

山西农业大学 生命科学学院,山西 晋中 030801
收稿日期:2019-09-26;接收日期:2019-11-26
基金项目:国家自然科学基金(Nos. 31601612,31772130)资助
摘要:大豆花叶病毒(Soybean mosaic virus,SMV)作为半夏(Pinellia ternata(Thunb.) Breit.)主要病毒病害之一,已对其产量和品质造成严重影响。构建病毒侵染性克隆是反向遗传学研究病毒基因功能、病毒与宿主相互作用的有力工具,为明确SMV侵染半夏的分子机制,开展SMV全长cDNA侵染性克隆的构建特别重要。因此文中利用Gibson体外重组系统对大豆花叶病毒山西半夏分离物(SMV-SXBX)侵染性克隆进行组装,通过农杆菌浸润法接种健康半夏;进一步通过机械传代、逆转录-聚合酶链式反应(RT-PCR)证实SMV-SXBX侵染性克隆3′末端含有poly(A)尾56 nt时具有稳定侵染性。该方法便捷、高效,且避免了SMV侵染性克隆在大肠杆菌中的不稳定问题。SMV全长侵染性cDNA克隆的构建,为进一步研究SMV复制和发病的分子机制奠定了基础。
关键词:侵染性克隆大豆花叶病毒Gibson组装多聚腺苷酸尾半夏
Construction and characterization of an infectious clone of Soybean mosaic virus isolate from Pinellia ternata
Li Zhang, Defu Wang, Yanni Pei, Shen Xian, Yanbing Niu


School of Life Sciences, Shanxi Agricultural University, Jinzhong 030801, Shanxi, China
Received: September 26, 2019; Accepted: November 26, 2019
Supported by: National Natural Science Foundation of China (Nos. 31601612, 31772130)
Corresponding author: Yanbing Niu. Tel: +86-354-6287205; E-mail: niuyanbingbest@163.com.
Abstract: Soybean mosaic virus (SMV), one of the major viral diseases of Pinellia ternata (Thunb.) Breit., has had a serious impact on its yield and quality. The construction of viral infectious clones is a powerful tool for reverse genetics research on viral gene function and interaction between virus and host. To clarify the molecular mechanism of SMV infection in Pinellia ternata, it is particularly important to construct the SMV full-length cDNA infectious clone. Therefore, the infectious clone of Soybean mosaic virus Shanxi Pinellia ternata isolate (SMV-SXBX) was constructed in this study by Gibson in vitro recombination system, and the healthy Pinellia ternata leaves were inoculated by Agrobacterium infiltration, further through mechanical passage and RT-PCR, confirming that the 3′ end of the SMV-SXBX infectious clone had a stable infectivity when it contained 56-nt of poly(A) tail. This method is not only convenient and efficient, but also avoids the instability of SMV infectious clones in Escherichia coli. The construction of SMV full-length infectious cDNA clones laid the foundation for further study on the molecular mechanism of SMV replication and pathogenesis.
