 3.2.1.8)能够作用于含有β-1, 4木糖苷键的木聚糖的主链并将其降解为木二糖及木二糖以上的寡聚和少量木糖[6]。主要分布在糖苷水解酶(Glycoside hydrolase,GH)第5、7、8、10、11和43家族[7],其中GH10和GH11家族研究最多,GH10家族木聚糖酶采用(β/α)8桶状结构,而GH11家族木聚糖酶为β-果冻卷结构,它们均以双保留机制对底物进行降解(催化氨基酸为两个谷氨酸)。β-甘露聚糖酶(β-1, 4-mannanase,EC 3.2.1.78)能够水解含有β-1, 4-D-甘露糖苷键的甘露聚糖,主要分布在糖苷水解酶GH5和GH26家族[8-10],两个家族序列相似性通常不足20%,但它们均为规则的TIM桶结构且采用相同的双保留机制(催化氨基酸通常为位于第4个和第7个β-折叠片上的谷氨酸)[11-12],此外在GH113和GH134等家族甘露聚糖酶也有少数分布。糖苷水解酶除了含有一个成熟的催化结构域,通常还含有一个碳水化合物结合结构域,目前根据氨基酸序列,结合特异性和结构被分为86个家族(CAZy数据库:www.cazy.org),它们之间通常借助一个较短的连接区进行连接,CBM通常与底物的水解和酶的稳定性相关,已有报道的甘露聚糖酶中含有的CBM结构域分布在CBM1、2、27、35等家族中,通常由β折叠片构成三明治结构[13-16]。
有的糖苷水解酶具有双功能或多功能,但天然存在的多功能酶普遍存在活性低、耐酸碱及高温能力差等特点[17-20]。蛋白质融合技术作为一种具有简化生产工艺、节约生产成本、改善酶学性质等优点的基因工程技术,近年来在蛋白的表达、纯化、性质改良、获得双功能或多功能蛋白、功能特异性的多功能抗体和蛋白的表面展示等方面均有应用[21]。在植物生物质的酶解方面蛋白质融合也得到了广泛的应用,Elleuche等将耐寒基因与木聚糖酶基因进行融合,获得的酶的耐低温能力大幅提升[22]。Lu等融合了葡聚糖酶和木聚糖酶,极大改善了融合酶的催化效率[23]。张献伟等通过将黑曲霉来源的木聚糖酶和甘露聚糖酶借助连接肽进行融合并在猪肾细胞(pK15)中进行表达,融合酶中的木聚糖酶和甘露聚糖酶的活性分别比单独表达时提高了54.0%和104.4%[24]。Adlakha等把从昆虫肠道中获得的木聚糖酶与纤维素酶融合表达,融合酶较亲本酶活性也都有一定提高[25]。
蓝状菌T. leycettanus JCM12802是一株耐热真菌,最适生长温度为40 ℃,目前已经从该菌株克隆获得了大量的生物质降解酶资源,且多数具有耐高温的特性[26],本实验室先前从该菌株中克隆获得了一个GH5家族甘露聚糖酶Man5A,其最适温度为90 ℃,且在80 ℃条件下稳定,同时获得了一个GH11家族木聚糖酶Tlxyn11B,最适温度为65 ℃,比活高达8 300.0 U/mg,但其热稳定性较差。本研究将木聚糖酶基因Tlxyn11B成熟蛋白编码区序列与去除CBM区的甘露糖酶基因man5A通过甘露糖酶连接区相融合,并在毕赤酵母当中进行表达和性质测定,最终融合酶同时具有较高的甘露聚糖酶和木聚糖酶活性,木聚糖酶的热稳定性得到了明显的提升。
1 材料与方法1.1 材料1.1.1 菌株和载体带有木聚糖酶基因的质粒pPIC9-Tlxyn11B和带有甘露聚糖酶基因的质粒pPIC9-man5A均由本实验室保存[27-28]。质粒pEASY-T3和宿主菌大肠杆菌Escherichia coli Trans1-T1购自北京全式金生物技术有限公司,用于目的基因的克隆。质粒pPIC9和表达宿主毕赤酵母Pichia pastoris GS115购自Invitrogen,用于表达载体的构建和外源基因的表达。
1.1.2 试剂和试剂盒限制性内切酶购自TaKaRa有限公司;重组酶和DNA纯化试剂盒购自南京诺唯赞生物公司;质粒提取试剂盒购自OMEGA公司;底物角豆胶和榉木木聚糖购自Sigma-Aldrich;蛋白Marker购自Genestar生物公司;DNA高保真聚合酶FastPfu DNA Polymerase购自北京全式金生物技术有限公司;其他化学试剂均为国产分析纯。
1.1.3 主要培养基及培养条件实验中所用的培养基有LB (Luria-Bertani)液体培养基和固体培养基、YPD (Yeast extract peptone dextrose medium)液体培养基和固体培养基、MD (Minimal dextrose)固体培养基、BMGY (Buffered glycerol-complex medium)培养基、BMMY (Buffered methanol-complex medium)培养基。其详细制备方法可参照文献[19]。
1.2 甘露聚糖酶和木聚糖酶融合基因的获得以带有木聚糖酶TlXYN11B编码基因Tlxyn11B的质粒pPIC9-Tlxyn11B为模板,以xyn11-F1和xyn11-R1为引物进行PCR扩增,获得木聚糖酶成熟区编码基因Tlxyn11B;以质粒pPIC9-man5A为模板,以man-F1和man-R1为引物进行PCR扩增,获得去除了CBM区的甘露聚糖酶基因man5A。PCR反应程序为:95 ℃保温5 min;95 ℃保温30 s,55 ℃退火30 s,72 ℃延伸1 min,30个循环;72 ℃保温10 min。引物序列见表 1,下划线部分代表限制性酶切位点EcoRⅠ和NotⅠ,其中man-F1和xyn11-R1含有15 bp的同源序列以便于后续通过重叠延伸PCR进行木聚糖酶与甘露聚糖酶基因的融合。
