

甘肃农业大学 动物医学院 甘肃省牛羊胚胎工程技术研究中心,甘肃 兰州 730070
收稿日期:2019-08-12;接收日期:2019-12-31;网络出版时间:2020-01-07
基金项目:国家重点研发计划(No. 2017YFD0502200)资助
摘要:本研究旨在建立一种多重PCR方法检测青海藏绵羊子宫内膜炎主要的病原菌。首先,提取5种标准菌株基因组,筛选出特异性引物;然后以标准菌株的基因组为模板,建立多重PCR方法。用无菌棉拭子涂抹藏绵羊子宫,置于LB培养液中培养并编号,48 h后提取样品基因组。运用单一PCR法对600份样品基因组进行检测,记录阳性样品;再挑取单一PCR法检测的阳性样品进行多重PCR检测,再次记录阳性样品,通过计算两种检测方法的符合率验证多重PCR方法;随机挑出30份阳性样品,进行病原菌分离鉴定菌种种类。单一PCR检测的样品中,无乳链球菌感染比例占47.33%,大肠杆菌占34.83%,金黄色葡萄球菌占6.5%,未检出沙门氏菌和化脓隐秘杆菌;多重PCR检测的阳性样品中,无乳链球菌感染比例占45.50%,大肠杆菌占33.50%,金黄色葡萄球菌占6.5%;两种检测结果相比较,多重PCR检测出的符合率均高于95%;分离鉴定的病原菌与两种PCR方法检测出的菌种结果基本一致。成功建立了多重PCR方法并检测出引起青海藏绵羊子宫内膜炎的主要病原菌为无乳链球菌、大肠杆菌和金黄色葡萄球菌。
关键词:藏绵羊子宫内膜炎无乳链球菌大肠杆菌金黄色葡萄球菌多重PCR
Establishment and evaluation of multiplex PCR for detection of main pathogenic bacteria of endometritis in Tibetan sheep
Jinhui Han, Meng Wang, Yangyang Pan, Xuequan Hu, Xingyun Zhang, Yan Cui, Gengquan Xu, Libin Wang, Sijiu Yu


Technology and Research Center of Gansu Province for Embryo Engineering of Bovine and Sheep & Goat, College of Veterinary Medicine, Gansu Agricultural University, Lanzhou 730070, Gansu, China
Received: August 12, 2019; Accepted: December 31, 2019; Published: January 7, 2020
Supported by: National Key Research and Development Program of China (No. 2017YFD0502200)
Corresponding author: Sijiu Yu. Tel: +86-931-7631133; E-mail: sjyu@163.com.
Abstract: A multiplex PCR method was developed to detect the main pathogens of Qinghai Tibetan sheep endometritis. First, the genomes of five standard bacterial strains were extracted and specific primers were selected; the multiplex PCR method was established by using the genome of the standard strain as a template. The samples were collected by sterile cotton swab from Tibetan sheep uterus, and then placed in LB medium and numbered. After 48 h, the genomes of cultured bacteria were extracted and detected by single PCR method, then the positive samples were recorded. The positive samples detected by single PCR were selected for multiplex PCR detection and recorded again. The coincidence rate between these two methods was calculated to measure the accuracy of multiplex PCR. In order to identify the species of the pathogen, 30 positive samples verified by single and multiplex PCR were randomly selected for bacterial isolation and identification. In the 600 samples, the infected ratio of Streptococcus agalactiae (GBS) was 47.33%, Escherichia coli 34.83%, Staphylococcus aureus 6.5%, Salmonella and Trueperella pyogenes were negatively detected. Among the positive samples detected by multiplex PCR, the positive ratio of GBS was 45.50%, E. coli 33.50%, S. aureus 6.5%. Comparison of two detection results, Multiplex PCR detection coincidence rate is more than 95%. The isolated pathogens were identified as E. coli, GBS and S. aureus, which was consistent with the results of two methods. The multiplex PCR method was successfully established and the main pathogens of endometritis in Qinghai Tibetan sheep were GBS, E. coli and S. aureus.
Keywords: Tibetan sheep endometritisStreptococcus agalactiaeEscherichia coliStaphylococcus aureusmultiplex PCR