Keywords: infectious cloningsoybean mosaic virusGibson assemblypoly(A) tailPinellia ternata (Thunb.) Breit
植物病毒病俗称植物“癌症”,是危害粮食作物、蔬菜水果、观赏植物、中药材等的重要病原物,在世界范围内造成严重的经济损失。1915年,Clinton从大豆植株上发现大豆花叶病毒(Soybean mosaic virus,SMV),Gardner和Kendrick在1921年为其正式命名[1]。SMV是马铃薯Y病毒属(Potyvirus)成员。SMV的宿主范围非常窄,除大豆外,自然侵染西番莲属、半夏属[2]等,其基因组为正义单链RNA,长约10 kb,被1个外壳蛋白包裹着。5′-末端共价结合基因组连接蛋白(Viral protein genome-linked,VPg),3′-末端为多聚腺苷酸尾[3]。SMV基因组编码一个大型开放阅读框(Open reading frame,ORF),翻译后的多聚蛋白经病毒编码的蛋白酶处理,产生一系列成熟蛋白,包括P1、HC-Pro、P3、P3-PIPO、6K1、CI、6K2、NIa-VPg、NIa-Pro、NIb和CP[2]。
侵染性克隆是指为了实现对病毒进行遗传操作,将其病毒全长基因组序列构建在质粒载体上,且具有感染宿主的功能。由于RNA病毒在其基因组RNA水平难以利用常规的遗传学手法进行研究[4],构建RNA病毒基因组全长cDNA侵染性克隆是进行反向遗传研究的关键[5]。传统的侵染性克隆构建方法依赖于限制性内切酶消化,这通常需要繁琐的亚克隆步骤[1],这些步骤可能会受到插入物和载体共用限制位点的局限进而改变病毒生存能力和传染性。1984年首次获得植物RNA病毒雀麦花叶病毒(Brome mosaic virus,BMV)全长cDNA侵染性克隆,但马铃薯Y病毒属病毒的体内转录侵染性克隆成功构建面临的重要问题是较大病毒基因组cDNA组装及其在大肠杆菌中的不稳定性,导致构建侵染性克隆费时费力,且成功率较低[6]。为了避免这些问题使侵染性克隆构建方法简单化,本研究采用Gibson组装法成功组装了SMV-SXBX全长cDNA侵染性克隆,实现了基因片段的精确快速组装,这对SMV-SXBX致病机制的研究、病毒开发利用和基因功能的研究具有重要意义[7]。
Gibson组装法又被称为“Gibson等温一步拼接法”,该方法不用考虑片段的长度或末端的互补性,即可利用T5核酸外切酶、Phusion聚合酶及Taq DNA连接酶的协同作用,在体外将多个带有末端重叠序列的DNA片段在单温反应管内实现片段的连接组装。其中T5核酸外切酶具有5′→3′核酸外切酶活性,能够从5′端切割有重叠区的DNA片段,使其产生3′突出末端,且不与Phusion聚合酶竞争。该单链DNA的重叠序列在50 ℃特异性退火,并使外切酶逐渐热失活[8]。由Phusion聚合酶和Taq DNA连接酶修复连接,而形成完整的双链DNA分子,从而实现无痕拼接,这种稳健的一步DNA组装方法简单、高效,适用于不依赖于限制性内切酶位点的快速插入[9]。
在本研究中利用Gibson体外重组系统对3个重叠长距离PCR片段进行组装,用含有病毒基因组的根癌农杆菌克隆体通过农杆菌浸润法接种健康半夏的方法进行SMV-SXBX侵染性克隆构建,进一步采用机械传代、逆转录-聚合酶链式反应(RT-PCR)证实了由侵染性克隆产生的后代病毒具有侵染性,获得稳定侵染性克隆。SMV-SXBX侵染性克隆可以对SMV序列进行有目的、精确的定位诱变,进而确定SMV的感病基因,分析病毒复制、运动、症状发展、宿主范围和病毒与宿主的相互作用,以期为防御和控制SMV提供理论依据。
1 材料与方法1.1 材料1.1.1 供试材料自山西省半夏主要种植区采集检测具有大豆花叶病毒的半夏植株将其种植于试验田中获得大豆花叶病毒侵染的半夏病株,作为毒源植株。半夏脱毒幼苗由作者实验室保存。本试验所用质粒pGreen Ⅱ-35S为中国热带农业科学院热带生物技术研究所庹德财老师惠赠、根癌农杆菌菌株GV3101 (pSoup)购自上海唯地生物技术有限公司。
1.1.2 酶与试剂PrimeSTAR? GXL DNA Polymerase、pMD 18-T购自TaKaRa公司;Trizol试剂购自Invitrogen公司;Gibson Assembly Master Mix购自NEB公司;Taq DNA polymerse、TransScript? first-stand cDNA Synthesis SuperMix、大肠杆菌DH5α、pEASY?-Blunt Cloning Kit购自TransGen公司;Gel Extraction Kit和Plasmid Mini KitⅠ试剂盒购自OMEGA公司。
1.2 方法1.2.1 提取总RNA、合成cDNA用Trizol试剂从SMV侵染的半夏叶片中提取总RNA,?80 ℃备用。利用TransScript? first-stand cDNA Synthesis SuperMix将SMV-SXBX的RNA转化为cDNA。