表 1 构建木聚糖酶-甘露聚糖酶融合基因的引物序列Table 1 Primer sequences for constructing xylanase-mannanase fusion gene
| Primers | Sequences (5′–3′) |
| man -F1 | ATTACTGTTTCCACCCAAACGACCACGACC ACTCTG |
| man-R1 | ATGCGGCCGCTCACTGAAACTGGCTAATAT |
| xyn11-F1 | TAGAATTCGCTCCTAGCGAGCTCTCCAAAC |
| xyn11-R1 | GGTCGTTTGGGTGGAAACAGTAATGGAAG AA |
| The underline represents the restriction enzyme cleavage site. | |
表选项
上述PCR扩增产物进行凝胶电泳并分别回收目标条带,以回收条带混合物为模板,以xyn11-F1和man-R1为引物进行PCR扩增,反应条件同上。PCR扩增产物连接pEASY-T3热激转化到Trans1-T1感受态细胞,并涂布氨苄青霉素抗性的LB固体培养基平板,37 ℃过夜培养后进行菌落PCR验证,并挑选阳性转化子进行测序验证。
1.3 融合表达质粒pPIC9-Tlxyn11B-man5A的构建、转化与阳性克隆筛选测序正确的克隆进行质粒提取经EcoRⅠ和NotⅠ双酶切后回收基因片段,连入同样经EcoRⅠ和NotⅠ处理的pPIC9载体,转化Trans1-T1大肠杆菌感受态,挑选阳性克隆并测序验证。获得重组载体pPIC9-Tlxyn11B-man5A用于毕赤酵母的转化。
重组载体pPIC9-Tlxyn11B-man5A用限制性内切酶BglⅡ线性化处理,经凝胶电泳纯化后回收线性化产物,利用电击转化毕赤酵母GS115感受态细胞,用1 mol/L的山梨醇重悬,30 ℃静置孵育1 h使细胞复苏,然后涂布于组氨酸缺陷型MD平板,30 ℃恒温箱内倒置培养2–3 d至长出肉眼可见的单菌落,随机用牙签挑选48个克隆子后点种到标有数字1–100的MD和MM平板上30 ℃恒温箱内倒置培养1–2 d,用牙签挑取克隆接种于有相应标号的含有3 mL BMGY培养基的15 mL酵母管中,30 ℃、220 r/min培养48 h后离心去除上清液,菌体用1 mL BMMY培养基重悬,诱导培养48 h后离心收集上清液进行酶活测定,筛选出高活性的转化子。
1.4 重组酶的获得和纯化筛选获得的活性高的转化子进行摇瓶培养。将阳性转化子在YPD培养基活化后按1%接种量接种于300 mL BMGY液体培养基中,30 ℃、220 r/min培养48 h,4 500 r/min离心5 min弃上清,菌体重悬于150 mL含有0.5%甲醇的BMMY液体培养基继续培养48 h,期间每隔12 h补加0.5%的甲醇。培养结束后离心收集上清液,经截留分子量10 kDa的膜包浓缩,用10 mmol/L的柠檬酸-磷酸氢二钠缓冲液(pH 6.5)进行过夜透析以去除多余的盐分。透析处理后的酶液加载到经过平衡的HiTrap QXL阴离子交换层析柱中,并用10 mmol/L的pH 6.5的柠檬酸-磷酸氢二钠缓冲液的配制的1 mol/L NaCl进行梯度洗脱,收集相应的峰所对应的蛋白并进行酶活性的测定和SDS-PAGE分析。
1.5 酶活力的测定木聚糖酶及甘露聚糖酶的酶活性测定均采用3, 5-二硝基水杨酸(DNS)法,具体参照Miller等[29]的方法并有所改进。具体反应体系为:100 μL适当稀释的酶液,900 μL质量体积比为1%的榉木木聚糖(用于木聚糖酶酶活的测定)或0.5%角豆胶(用于甘露聚糖酶酶活的测定)的柠檬酸-磷酸氢二钠溶液,恒温反应10 min后加入1.5 mL DNS终止反应,沸水浴中煮沸5 min后立即置于冷水中冷却,取250 μL反应终止液于540 nm下测定吸光值,并根据相应的标准曲线计算出酶活力。木聚糖酶活力在pH 4.0、70 ℃条件下测定,甘露聚糖酶活力在pH 5.0、90 ℃条件下测定。酶活定义为:在上述条件下,每分钟产生1 μmol还原糖所需的酶量为1个单位(U)。
1.6 酶学性质分析最适温度及热稳定性的测定:将纯化后的融合酶进行适当稀释,在30 ℃–95 ℃温度条件下及pH 4.0或pH 5.0的条件下分别测定木聚糖酶和甘露聚糖酶的酶活,以确定融合酶中木聚糖酶和甘露聚糖酶的最适温度。将稀释酶液(100 ng/μL)分别在60 ℃、70 ℃、80 ℃三个不同温度下处理2、5、8、10、20、30、60 min后立即置于冰上,之后稀释合适的倍数并在最适条件下测定处理过的酶液的剩余酶活。以未经处理的酶的酶活力为100%,分别计算不同温度条件、处理不同时间后样品的剩余酶活以测定酶在不同温度下的稳定性。
最适pH及pH稳定性的测定:分别在甘露聚糖酶和木聚糖酶的最适温度条件下测定不同pH (1.0–12.0)条件下的酶活力来确定最适pH;将酶用不同pH的缓冲液进行稀释,37 ℃恒温孵育1 h,在最适条件下测定剩余酶活力,并以未处理酶液的酶活力为100%,计算不同pH处理下的相对剩余酶活以测定酶在不同pH下的稳定性。