近20年来,随着养羊业的集约化、规模化的不断发展,我国羊的品种和数量越来越多[1-2]。藏绵羊在我国西藏和青海分布最广、数量最多,是高原农牧民重要的生产和生活资料,对牧区的经济发展具有极其重要的作用。藏绵羊终年放牧于天然草场,所产羊肉是纯天然、绿色、无污染、安全、品质好、营养价值高的食品,备受牧民和消费者青睐[3]。发展藏绵羊产业对提高牧民收益、加快牧区建设、促进民族经济繁荣和保持生态平衡具有极其重要的意义。
由于饲养环境以及季节的变化加上管理水平的不足[4-5],导致藏绵羊各类疾病的发病率不断上升,特别是生殖类疾病。子宫内膜炎是严重威胁藏绵羊健康的重要疾病之一,严重阻碍藏羊养殖业的健康发展,大多数是由于围产期绵羊病原微生物感染所引起的一种繁殖障碍性疾病,主要导致产犊间期延长、产奶量降低、绵羊消瘦、体重降低,进而影响绵羊的繁殖性能,也是绵羊不孕不育的主要原因之一,给绵羊养殖业造成严重的经济损失。由于子宫内膜炎不出现全身性的发热、炎症、采食量下降和疼痛等临床症状,特别是隐性子宫内膜炎,易被饲养者忽视,也是淘汰率升高的主要原因[6-7]。因此,快速准确地检测病原菌是防治藏绵羊子宫内膜炎的关键环节。多重PCR法能在同一反应管内同时检出多种病原菌,能极大地节省时间,节省试剂,节约经费开支,为临床提供更快更准确的诊断信息,在临床疾病诊断方面具有实际意义。因此本研究拟建立一种快速简单、准确、重复性好的多重PCR方法对引起青海藏绵羊子宫内膜炎的病原菌进行检测,以期为藏绵羊子宫内膜炎的快速诊断提供可靠的工具,并为青海地区藏绵羊子宫内膜炎的诊断和防治提供理论依据。
1 材料与方法1.1 材料1.1.1 标准菌株大肠杆菌(ATCC25922)、金黄色葡菌球菌(ATCC6538)、沙门氏菌(BNCC103134)保存在甘肃省牛羊胚胎工程技术中心,无乳链球菌(ATCC13813TM)购自北纳生物公司,化脓隐秘杆菌是西南大学荣昌校区动物医学系程方俊老师馈赠的分离株。
1.1.2 试验样品本试验藏绵羊子宫样品采自西宁市百德屠宰场,藏绵羊来源于青海省周边牧区,共600份,均为2–3岁,4 h内运回实验室。用无菌棉拭子涂抹子宫,置于LB培养液中培养并编号,48 h后提取样品基因组。
1.1.3 主要试剂及仪器主要试剂:细菌基因组DNA提取试剂盒(天根,北京)、Taq酶(Promega,美国)、血琼脂平板(HKM,广东)等。主要仪器:PCR仪(RioRad,美国)、台式冷冻高速离心机(Hermle Z323K,上海)等。
1.2 方法1.2.1 引物的设计与合成根据GenBank数据库中已经发表的序列:无乳链球菌(GI No.X72754)、大肠杆菌(GI No.V00331)、金黄色葡萄球菌(GI No.288516)、化脓隐秘杆菌(GI No.372925.1)和沙门氏菌(GI No.161793)在16S rRNA与23S rRNA之间的特异性区域设计引物,选取的病原基因分别为cfb、phoA、nuc、ISR、invA,扩增片段长度分别为150、385、1 319、340、495 bp,由生工生物工程(上海)股份有限公司合成(表 1)。
表 1 PCR试验所用引物Table 1 Primers used in the study
| Test strain | Target genes | Primer sequence (5′–3′) | Size (bp) |
| GBS | cfb | cfb-F: TTCACCAGCTGTATTAGAAGTACATGC cfb-R: CCCTGAACATTATCTTTGATATTTCTCA | 150 |
| E. coli | phoA | phoA-F: CTTGCTGGTTTGTGAGTGAAAG phoA-R: GATTCAGTTAATGATAGTGTGTCG | 385 |
| S. aureus | nuc | muc-F: GGACGACATTAGACGAATCA muc-R: CGGGCACCTATTTTCTATCT | 1 319 |
| Trueperella pyogenes | ISR | ISR-F: CTTGTCTTTGGGATAAGCCTGGGAA ISR-R: GCACATACCGTCACAA | 340 |
| Salmonella | invA | invA-F: ACTGGCGTTATCCCTTTCTCTGGTG invA-R: ATGTTGTCCTGCCCCTGGTAAGAGA | 495 |
表选项
1.2.2 标准菌株的培养及基因组的提取用接种环分别挑取大肠杆菌、金黄色葡萄球菌、化脓隐秘杆菌、无乳链球菌、沙门氏菌划线培养在LB固体培养皿中,放入37 ℃培养箱中培养24 h。挑取单个菌落,接入LB液体培养基中,于37 ℃恒温振荡器培养24 h。按照细菌基因组DNA提取试剂盒的步骤提取菌株基因组。
1.2.3 PCR检测的特异性试验分别以大肠杆菌、金黄色葡萄球菌、化脓隐秘杆菌、无乳链球菌、沙门氏菌的基因组为模板,加入金黄色葡萄球菌引物,按照上述PCR反应程序进行扩增,通过琼脂糖凝胶电泳观察金黄色葡萄球菌引物的特异性。用同样的方法检测大肠杆菌、化脓隐秘杆菌、无乳链球菌、沙门氏菌引物的特异性,通过电泳观察各个引物的特异性。
1.2.4 PCR检测的灵敏性试验通过活菌计数法对大肠杆菌、金黄色葡萄球菌、化脓隐秘杆菌、无乳链球菌、沙门氏菌进行菌液浓度测定,计算1 mL菌液的浓度。将各菌液稀释成10–1、10–2、10–3、10–4、10–5、10–6、10–7七个浓度梯度,提取基因组,分别为模板进行PCR反应,检测其灵敏性。
1.2.5 单一PCR检测单一PCR的反应体积是20 μL,其中Taq DNA聚合酶(Mix) 10 μL、上游引物和下游引物各0.5 μL、细菌DNA 2 μL、双蒸水7 μL。反应程序为:95 ℃预变性5 min;95 ℃ 30 s;58 ℃ 30 s,72 ℃ 30 s,30个循环;终延伸72 ℃ 5 min。
1.2.6 多重PCR反应条件及体系的建立根据单一PCR的反应条件,建立25 μL的多重PCR反应体系:3种菌株(大肠杆菌、金黄色葡萄球菌、无乳链球菌)的DNA模板各1 μL,大肠杆菌上下游引物各0.5 μL,金黄色葡萄球菌上下游引物各0.6 μL、无乳链球菌上下游引物各0.4 μL,Premix Taq DNA聚合酶12.5 μL,双蒸水6.5 μL。反应程序为:95 ℃预变性5 min;95 ℃30 s,60 ℃ 30 s,72 ℃ 30 s,40个循环;终延伸72 ℃ 5 min。
1.3 病原菌分离和鉴定1.3.1 病原菌的分离培养分别用适量的生理盐水滴入30份藏绵羊子宫内部,吹打均匀,分别接种于血琼脂培养基、麦康凯培养基、SS培养基和伊红美兰培养基,于37 ℃恒温培养箱培养24 h,观察菌落生长情况及形态特征。挑取单个典型菌落,进行涂片、革兰氏染色、镜检,同时接种于2%犊牛血清的营养肉汤中,37 ℃、200 r/min培养18 h,根据菌落形态特征、溶血性、染色特性等初步推断为链球菌、葡萄球菌和肠杆菌等。
1.3.2 病原菌的生化鉴定根据杭州滨和微生物微量生化试剂盒说明书进行生化实验。对疑似为链球菌的细菌进行吡咯烷酮释放剂、VP、七叶苷和马尿酸钠发酵等试验进行鉴定;对疑似为葡萄球菌的细菌通过乳糖、麦芽糖、甘露醇等试验进行鉴定;对疑似大肠杆菌的细菌通过枸橼酸盐(西蒙氏)、葡萄糖产气、乳糖、麦芽糖、甘露糖、蔗糖、H2S (TSI)、尿素酶等试验进行鉴定。