1.2.2 引物设计利用Primer Premier 5.0软件设计引物。其中pGreen SMV1F与SMV1R、SMV2F与pGreen SMV2R (pGreen SMV2R′)两对特异引物分别用于扩增大片段SMV-SXBX1和A尾28 nt (56 nt)的大片段SMV-SXBX2 (图 1A);pGr35S-SMVF (pGreen SMVF′)和pGr35S-SMVR用于质粒载体线性化;SMV3F和SMV3R用于验证SMV-SXBX全长的有效性。在引物设计时,pGreen SMV1F与pGr35S-SMVR、pGr35S-SMVF (pGr35S-SMVF′)与pGreen SMV2R (pGreen SMV2R′)、SMV1R与SMV2F之间都分别有25-30 bp的重叠序列,以便于完成Gibson组装。引物均由生工生物工程(上海)股份有限公司合成(表 1)。
 |
| 图 1 SMV基因组结构及其SMV-SXBX全长不同长度A尾cDNA克隆构建示意图 Fig. 1 Schematic representation genome organization of soybean mosaic virus and construction of full-length cDNA clone of SMV-SXBX with different lengths of poly(A). (A) Schematic illustration of the genome organization of SMV and SMV-SXBX full-length cDNA clones with different lengths of poly(A). Filled blue boxes represent poly(A) with different lengths. The large ORF are shown as white filled boxes and PIPO are shown as line-box. Boxes represent encoded mature protein in the polyproteins: P1, HC-Pro, P3, P3-PIPO, 6K1, CI, 6K2, NIa-VPg, NIa-Pro, NIb and CP. The 5′-and 3′-untranslated regions indicated with black and blue boxes. (B) Digestion of vector with primer pGr35s-SMVF/F′ and pGr35s-SMVR to produce linearized vector. (C) Two overlapping SMV-SXBX cDNA fragments (1: 6 627 bp; 2: 2 960 bp). (D) Construct viral genome-encoding plasmids in vitro by one-step Gibson assembly. The overlapping regions are depicted by green, blue and grey shadow boxes, pink lines with the number of overlapping nucleotides indicated. Note that all of the fragments in the figure are not drawn exactly to scale. |
| 图选项 |
表 1 引物序列Table 1 The primer sequences
| Primer names | Primer sequence (5′-3′) | Description |
| pGr35S-SMVF | CCTAAAAAAAAAAAAAAAAAAAAAAAAAAAATCGGTACGCTGAAATCACCAGTCTC | For vector of SMV |
| pGr35S-SMVF′ | CCTAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAAATCGGTACGCTGAAATCACCAGTCTC | |
| pGr35S-SMVR | CCTCTCCAAATGAAATGAACTTCCT | |
| pGreen SMV1F | AGGAAGTTCATTTCATTTGGAGAGGAAAAAATTAAAACATCTCAACAAAACC | For SMV fragment 1 |
| SMV1R | CAGAAACAAGAGGCAACCCACAGA | |
| SMV2F | CTTCTGTGGGTTGCCTCTTGTTTCT | For SMV fragment 2 |