动力学常数的测定:分别以浓度范围为0.1–10.0 mg/mL的榉木木聚糖和0.5–5.0 mg/mL的角豆胶为底物,在pH 4.0、70 ℃条件下测定木聚糖酶活性,在pH 5.0、90 ℃条件下测定甘露聚糖酶活性,反应时间均为5 min,利用双倒数法作图并计算得到Km及Vmax的值。
金属离子及化学试剂对酶活性的影响测定:将5 mmol/L的不同的金属离子和化学试剂添加到酶促反应体系中,以不添加任何离子和化学试剂作为对照,在酶的最适条件下测定它们对酶活性的影响。
2 结果与分析2.1 重组载体的构建与融合蛋白的表达与纯化通过PCR将594 bp的木聚糖酶成熟区编码基因Tlxyn11B和1 131 bp的甘露聚糖酶基因man5A (不含N端CBM区)进行拼接,获得1 725 bp的融合基因Tlxyn11B-linker-man5A,融合基因编码的蛋白理论分子量为62.6 kDa,理论等电点为4.82。融合基因Tlxyn11B-linker-man5A在毕赤酵母GS115中实现了分泌表达,获得融合蛋白Tlxyn11B-Man5A。其表观分子量约为70 kDa,较理论分子量稍大(图 1),该融合酶有2个可能的N糖基化位点,在毕赤酵母中表达可能发生了糖基化从而造成表观分子量大于理论分子量。
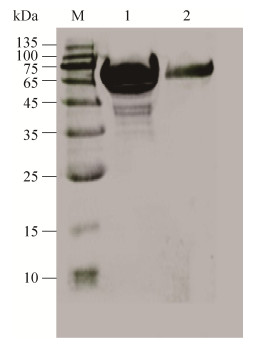 |
| 图 1 融合蛋白Tlxyn11B-Man5A的SDS-PAGE分析 Fig. 1 SDS-PAGE analysis of fusion protein Tlxyn11B-Man5A. Lane M: the standard modecule weight; lane 1: concentrated Tlxyn11B-Man5A; lane 2: purified Tlxyn11B-Man5A. |
| 图选项 |
2.2 融合酶的酶学性质2.2.1 融合酶的最适pH及pH稳定性在pH 1.0–8.0范围内测定甘露聚糖酶和木聚糖酶的最适pH,结果显示融合酶的木聚糖酶的最适pH (图 2A)为4.0,在pH 3.0–5.0范围内能够维持60%以上的酶活力。融合酶中的甘露聚糖酶的最适pH (图 2B)为5.0,在pH 3.0–5.5范围内甘露聚糖酶的相对酶活力维持在50%以上。在pH 1.0–12.0范围内测定融合酶的pH稳定性,结果显示木聚糖酶在pH 2.0–9.0时稳定,pH 2.0时剩余酶活仍达90% (图 2C)。甘露聚糖酶(图 2D)在pH 2.0–9.0范围内,剩余酶活力能够维持在60%以上(图中Tlxyn11B-W和TlxyB分别代表融合前后的木聚糖醇,Man5A-W和Man5A分别代表融合前后的甘露聚糖醇)。
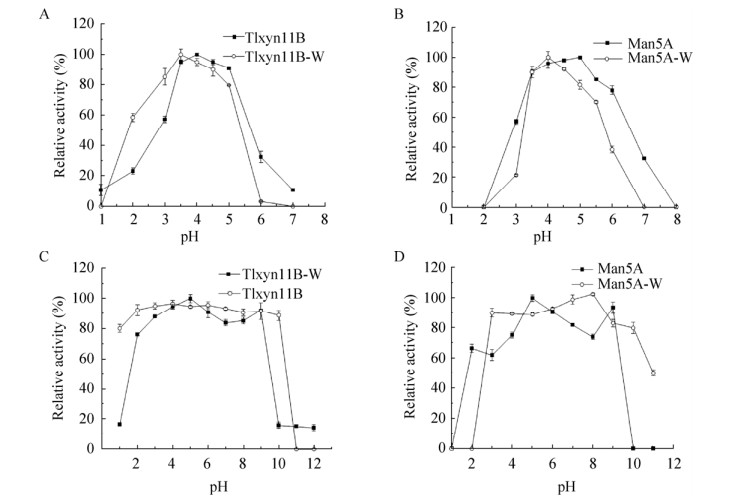 |
| 图 2 融合前后木聚糖酶和甘露聚糖酶的最适pH及pH稳定性 Fig. 2 Optimum pH and pH stability of xylanase and mannanase before and after fusion. (A) Effect of pH on the activities of xylanase Tlxyn11B-W and Tlxyn11B. (B) Effect of pH on the activities of mannanase Man5A-W and Man5A. (C) pH stabilities of xylanase Tlxyn11B-W and Tlxyn11B. (D) pH stabilities of mannanase Man5A-W and Man5A. |
| 图选项 |
2.2.2 融合酶的最适温度及温度稳定性分别在pH 4.0和pH 5.0条件下测定融合酶的木聚糖酶和甘露聚糖酶在不同温度(30–95 ℃)的酶活力,结果显示(图 3A–B)融合酶中木聚糖酶和甘露聚糖酶分别在70 ℃和90 ℃时具有最高酶活力。甘露聚糖酶在80–95 ℃范围内维持在50%以上酶活力。木聚糖酶在低于70 ℃时酶活随温度升高而升高,70 ℃以上酶活急剧下降。在最适pH 4.0的条件下将酶液分别在60 ℃、70 ℃条件下处理不同时间测定木聚糖酶的热稳定性,结果显示(图 3C),60 ℃处理1 h还剩余48%的酶活力,70 ℃处理2 min还剩60%的酶活力,70 ℃处理5 min还剩23%的酶活力。在最适pH 5.0的条件下将酶液分别在70 ℃、80 ℃条件下处理不同的时间测定甘露聚糖酶的温度稳定性,结果表明(图 3D),70 ℃处理2 min还剩余77.5%的酶活,80 ℃处理2 min还剩余71.26%的酶活。
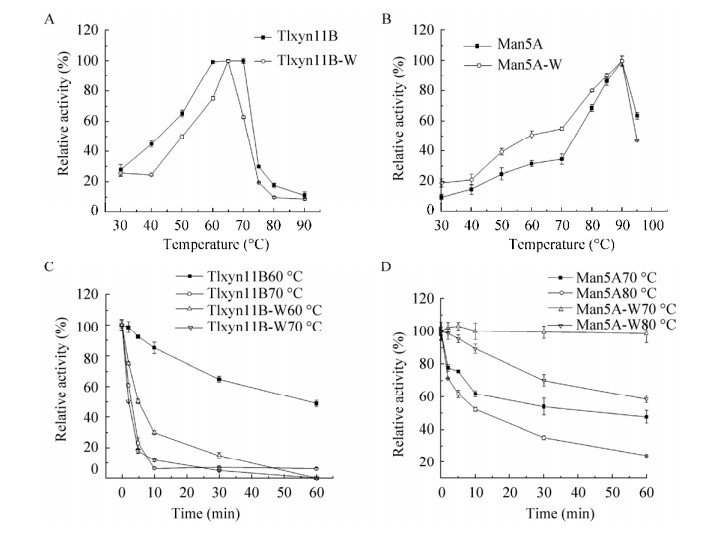 |
| 图 3 融合前后木聚糖酶和甘露聚糖酶的最适温度及热稳定性 Fig. 3 Optimum temperature and thermostability of xylanase and mannanase before and after fusion. (A) Effect of temperature on the activities of xylanase Tlxyn11B-W and Tlxyn11B. (B) Effect of temperature on the activities of mannanase Man5A-W and Man5A. (C)Thermostability of xylanase Tlxyn11B-W and Tlxyn11B. (D) Thermostability of mannanase Man5A-W and Man5A. |