2 结果与分析2.1 PCR引物特异性检测2.1.1 金黄色葡萄球菌引物特异性检测分别以化脓隐秘杆菌、沙门氏菌、无乳链球菌、大肠杆菌和金黄色葡萄球菌的基因组为模板,检测金黄色葡萄球菌引物特异性,泳道6–8的金黄色葡萄球菌在1 319 bp有特异性条带,其余泳道均无条带,说明其引物特异性好(图 1)。
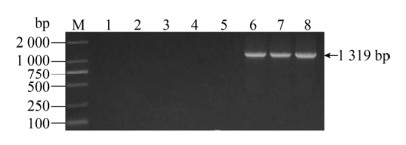 |
| 图 1 金黄色葡萄球菌特异性结果 Fig. 1 The primer specificity for S. aureus. M: DNA marker; 1: negative control; 2: E. coli; 3: Trueperella pyogenes; 4: GBS; 5: Salmonella; 6–8: S. aureus. |
| 图选项 |
2.1.2 化脓隐秘杆菌引物特异性检测分别以沙门氏菌、无乳链球菌、大肠杆菌、金黄色葡萄球菌和化脓隐秘杆菌的基因组为模板,检测化脓隐秘杆菌引物特异性,泳道6–8的化脓隐秘杆菌在340 bp有特异性条带,其余泳道均无条带,说明其引物特异性好(图 2)。
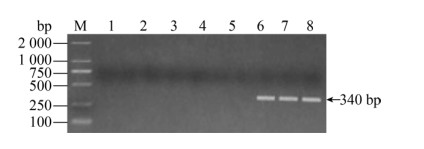 |
| 图 2 化脓隐秘杆菌特异性结果 Fig. 2 The primer specificity for Trueperella pyogenes. M: DNA marker; 1: negative control; 2: Salmonella; 3: GBS; 4: E. coli; 5: S. aureus; 6–8: Trueperella pyogenes. |
| 图选项 |
2.1.3 沙门氏菌引物特异性检测分别以无乳链球菌、大肠杆菌、金黄色葡萄球菌、化脓隐秘杆菌和沙门氏菌的基因组为模板,检测沙门氏菌引物特异性,泳道6–8的沙门氏菌在495 bp有特异性条带,其余泳道均无条带,说明其引物特异性好(图 3)。
 |
| 图 3 沙门氏菌特异性结果 Fig. 3 The primer specificity for Salmonella. M: DNA marker; 1: negative control; 2: GBS; 3: E. coli; 4: S. aureus; 5: Trueperella pyogenes; 6–8: Salmonella. |
| 图选项 |
2.1.4 无乳链球菌引物特异性检测分别以大肠杆菌、金黄色葡萄球菌、化脓隐秘杆菌、沙门氏菌和无乳链球菌的基因组为模板,检测无乳链球菌引物特异性,泳道6–8的无乳链球菌在150 bp有特异性条带,其余泳道均无条带,说明其引物特异性好(图 4)。
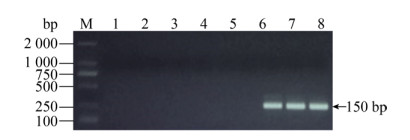 |
| 图 4 无乳链球菌特异性结果 Fig. 4 The primer specificity for GBS. M: DNA marker; 1: negative control; 2: E. coli; 3: S. aureus; 4: Trueperella pyogenes; 5: Salmonella; 6–8: GBS. |
| 图选项 |
2.1.5 大肠杆菌特异性结果分别以无乳链球菌、金黄色葡萄球菌、化脓隐秘杆菌、沙门氏菌和大肠杆菌的基因组为模板,检测大肠杆菌引物特异性,泳道6–8的大肠杆菌在385 bp有特异性条带,其余泳道均无条带,说明其引物特异性好(图 5)。
 |
| 图 5 大肠杆菌特异性结果 Fig. 5 The primer specificity for E. coli. M: DNA marker; 1: negative control; 2: GBS; 3: S. aureus; 4: Trueperella pyogenes; 5: Salmonella; 6–8: E. coli. |
| 图选项 |
2.2 单一PCR灵敏性试验分别将金黄色葡萄球菌、化脓隐秘杆菌、沙门氏菌、无乳链球菌、大肠杆菌接种在1 mL LB液体培养基中,37 ℃培养24 h,经过活菌计数法计算出各种菌株的浓度,分别为2.4×109、1.7×108、1.43×109、2.8×108、1.03×109 CFU/mL。接着提取5种菌株菌液的基因组,稀释成不同倍数作为模板进行单一PCR,1%琼脂糖凝胶电泳后观察结果。如图 6–10所示,5种菌株单一PCR最低检测限:金黄色葡萄球菌最低检测浓度为2.4×104 CFU/mL,化脓隐秘杆菌最低检测浓度为1.7×102 CFU/mL,沙门氏菌最低检测浓度为1.43×102 CFU/mL,无乳链球菌最低检测浓度为2.8×104 CFU/mL,大肠杆菌最低检测浓度为1.03×103 CFU/mL。
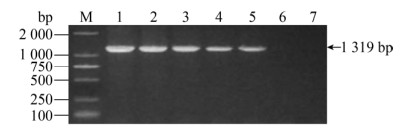 |
| 图 6 金黄色葡萄球菌引物的敏感性 Fig. 6 The sensitivity for S. aureus. M: DNA marker; 1: 2.4×108 CFU/mL; 2: 2.4×107 CFU/mL; 3: 2.4×106 CFU/mL; 4: 2.4×105 CFU/mL; 5: 2.4×104 CFU/mL; 6: 2.4×103 CFU/mL; 7: 2.4×102 CFU/mL. |
| 图选项 |
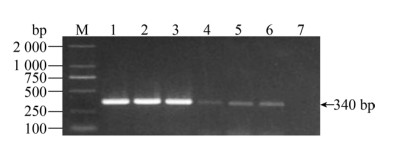 |
| 图 7 化脓隐秘杆菌引物的敏感性 Fig. 7 The sensitivity for Trueperella pyogenes. M: DNA marker; 1: 1.7×107 CFU/mL; 2: 1.7×106 CFU/mL; 3: 1.7×105 CFU/mL; 4: 1.7×104 CFU/mL; 5: 1.7×103 CFU/mL; 6: 1.7×102 CFU/mL; 7: 1.7×101 CFU/mL. |
| 图选项 |
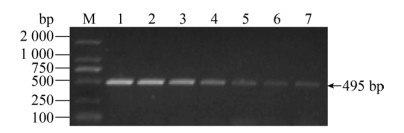 |
| 图 8 沙门氏菌引物的敏感性 Fig. 8 The sensitivity for Salmonella. M: DNA marker; 1: 1.43×108 CFU/mL; 2: 1.43×107 CFU/mL; 3: 1.43× 106 CFU/mL; 4: 1.43×105 CFU/mL; 5: 1.43×104 CFU/mL; 6: 1.43×103 CFU/mL; 7: 1.43×102 CFU/mL. |
| 图选项 |
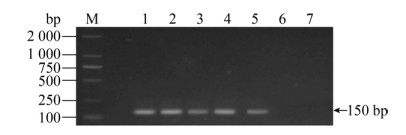 |
| 图 9 无乳链球菌引物的敏感性 Fig. 9 The sensitivity for GBS. M: DNA marker; 1: 2.8×108 CFU/mL; 2: 2.8×107 CFU/mL; 3: 2.8× 106 CFU/mL; 4: 2.8×105 CFU/mL; 5: 2.8×104 CFU/mL; 6: 2.8×103 CFU/mL; 7: 2.8×102 CFU/mL. |
| 图选项 |
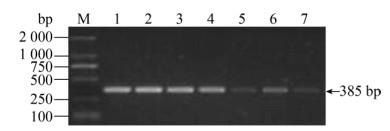 |
| 图 10 大肠杆菌的敏感性 Fig. 10 The sensitivity for E. coli. M: DNA marker; 1: 1.03×108 CFU/mL; 2: 1.03×107 CFU/mL; 3: 1.03× 106 CFU/mL; 4: 1.03×105 CFU/mL; 5: 1.03×104 CFU/mL; 6: 1.03×103 CFU/mL; 7: 1.03×102 CFU/mL. |
| 图选项 |
2.3 多重PCR优化反应条件的结果多重PCR的反应体系是25 μL,其中Taq DNA聚合酶(Mix) 12.5 μL,多重PCR模板:无乳链球菌、大肠杆菌、金黄色葡萄球菌基因组模板各1 μL;无乳链球菌上下游引物各为0.4 μL、大肠杆菌上下游引物各为0.5 μL、金黄色葡萄球菌上下游引物各为0.6 μL;双蒸水6.5 μL。根据单一PCR退火温度,将多重PCR退火温度分别设定在55–60 ℃进行温度梯度多重PCR扩增,故反应程序为:预变性95 ℃ 5 min;95 ℃ 30 s,55–60 ℃ 30 s,72 ℃ 30 s,40个循环;终延伸72 ℃ 5 min。随着退火温度的增高,在60 ℃时,3种产物的条带非常清晰,故将60 ℃作为多重PCR的最佳退火温度(图 11)。
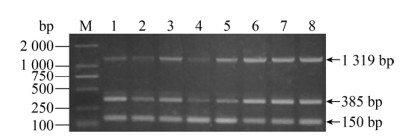 |
| 图 11 多重PCR退火温度的筛选 Fig. 11 The screening of annealing temperature for multiplex PCR. M: DNA marker; 1: 55.0 ℃; 2: 55.3 ℃; 3: 56.0 ℃; 4: 56.9 ℃; 5: 58.1 ℃; 6: 59.0 ℃; 7: 59.6 ℃; 8: 60.0 ℃. |
| 图选项 |
2.4 单一PCR检测样品的结果运用单一PCR法对600份样品基因组进行检测,其中混合感染高于单一感染,大肠杆菌和无乳链球菌混合感染比例最高,占检测样品24.67%,单一感染样品中,无乳链球菌感染比例最高,占检测样品的19.17% (表 3)。
表 3 单一PCR检测结果Table 3 Single PCR test results
| Bacterial species | E. coli | GBS | S. aureus | E. coli+S. aureus | E. coli+GBS | S. aureus +GBS | E. coli+GBS+S. aureus |
| Number of infections | 41.00 | 115.00 | 7.00 | 11.00 | 148.00 | 12.00 | 9.00 |
| The proportion (%) | 6.83 | 19.17 | 1.17 | 1.83 | 24.67 | 2.00 | 1.50 |
表选项
2.5 多重PCR检测的结果采用建立好的多重PCR方法,对标记好的单一PCR检测的阳性样品进行检测,无乳链球菌感染比例占45.50%,大肠杆菌占33.50%,金黄色葡萄球菌占6.5%,大肠杆菌和无乳链球菌混合感染占23.67% (表 4)。
表 4 多重PCR检测结果Table 4 Multiplex PCR results
| Bacterial species | E. coli | GBS | S. aureus | E. coli+S. aureus | E. coli+GBS | S. aureus+GBS | E. coli+GBS+S. aureus |
| Number of infections | 39.00 | 110.00 | 7.00 | 11.00 | 142.00 | 12.00 | 9.00 |
| The proportion (%) | 6.50 | 18.33 | 1.17 | 1.83 | 23.67 | 2.00 | 1.50 |
| Note: Multiplex PCR test sample number is consistent with single PCR. | |||||||
表选项
2.6 多重PCR与单一PCR检测的符合率多重PCR与单一PCR对无乳链球菌检出结果的符合率为95.65%,大肠杆菌为95.12%,金黄色葡萄球菌为100%,大肠杆菌和无乳链球菌混合感染的符合率为95.95%,大肠杆菌和金黄色葡萄球菌、无乳链球菌和金黄色葡萄球菌、大肠杆菌和金黄色葡萄球菌和无乳链球菌混合感染的符合率为100%。由此可见,多重PCR检测的符合率均高于95% (表 5)。
表 5 多重PCR符合率分析结果Table 5 Multiplex PCR compliance analysis results
| Bacterial species | E. coli | GBS | S. aureus | E. coli+S. aureus | E. coli+GBS | S. aureus+GBS | E. coli+GBS+S. aureus |
| Single PCR detection | 41.00 | 115.00 | 7.00 | 11.00 | 148.00 | 12.00 | 9.00 |
| Multiplex PCR detection | 39.00 | 110.00 | 7.00 | 11.00 | 142.00 | 12.00 | 9.00 |
| Compliance rate (%) | 95.12 | 95.65 | 100.00 | 100.00 | 95.95 | 100.00 | 100.00 |
| Note: Compliance rate is the ratio of the number of positive samples detected by multiplex PCR to the number of positive samples detected by a single PCR. The positive sample number of single PCR detection is in one-to-one correspondence with the positive samples detected by multiplex PCR. | |||||||
表选项
2.7 病原菌分离和鉴定结果2.7.1 病原菌的镜检结果被检测的30份样品中,经细菌分离纯化培养、革兰氏染色、镜检试验结果,在油镜下观察,疑似为链球菌的菌种呈蓝紫色串珠状,为革兰氏阳性菌(图 12);疑似为金黄色葡萄球菌的菌种呈蓝紫色葡萄状,为革兰氏阳性菌(图 13);疑似为大肠杆菌的菌种呈两端钝圆的、粉红色的短杆状,为革兰氏阴性菌(图 14)。
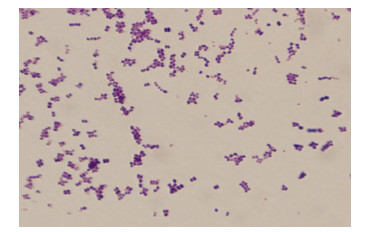 |
| 图 12 链球菌的革兰氏染色 Fig. 12 The Gram stain of Streptococcus. |
| 图选项 |
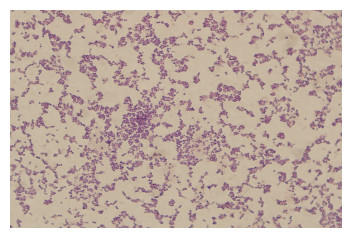 |
| 图 13 金黄色葡萄球菌的革兰氏染色 Fig. 13 The Gram of S. aureus. |
| 图选项 |
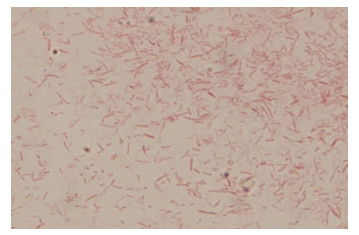 |