| 图选项 |
2.2.3 融合酶的动力学参数测定分别在甘露聚糖酶和木聚糖酶的最适条件下以角豆胶和榉木木聚糖为底物进行测定,经Michaelis-Menten模型计算出融合酶中甘露聚糖酶和木聚糖酶的动力学参数(表 2),最终得到融合酶的甘露聚糖酶的Km和Vmax分别为1.7 mg/mL、2 831.6 μmol/(min·mg),木聚糖酶的Km和Vmax分别为1.2 mg/mL、2 000.0 μmol/ (min·mg)。
表 2 木聚糖酶和甘露聚糖酶融合前后的动力学参数Table 2 Kinetic parameters before and after fusion of xylanase and mannanase
| Vmax (μmol/(min·mg)) | Km (mg/mL) | Kcat/Km (s/(mL·mg)) | U (U/mg) | |
| Tlxyn11B | 2 000.0 | 1.2 | 1 805.6 | 1 784.3 |
| Tlxyn11B-W | 12 800.0 | 3.3 | 1 426.0 | 8 300.0 |
| Man5A | 2 831.6 | 1.7 | 2 921.3 | 1 639.6 |
| Man5A-W | 3 200.0 | 2.0 | 2 400.0 | 1 979.0 |
表选项
2.2.4 金属离子及化学试剂对酶活性的影响如表 3所示,融合前后金属离子和化学试剂对酶的活性的影响基本无变化,对于甘露聚糖酶Cr3+、Cu2+、Fe3+、SDS能够抑制酶活,而其他离子和化学试剂对酶活几乎没影响。对于木聚糖酶Pb2+、Cu2+、Fe3+、SDS能够抑制酶活,其余离子和化学试剂对其无明显影响。
表 3 不同金属离子和化学试剂对融合前后甘露聚糖酶和木聚糖酶活性的影响Table 3 Effects of different metal ions and chemical reagents on mannanase and xylanase activities before and after fusion
| Chemical | Man5A-W Relative activity (%) | Man5A Relative activity (%) | Tlxyn11B-W Relative activity (%) | Tlxyn11B Relative activity (%) |
| Control | 100.0±1.9 | 100.0±3.2 | 100.0±1.7 | 100.0±0.5 |
| Cr3+ | 57.8±5.6 | 68.1±1.3 | 101.8±5.9 | 100.7±3.1 |
| Zn2+ | 105.7±2.8 | 102.5±2.1 | 99.9±1.2 | 97.7±2.2 |
| Mg2+ | 121.4±2.0 | 110.2±3.4 | 106.9±4.0 | 100.6±5.6 |
| Na2+ | 119.6±0.4 | 112.5±2.6 | 107.5±12.3 | 98.6±6.3 |
| Pb2+ | 92.7±3.7 | 85.3±4.1 | 86.5±2.3 | 89.3±1.8 |
| Ni2+ | 94.9±1.0 | 100.3±1.9 | 107.8±0.4 | 94.1±6.5 |
| Cu2+ | 68.7±0.6 | 80.5±2.4 | 85.6±8.6 | 80.6±3.1 |
| K+ | 117.0±2.2 | 100.6±4.8 | 112.8±1.7 | 108.8±4.0 |
| Fe3+ | 50.7±1.7 | 65.5±5.3 | 54.7±1.3 | 66.4±6.2 |
| Mn2+ | 91.5±2.0 | 100.3±2.5 | 96.3±3.2 | 90.1±2.9 |
| EDTA | 120.9±1.0 | 105.4±6.1 | 113.3±8.9 | 97.2±3.6 |
| SDS | 20.8±1.0 | 10.3±0.2 | 33.1±5.6 | 18.6±1.2 |
| Ca2+ | 99.4±1.1 | 110.2±5.3 | 109.3±0.9 | 90.8±1.5 |
表选项
3 讨论研究通过甘露聚糖酶自身的连接区将木聚糖酶和甘露聚糖酶相连,从而实现了融合表达,获得了具有高活性的木聚糖酶和甘露聚糖酶的双功能融合蛋白,为半纤维素的降解及饲料和食品中的应用提供了便利。基于蛋白质融合来获得多功能酶的研究不乏报道[30-31],但融合设计并非都能成功,如涉及纤维素酶-木聚糖酶的研究中,与淀粉酶C末端融合的葡聚糖酶失去活性[32]。融合酶的种类、来源、结构、融合顺序、连接肽的长短和种类对融合酶的性质都可能有重要的影响[24, 32-33]。多数糖苷水解酶从结构上来看其N端和C端相距较近并发生相互作用,任意一端的变动都可能影响酶的整体结构进而影响酶学性质。本研究中甘露聚糖酶Man5A的CBM区属于CBM1家族,主要是由β折叠片构成的三明治结构,而木聚糖酶Tlxyn11B从三维结构上来看同样主要是由β折叠片构成,在结构上与CBM1有一定的相似度,从而保证了酶结构功能的正常,该项工作为融合酶的选择提供了一定的指导。