| 图 14 大肠杆菌的革兰氏染色 Fig. 14 The Gram of E. coli. |
| 图选项 |
2.7.2 病原菌菌株的分离结果在30份阳性样品中,通过细菌的分离纯化培养,根据菌落形态特征、溶血性、染色特性等初步推断为链球菌、葡萄球菌、大肠杆菌等。其中,在革兰氏阳性菌中链球菌属最多,在革兰氏阴性菌中大肠杆菌最多(表 6)。
表 6 样品中分离菌株的统计表Table 6 Statistical table of isolated strains in samples
| Strain | Gram-positive bacteria | Gram-negative bacteria | Other genus | |
| Streptococcus | Staphylococcus | E. coli | ||
| Number of infections | 20 | 2 | 7 | 3 |
表选项
2.7.3 病原菌的生化鉴定结果对疑似链球菌的菌种生化鉴定结果(表 7)表明,该菌株能分解海藻糖、蔗糖、山梨醇,能产生精氨酸,对七叶苷、甘露醇、VP无任何反应及对吡咯烷酮释放剂不显红色,因此判定该菌株为无乳链球菌;对疑似葡萄球菌的菌种生化鉴定结果(表 8)表明,该菌株能分解乳糖、麦芽糖、蔗糖、甘露糖、海藻糖,能使尿素发生反应,加入指示剂后变红色,不能分解甘露醇,不能还原硝酸盐,因此判定该菌株为金黄色葡萄球菌;对疑似大肠杆菌的菌种生化鉴定结果(表 9)表明,该菌株能使葡萄糖盐酸产气,能产生鸟氨酸和赖氨酸,能分解山梨醇、木胶糖、乳糖、麦芽糖、甘露糖,不产生H2S,因此判定该菌株为大肠杆菌。
表 7 链球菌的生化试验结果Table 7 Biochemical test results of Streptococcus
| Experimental project | Results | Experimental project | Results |
| Pyrrolidone release agent | – | vp | – |
| Arginine | + | DPP | – |
| PMG | – | Heptaside | – |
| MAG | – | TMZ | – |
| Trehalose | + | Sucrose | + |
| Sorbitol | + | Mannitol | – |
| Note: “+”: Means positive/Acid production; “–”: Means negative. | |||
表选项
表 8 金黄色葡萄球菌的生化试验结果Table 8 Biochemical test results of Staphylococcus aureus
| Experimental project | Results | Experimental project | Results |
| Lactose | + | Maltose | + |
| Mannitol | – | Sucrose | + |
| Mannose | + | Wood gum | – |
| Urea | + | Trehalose | + |
| N-acetylglucosamine | – | Nitrate | – |
| Note: “+”: Means positive/Acid production; “–”: Means negative. | |||
表选项
表 9 大肠杆菌的生化试验结果Table 9 Biochemical test results of E. coli
| Experimental project | Results | Experimental project | Results |
| Hydrogen sulfide | – | Phenyla lanine | – |
| Peptone water | – | Citrate | – |
| Semi-solid | + | Ornithine | + |
| Raffinose | – | Sorbitol | + |
| Wood gum | + | Lactose | + |
| Gluconate | Gas production | Urea | – |
| Mannose | + | Calendula | – |
| Maltose | + | Amino acid blankcontrol tube | + |
| Note: “+”: Means positive/Acid production; “–”: Means negative. | |||
表选项
3 讨论与结论羊子宫内膜炎是指子宫内膜的化脓性和坏死性炎症,主要是由于母羊分娩后,子宫受损或机体抵抗力下降[8-10],细菌等微生物趁机侵入感染而引起,其临床特征为阴道流出浆液性或脓性分泌物,可导致母绵羊屡配不孕、空怀、死胎或流产,甚至交配时引起公羊生殖系统疾病[11-14]。在青海藏区,由于冬季气候变化及补饲不当,造成围产期藏绵羊抵抗力较低[15],牧场及产房环境脏乱,导致藏羊分娩时阴门接触地面感染并引发子宫内膜炎,造成重大的经济损失。截止目前,最有效的药物是抗生素,但抗生素的滥用使得动物机体产生耐药性,机体抵抗力差,失去抵御细菌入侵能力,进而危害人们健康。因此,本研究针对青海藏绵羊子宫内膜炎的病原菌检测建立了多重PCR方法,可以在一个反应体系中同时检测多种病原,为临床上对藏绵羊子宫内膜炎的诊断、病原菌的检测等研究提供了技术支持。
目前用于鉴别诊断动物病原菌的检测方法有很多种,如病原菌分离、ELISA方法等。但这些方法在临床实际应用中仍然存在着不足之处:病原菌分离培养技术特异性强,通过细菌分离培养、涂片、革兰氏染色、镜检及生化鉴定等进行检测,准确率高但是其耗时长;ELISA方法特异性好,但只能检测单一病原菌。PCR技术因具有快速、特异、敏感和易于操作等特点,在畜禽传染病的检测、鉴别诊断方面有广泛应用前景且已涉及到各个领域,如医学研究与应用、植物生物学研究、海洋生物学研究、遗传学研究等,具有快速准确、简便、特异性高的优点[16]。多重PCR技术的优势在于它的高效性,通过一次扩增反应即可实现对多种病原体进行检测和鉴别,相较多个单一PCR检测方法成本显著降低。多重PCR法能在同一反应管内同时检出多种病原体,极大地节省时间、节省试剂、节约经费开支,为临床提供更多更准确的诊断信息。多重PCR是基于普通PCR的一种可同时对多个目的片段进行扩增的PCR检测技术,该技术在临床上疾病诊断方面具有实际意义。
在我国关于引起藏绵羊子宫内膜炎病原菌的报道寥寥无几。据国内外报道,引起羊子宫内膜炎的病原菌有大肠杆菌、化脓隐秘杆菌(又称化脓性棒状杆菌)、链球菌、金黄色葡萄球菌等[17-20];引起牦牛子宫内膜炎的病原菌有大肠杆菌等[21];引起甘肃省地区奶牛子宫内膜炎病原菌有化脓隐秘杆菌、大肠杆菌、金黄色葡萄球菌、无乳链球菌等[22-23];引起内蒙古和宁夏地区奶牛子宫内膜炎病原菌有葡萄球菌等[24-26];引起国外地区奶牛子宫内膜炎病原菌有大肠杆菌、化脓隐秘杆菌、坏死梭杆菌、乳房链球菌等[27-30]。据报道,王爽、孙东波、王宁等成功应用多重PCR方法检测出引起奶牛子宫内膜炎的主要病原菌为大肠杆菌、化脓隐秘杆菌、金黄色葡萄球菌、乳房链球菌等[31-33];万圣等成功应用多重PCR方法在蓄脓的犬子宫中检测出链球菌、沙门氏菌、金黄色葡萄球菌和大肠杆菌[34];国外研究者Aghamiri等在伊朗某奶牛场成功应用多重PCR方法检测出引起该地区奶牛子宫内膜炎的主要病原菌为大肠杆菌、化脓隐秘杆菌、坏死梭杆菌和普氏菌[35]。本研究先提取标准菌株基因组,筛选出特异性引物;然后以标准菌株的基因组为模板,建立多重PCR方法。用无菌棉拭子涂抹藏绵羊子宫,置于LB培养液中培养并编号,48 h后提取样品基因组。运用单一PCR法对600份样品基因组进行检测,记录阳性样品;挑取单一PCR法检测的阳性样品进行多重PCR检测,再次记录阳性样品,通过计算两种方法检测的符合率验证多重PCR方法;并随机挑出30份阳性样品,进行病原菌分离鉴定菌种种类。结果成功建立了能快速检测3种细菌的多重PCR方法,且多重PCR检测结果与单一PCR基本一致,为藏绵羊子宫内膜炎的诊断和流行病学监测,提供了一种新的实用方法。
本试验结果表明,引起藏绵羊子宫内膜炎的主要病原菌为无乳链球菌、大肠杆菌和金黄色葡萄球菌等。通过细菌分离纯化培养与生化鉴定,在30份样品中,分离出了无乳链球菌、大肠杆菌、金黄色葡萄球菌,其中无乳链球菌感染比例最高,在分离鉴定的试验中,混合感染主要以无乳链球菌、大肠杆菌为主,与PCR法检测出的菌种结果一致。青海地区地域辽阔,羊群颇多,在后期可以生产专门针对检测藏绵羊的子宫内膜炎的试剂盒,无论是基层技术人员还是相关研究者均可以方便快捷地检测该病,进而能够第一时间预防子宫内膜炎的发生,减少经济损失。
参考文献
| [1] | Wang XR. Study on genetic diversity and inbreeding degree of domestic yak and Tibetan sheep in Qinghai Province based on SSR markers[D]. Xining: Qinghai Normal University, 2019 (in Chinese). 王秀蓉.基于SSR标记评估青海省家牦牛和藏绵羊遗传多样性及近交程度[D].西宁: 青海师范大学, 2019. |
| [2] | Tang XH, Qiangba YZ. Research progress in Tibetan sheep adaptability to hypoxia and disease related genes. Anim Husb & Vet Med, 2017, 49(2): 115-118 (in Chinese). 唐晓惠, 强巴央宗. 藏绵羊对高原低氧环境的适应性及抗病性相关基因研究进展. 畜牧与兽医, 2017, 49(2): 115-118. |
| [3] | Lu XL, Zhao YL, Wu ZW, et al. Study on blood physiological characteristics of plateau adaptation in Tibetan sawa sheep. Southwest China J Agri Sci, 2019, 32(6): 1443-1447 (in Chinese). 卢晓丽, 赵彦玲, 吴征王, 等. 西藏色瓦藏绵羊高原适应性的血液生理学特性研究. 西南农业学报, 2019, 32(6): 1443-1447. |
| [4] | Li MS, Guo WC. Feeding management of Tibetan sheep in different periods (2). Spec Econ Anim Plant, 2016, 19(8): 4-5 (in Chinese). 李沐森, 郭文场. 藏羊不同时期的饲养管理(2). 特种经济动植物, 2016, 19(8): 4-5. |
| [5] | Li FH, Wang ZF, Hou FJ, et al. Research progress on characteristics of oat hay supplementary feeding on Tibetan sheep in cold season. Chin Anim Husb Vet Med, 2016, 43(9): 2358-2365 (in Chinese). 李福厚, 王召锋, 侯扶江, 等. 藏绵羊燕麦青干草冷季补饲特征研究进展. 中国畜牧兽医, 2016, 43(9): 2358-2365. |
| [6] | Hemingway RG. The Influences of dietary intakes and supplementation with Selenium and Vitamin E on reproduction diseases and reproductive efficiency in cattle and sheep. Vet Res Commun, 2003, 27(2): 159-174. |
| [7] | Yang SH, Wang JM, Li P, et al. Intervention mechanisms of Lactobacillus fermentum on LTA-induced inflammatory injury of endometrial cells in bos taurus. J Shenyang Agri Univ, 2019, 50(1): 1-9 (in Chinese). 杨淑华, 王佳美, 李鹏, 等. 发酵乳杆菌对LTA诱导的牛子宫内膜细胞炎性损伤干预作用的研究. 沈阳农业大学学报, 2019, 50(1): 1-9. |
| [8] | Wang JM. Research on the intervention mechanisms of Lactobacillus fermentum on LTA-induced inflammatory injury of endometrial cells in bos taurus[D]. Shenyang: Shenyang Agricultural University, 2018 (in Chinese). 王佳美.发酵乳杆菌对LTA诱导的牛子宫内膜细胞炎性损伤干预作用的研究[D].沈阳: 沈阳农业大学, 2018. http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CMFD&filename=1018993746.nh |
| [9] | Li MQ. Research on the Intervention mechanisms of Lactobacillus fermentum on LPS-induced inflammatory injury of endometrial epithelial cells in bos taurus[D]. Shenyang: Shenyang Agricultural University, 2018 (in Chinese). 李美琦.发酵乳杆菌对LPS诱导的牛子宫内膜细胞炎性损伤调节作用的研究[D].沈阳: 沈阳农业大学, 2018. http://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CMFD&filename=1018993745.nh |