甘露聚糖酶Man5A有非常好的热稳定性[27],而木聚糖酶Tlxyn11B热稳定性相对较差。当将Man5A的CBM替换为木聚糖酶Tlxyn11B进行融合时,使木聚糖酶的热稳定性明显提高[28],而甘露聚糖酶热稳定性有所降低,但优于去掉CBM的Man5A (Man5AΔCBM)热稳定性[27]。Wang等报道Man5A在80 ℃处理60 min后,仍然保留50%的酶活力,而Man5A-CBM在80 ℃处理30 min后完全失活。Tlxyn11B-Man5A的稳定性处于Man5A和Man5A-CBM之间,可见CBM在甘露聚糖酶Man5A热稳定性的维持中发挥重要的作用,且与CBM的结构相关。木聚糖酶Tlxyn11B热稳提高的机制尚不明确,推测与连接区对木聚糖酶的保护相关。同时,我们发现木聚糖酶Tlxyn11B作用的pH范围在融合表达后也得到了拓宽。而融合前后木聚糖酶和甘露聚糖酶对不同金属离子和化学试剂的抗性没有明显区别,Cu2+、Fe3+、SDS能够同时抑制两种酶的活性,此外Cr3+对甘露聚糖酶,Pb2+对木聚糖酶也有部分抑制作用,说明融合表达并不影响两种酶对金属离子和化学试剂的抗性。动力学参数方面,融合后甘露聚糖酶和木聚糖酶的Km值均降低,说明融合后有利于酶与底物的结合,从融合后Kcat/Km值均增加来看,融合后两种酶的催化效率也都有提高。这类通过蛋白质融合改变酶的活力和热稳定性的研究,目前大多认为是连接肽的种类和长短影响了融合后蛋白单个结构域及各结构域间相互作用[23, 34]。
综上,通过不同活性酶编码基因的融合表达,我们获得了一个同时具有高的甘露聚糖酶和木聚糖酶的双功能蛋白,并提高了木聚糖酶的热稳定性。这为后续的应用提供了便利、节约了成本,也为酶性能的改良提供了思路。
参考文献
| [1] | Beukes N, Pletschke BI. Effect of lime pre-treatment on the synergistic hydrolysis of sugarcane bagasse by hemicellulases. Bioresour Technol, 2010, 101(12): 4472-4478. DOI:10.1016/j.biortech.2010.01.081 |
| [2] | Yamamoto M, Iakovlev M, Bankar S, et al. Enzymatic hydrolysis of hardwood and softwood harvest residue fibers released by sulfur dioxide-ethanol-water fractionation. Bioresour Technol, 2014, 167: 530-538. DOI:10.1016/j.biortech.2014.06.054 |
| [3] | Várnai A, Huikko L, Pere J, et al. Synergistic action of xylanase and mannanase improves the total hydrolysis of softwood. Bioresour Technol, 2011, 102(19): 9096-9104. DOI:10.1016/j.biortech.2011.06.059 |
| [4] | Soni H, Ganaie MA, Pranaw K, et al. Design-of-experiment strategy for the production of mannanase biocatalysts using plam karnel cake and its application to degrade locust bean and guar gum. Biocatal Agric Biotechnol, 2015, 4(2): 229-234. DOI:10.1016/j.bcab.2015.01.001 |
| [5] | Benech RO, Li XM, Patton D, et al. Recombinant expression, characterization, and pulp prebleaching property of a Phanerochaete chrysosporium endo-β-1, 4-mannanase. Enzyme Microb Technol, 2007, 41(6/7): 740-747. |
| [6] | Chen CC, Luo HY, Han X, et al. Structural perspectives of an engineered β-1, 4-xylanase with enhanced thermostability. J Biotechnol, 2014, 189: 175-182. DOI:10.1016/j.jbiotec.2014.08.030 |
| [7] | Pollet A, Delcour JA, Courtin CM. Structural determinants of the substrate specificities of xylanases from different glycoside hydrolase families. Crit Rev Biotechnol, 2010, 30(3): 176-191. DOI:10.3109/07388551003645599 |
| [8] | Luo HY, Wang YR, Wang H, et al. A novel highly acidic β-mannanase from the acidophilic fungus Bispora sp. MEY-1: gene cloning and overexpression in Pichia pastoris. Appl Microbiol Biotechnol, 2009, 82(3): 453-461. |
| [9] | Couturier M, Roussel A, Rosengren A, et al. Structural and biochemical analyses of glycoside hydrolase families 5 and 26 β-(1, 4)-mannanases from Podospora anserina reveal differences upon manno-oligosaccharide catalysis. J Biol Chem, 2013, 288(20): 14624-14635. DOI:10.1074/jbc.M113.459438 |
| [10] | Li JF, Wei XH, Tang CD, et al. Directed modification of the Aspergillus usamii β-mannanase to improve its substrate affinity by in silico design and site-directed mutagenesis. J Ind Microbiol Biotechnol, 2014, 41(4): 693-700. DOI:10.1007/s10295-014-1406-7 |
| [11] | Hilge M, Gloor SM, Rypniewski W, et al. High-resolution native and complex structures of thermostable β-mannanase from Thermomonospora fusca-Substrate specificity in glycosyl hydrolase family 5. Structure, 1998, 6(11): 1433-1444. DOI:10.1016/S0969-2126(98)00142-7 |
| [12] | Zhang YL, Ju JS, Peng H, et al. Biochemical and structural characterization of the intracellular mannanase AaManA of Alicyclobacillus acidocaldarius reveals a novel glycoside hydrolase family belonging to clan GH-A. J Biol Chem, 2008, 283(46): 31551-31558. DOI:10.1074/jbc.M803409200 |
| [13] | Ghosh A, Verma AK, Gautam S, et al. Structure and functional investigation of ligand binding by a family 35 carbohydrate binding module (CtCBM35) of β-mannanase of family 26 glycoside hydrolase from Clostridium thermocellum. Biochemistry (Mosc), 2014, 79(7): 672-686. DOI:10.1134/S0006297914070098 |
| [14] | Von Freiesleben P, Spodsberg N, Stenb?k A, et al. Boosting of enzymatic softwood saccharification by fungal GH5 and GH26 endomannanases. Biotechnol Biofuels, 2018, 11: 194. DOI:10.1186/s13068-018-1184-y |
| [15] | Dos Santos CR, Paiva JH, Meza AN, et al. Molecular insights into substrate specificity and thermal stability of a bacterial GH5-CBM27 endo-1, 4-β-D- mannanase. J Struct Biol, 2012, 177(2): 469-476. DOI:10.1016/j.jsb.2011.11.021 |