| [10] | Adnane M, Chapwanya A, Kaidi R, et al. Profiling inflammatory biomarkers in cervico-vaginal mucus (CVM) postpartum: potential early indicators of bovine clinical endometritis?. Theriogenology, 2017, 103: 117-122. |
| [11] | Okawa H, Fujikura A, Wijayagunawardane MMP, et al. Effect of diagnosis and treatment of clinical endometritis based on vaginal discharge score grading system in postpartum Holstein cows. J Vet Med Sci, 2017, 79(9): 1545-1551. |
| [12] | Xu TW, Xu SX, Hu LY, et al. Effect of dietary types on feed intakes, growth performance and economic benefit in Tibetan sheep and yaks on the Qinghai-Tibet plateau during cold season. PLoS ONE, 2017, 12(1): e0169187. |
| [13] | Jing XP, Peng QH, Hu R, et al. Effect of supplements during the cold season on the reproductive system in prepubertal Tibetan sheep ewes. Anim Sci J, 2017, 88(9): 1269-1278. |
| [14] | de Boer M, Buddle BM, Heuer C, et al. Associations between intrauterine bacterial infection, reproductive tract inflammation, and reproductive performance in pasture-based dairy cows. Theriogenology, 2015, 83(9): 1514-1524. |
| [15] | Zhang L, Yang J, Luosang CC, et al. Experiment on supplementary feeding for Tibetan Sheep of plateau type in Spring. Southwest China J Agri Sci, 2017, 30(4): 975-977 (in Chinese). 张立, 央金, 洛桑催成, 等. 高原型藏绵羊春季补饲试验. 西南农业学报, 2017, 30(4): 975-977. |
| [16] | Chen MJ, Fang T, Ke T, et al. Multiplex PCR- a molecular biotechnique of high efficiency and speed. J Wuhan Univ Technol, 2005, 27(10): 33-36 (in Chinese). 陈明洁, 方倜, 柯涛, 等. 多重PCR-一种高效快速的分子生物学技术. 武汉理工大学学报, 2005, 27(10): 33-36. |
| [17] | Sokkar SM, Kubba MA, Al-Augaidy F. Studies on Natural and Experimental Endometritis in Ewes. Vet Pathol, 1980, 17(6): 693-698. |
| [18] | Adams NR. A pathological and bacteriological abattoir survey of the reproductive tract of Merino ewes in Western Australia. Austral Vet J, 1975, 51(7): 351-354. |
| [19] | Regassa F, Sheldon IM, Noakes DE. Effect of experimentally induced metritis on uterine involution, acute phase protein response and PGFM secretion in the postpartum ewe. Vet Rec, 2002, 150(19): 605-607. |
| [20] | Bao XJ, Lü JJ, Sun L, et al. Effects of endometritis with E. coli on blood and endometrium in goats. Chin J Vet Med, 2010, 30(2): 219-221 (in Chinese). 包喜军, 吕俊锦, 孙琳, 等. 大肠杆菌性子宫内膜炎对山羊血液和子宫内膜的影响. 中国兽医学报, 2010, 30(2): 219-221. |
| [21] | Gan FB, Zhaxi ZM, Wu QX, et al. Establishment of yak endometritis model induced by LPS in vitro. J Plateau Agri, 2019, 3(4): 437-442 (in Chinese). 甘富斌, 扎西卓玛, 吴庆侠, 等. LPS体外诱导牦牛子宫内膜炎症模型的建立. 高原农业, 2019, 3(4): 437-442. |
| [22] | Li HS, Yang F, Wang XR, et al. Isolation and identification of endometritis pathogens in dairy farms located in Lanzhou city and its antibiotic resistance analysis. Chin Anim Husb Vet Med, 2014, 41(1): 222-226 (in Chinese). 李宏胜, 杨峰, 王旭荣, 等. 兰州地区部分奶牛场子宫内膜炎病原菌分离鉴定及抗生素耐药性研究. 中国畜牧兽医, 2014, 41(1): 222-226. |
| [23] | Wang XW, Wang XR, Yang ZQ, et al. Isolation, identification and drug-resistance of pathogenic bacteria from the dairy cows infected by endometritis in Gansu. Acta Agric Boreali-Occid Sin, 2014, 23(6): 16-21 (in Chinese). 王孝武, 王旭荣, 杨志强, 等. 甘肃省某牛场奶牛子宫内膜炎病原菌的分离鉴定及其耐药性分析. 西北农业学报, 2014, 23(6): 16-21. |