| [16] | Tanaka M, Umemoto Y, Okamura H, et al. Cloning and characterization of a beta-1, 4-mannanase 5C possessing a family 27 carbohydrate-binding module from a marine bacterium, Vibrio sp. strain MA-138. Biosci Biotechnol Biochem, 2009, 73(1): 109-116. DOI:10.1271/bbb.80521 |
| [17] | Cao H, Sun LC, Huang Y, et al. Structural insights into the dual-substrate recognition and catalytic mechanisms of a bifunctional acetyl ester-xyloside hydrolase from Caldicellulosiruptor lactoaceticus. ACS Catal, 2019, 9(3): 1739-1747. DOI:10.1021/acscatal.8b03383 |
| [18] | Wang K, Cao RT, Wang ML, et al. A novel thermostable GH10 xylanase with activities on a wide variety of cellulosic substrates from a xylanolytic Bacillus strain exhibiting significant synergy with commercial Celluclast 1.5 L in pretreated corn stover hydrolysis. Biotechnol Biofuels, 2009, 12: 48. |
| [19] | Li XL, Tu T, Yao B, et al. A novel bifunctional xylanase/cellulase TcXyn10A from Thermoascus crustaceus JCM12803. Chin J Biotech, 2018, 34(12): 1996-2006 (in Chinese). 李晓丽, 涂涛, 姚斌, 等. 嗜热子囊菌JCM12803来源的双功能木聚糖/纤维素酶. 生物工程学报, 2018, 34(12): 1996-2006. |
| [20] | Mosina NL, Schubert WD, Cowan DA. Characterization and homology modelling of a novel multi-modular and multi-functional Paenibacillus mucilaginosus glycoside hydrolase. Extremophiles, 2019, 23(6): 681-686. DOI:10.1007/s00792-019-01121-8 |
| [21] | Nie CM, Ning XY, Zhang YH, et al. improvement of lactase secretion level by fusion expression with tag in Pichia pastoris. J Agric Sci Technol, 2012, 14(5): 71-77 (in Chinese). 聂春明, 宁晓彦, 张宇宏, 等. 应用融合标签技术提高乳糖酶在毕赤酵母中的分泌表达. 中国农业科技导报, 2012, 14(5): 71-77. |
| [22] | Elleuche S, Piascheck H, Antranikian G. Fusion of the OsmC domain from esterase EstO confers thermolability to the cold-active xylanase Xyn8 from Pseudoalteromonas arctica. Extremophiles, 2011, 15(2): 311-317. DOI:10.1007/s00792-011-0361-8 |
| [23] | Lu P, Feng MG. Bifunctional enhancement of a β-glucanase-xylanase fusion enzyme by optimization of peptide linkers. Appl Microbiol Biotechnol, 2008, 79(4): 579-587. DOI:10.1007/s00253-008-1468-4 |
| [24] | Zhang XW, Zhang GG, Wu ZF, et al. Peptide linkers optimized recombinant enzyme gene of XynB-ManA and its co-expression in pK15 cells. Sci Agric Sin, 2013, 46(22): 4774-4783 (in Chinese). 张献伟, 张冠冠, 吴珍芳, 等. 木聚糖酶-甘露聚糖酶融合酶基因linker优化及其在猪肾pk15细胞中共表达. 中国农业科学, 2013, 46(22): 4774-4783. |
| [25] | Adlakha N, Rajagopal R, Kumar S, et al. Synthesis and characterization of chimeric proteins based on cellulase and xylanase from an insect gut bacterium. Appl Environ Microbiol, 2011, 77(14): 4859-4866. DOI:10.1128/AEM.02808-10 |
| [26] | Guo YJ, Tu T, Qiu J, et al. Characterization and structure of a novel thermostable glucoamylase from Talaromyces leycettanus JCM12802. Chin J Biotech, 2019, 35(4): 616-625 (in Chinese). 郭玉杰, 涂涛, 邱锦, 等. 篮状菌Talaromyces leycettanus JCM12802来源的高温葡萄糖淀粉酶性质与结构. 生物工程学报, 2019, 35(4): 616-625. |
| [27] | Wang CH, Luo HY, Niu CF, et al. Biochemical characterization of a thermophilic β-mannanase from Talaromyces leycettanus JCM12802 with high specific activity. Appl Microbiol Biotechnol, 2015, 99(3): 1217-1228. DOI:10.1007/s00253-014-5979-x |
| [28] | Wang XY, Ma R, Xie XM, et al. Thermostability improvement of a Talaromyces leycettanus xylanase by rational protein engineering. Sci Rep, 2017, 7(1): 15287. |
| [29] | Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem, 1959, 31(3): 426-428. DOI:10.1021/ac60147a030 |
| [30] | Lu P, Feng MG, Li WF, et al. Construction and characterization of a bifunctional fusion enzyme of Bacillus-sourced β-glucanase and xylanase expressed in Escherichia coli. FEMS Microbiol Lett, 2006, 261(2): 224-230. DOI:10.1111/j.1574-6968.2006.00367.x |
| [31] | Shibuya I, Tamura G, Shima H, et al. Construction of an α-amylase/glucoamylase fusion gene and its expression in Saccharomyces cerevisiae. Biosci Biotechnol Biochem, 1992, 56(6): 884-889. DOI:10.1271/bbb.56.884 |
| [32] | Hong SY, Lee JS, Cho KM, et al. Assembling a novel bifunctional cellulase-xylanase from Thermotoga maritima by end-to-end fusion. Biotechnol Lett, 2006, 28(22): 1857-1862. DOI:10.1007/s10529-006-9166-8 |
| [33] | Lu P. Construction, optimization and characterization of bifunctional β-glucanase and β-glucanase-phytase fusions[D]. Hangzhou: Zhejiang University, 2008 (in Chinese). 陆平.双功能β-葡聚糖酶-木聚糖酶与β-葡聚糖酶-植酸酶融合酶的优化构建及其酶学特性分析[D].杭州: 浙江大学, 2008. http://d.wanfangdata.com.cn/Thesis/Y1378899 |
| [34] | Qiao JY, Cao YH. In-fusion expression and characterization of β-xylanase and β-1, 3-1, 4-glucanase in Pichia pastoris. Biologia, 2012, 67(4): 649-653. |