| [24] | Li ZF, Zhao JL, Guan H, et al. Distribution of super antigen genes and antimicrobial resistance of pathogenic staphylococcus isolated from dairy cows with endometritis in Inner Mongolia. Progr Vet Med, 2013, 34(9): 18-23 (in Chinese). 李志芳, 赵俊利, 关红, 等. 内蒙古地区致奶牛子宫内膜炎葡萄球菌超抗原基因分布及耐药性研究. 动物医学进展, 2013, 34(9): 18-23. |
| [25] | Zhao JL, Ding YX, Zhao HX, et al. Presence of superantigen genes and antimicrobial resistance in Staphylococcus isolates obtained from the uteri of dairy cows with clinical endometritis. Vet Rec, 2014, 175(14): 352. |
| [26] | Zhao QM, Yu YT, Ren X. Isolation, identification and drug sensitivity tests of main pathogenic bacteria causing cow endometritis in dairy cow farms of Ningxia. Progr Vet Med, 2015(8): 114-119 (in Chinese). 赵清梅, 余永涛, 任贤. 宁夏地区奶牛子宫内膜炎主要病原菌的分离鉴定及药敏试验. 动物医学进展, 2015(8): 114-119. |
| [27] | Malinowski E, Lassa H, Markiewicz H, et al. Sensitivity to antibiotics of Arcanobacterium pyogenes and Escherichia coli from the uteri of cows with metritis/endometritis. Vet J, 2011, 187(2): 234-238. |
| [28] | Pohl A, Lübke-Becker A, Heuwieser W. Minimum inhibitory concentrations of frequently used antibiotics against Escherichia coli and Trueperella pyogenes isolated from uteri of postpartum dairy cows. J Dairy Sci, 2017, 101(2): 1355-1364. |
| [29] | Wagener K, Grunert T, Prunner I, et al. Dynamics of uterine infections with Escherichia coli, Streptococcus uberis and Trueperella pyogenes in post-partum dairy cows and their association with clinical endometritis. Vet J, 2014, 202(3): 527-532. |
| [30] | Bicalho MLS, Machado VS, Oikonomou G, et al. Association between virulence factors of Escherichia coli, Fusobacterium necrophorum, and Arcanobacterium pyogenes and uterine diseases of dairy cows. Vet Microbiol, 2012, 157(1/2): 125-131. |
| [31] | Wang S. Identification of the major pathogenic bacteria causing bovine endometritis and the establish of three main germs by multiplex polymerase chain reaction[D]. Daqing: Heilongjiang Bayi Agricultural University, 2008 (in Chinese). 王爽.奶牛子宫内膜炎主要病原菌分离鉴定及多重PCR诊断方法的建立[D].大庆: 黑龙江八一农垦大学, 2008. |
| [32] | Sun DB, Wu R, He XJ, et al. Development of a multiplex PCR for diagnosis of Staphylococcus aureus, Escherichia coli and Bacillus cereus from cows with endometritis. Agric Sci China, 2011, 10(10): 1624-1629. |
| [33] | Wang Ningning. Identification of the main pathogen in clinical endometritis of dairy cows and establishment of multiplex PCR detection method[D]. Yangling: Northwest A & F University, 2019 (in Chinese). 王宁宁.奶牛临床型子宫内膜炎主要致病菌的分离鉴定及多重PCR检测方法的建立[D].杨陵: 西北农林科技大学, 2019. |
| [34] | Wan S. The establishment of a multiplex PCR for distinguishing four microbes in pyometra and the tentative observation of the antibacterial effect of lysozyme[D]. Taian: Shandong Agricultural University, 2013 (in Chinese). 万圣.犬子宫蓄脓4种细菌多重PCR检测方法的建立及溶菌酶抑菌效果观察[D].泰安: 山东农业大学, 2013. |
| [35] | Aghamiri SM, Haghkhah M, Ahmadi MR, et al. Development of a multiplex PCR for the identification of major pathogenic bacteria of post-partum endometritis in dairy cows. Reprod Domest Anim, 2014, 49(2): 233-238. |