| pGreen SMV2R | CGATTTTTTTTTTTTTTTTTTTTTTTTTTTTAGGACAACAAATATTGCCGTACTTC | |
| pGreen SMV2R′ | CGATTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTAGGACAACAAATATTGCCGTACTTC | |
| SMV3F | GGGTTGGCTATGGTTCTT | 6 358-6 375 |
| SMV3R | TGGTGCTGCTGTGAATGT | 7 548-7 565 |
| SMV4F | AATCAGGCAAGGAGAAGGAAGGA | 8 535-8 558 |
| SMV4R | GGGGGTCAAAACCACACTAAACA | 9 447-9 469 |
| Note: pGr35S-SMVF' and pGreen SMV2R′ are amplification primers that increase the number of poly(A) tails. The single underlined sequences indicate homologous recombination sequences. | ||
表选项
1.2.3 目的基因获取及载体制备以1.2.1得到的cDNA为模板,利用两对特异引物pGreen SMV1F与SMV1R、SMV2F与pGreen SMV2R (pGreen SMV2R′)对SMV-SXBX基因组进行大片段扩增。以pGreen Ⅱ-35S质粒为模板,利用特异引物pGr35S-SMVF (pGr35S-SMVF′)和pGr35S-SMVR采用反向PCR的方法使载体线性化。
1.2.4 Gibson组装通过NanoDrop分光光度计对回收产物检测浓度后,按照Gibson Assembly Master Mix说明书计算两个SMV-SXBX大片段和线性化载体加入量并对其进行组装(图 1B-D)。
1.2.5 农杆菌化学转化将1.2.4组装质粒冻融转化进农杆菌GV3101 (pSoup)菌株,28 ℃、220 r/min振荡培养2-3 h,使其活化,活化菌液涂于含有卡那霉素(Kanamycin,Kan)和利福平(Rifampicin,Rif)的Luria Bertani (LB)平板上,28 ℃培养2-4 d。
1.2.6 克隆鉴定随机挑选白色单菌落,28 ℃下于含有Kan和Rif液体LB中培养2-3 h,利用特异引物SMV3F和SMV3R (表 1)进行菌落PCR鉴定。鉴定为阳性的菌落,提取农杆菌质粒,然后再利用pGreen SMV1F与SMV1R、SMV2F与pGreen SMV2R进行SMV-SXBX全长的鉴定,鉴定为全长的质粒,命名为pGreen-SMV-SXBX。以包含质粒pGreen-SMV-SXBX农杆菌菌液为模板分片段PCR鉴定全长cDNA侵染性克隆。
1.2.7 农杆菌浸润接种测序正确克隆接种于含有Kan和Rif固体LB培养基上,28 ℃培养,菌落长出后挑单克隆于含有相同抗生素和200 μmol/L乙酰丁香酮的LB液体培养基中扩大培养,10 μL培养物在相同条件下至菌液OD600为0.7左右,随后吸取1 mL菌液离心,收集菌体,无菌去离子水洗涤菌体,再次离心收集菌体[10],用细胞悬浮缓冲液(200 μmol/L乙酰丁香酮,10 mmol/L MES,pH 5.7,10 mmol/L MgCl2)重悬并在室温下避光处理2-3 h。使用1 mL注射器,无针头真空浸润法将重悬液轻轻接种到健康幼苗叶片背面,以pGreen Ⅱ-35S空载质粒的农杆菌作为阴性对照。每个克隆接种5株,接种植株置于光照培养箱中,25 ℃、16 h光照下培养,接种1-2周后观察统计发病情况。
1.2.8 机械摩擦接种SMV-SXBX病毒接种液的制备:取1.2.7农杆菌浸润接种半夏的病症叶片,称重,置于在冰上预冷的灭菌研钵中,按1︰10配比加入同样预冷的PBS缓冲液(pH 7.2),迅速研磨成浆液,用无菌纱布过滤掉叶片残渣获得病毒接种液备用。通过摩擦接种法将病毒接种液接种于脱毒半夏幼苗,接种后半夏保存于人工气候室。
1.2.9 RT-PCR法检测SMV-SXBX以提取接种半夏的总RNA为模板,用引物SMV4F、SMV4R进行RT-PCR扩增检测。
2 结果与分析2.1 SMV-SXBX的全长cDNA克隆与组装以cDNA第一链为模板,利用3对特异引物pGreen SMV1F与SMV1R、SMV2F与pGreen SMV2R、SMV2F与pGreen SMV2R′扩增分别得到长度为6 627 bp和2 960 bp的SMV-SXBX基因组大片段Ⅰ和Ⅱ;以pGreenⅡ-35S质粒为模板利用特异引物pGr35S-SMVF和pGr35S-SMVR、pGr35S-SMVF′和pGr35S-SMVR采用反向PCR的方法得到线性化载体3 233 bp,cDNA均得到预期大小的清晰条带(图 2A,2C)。
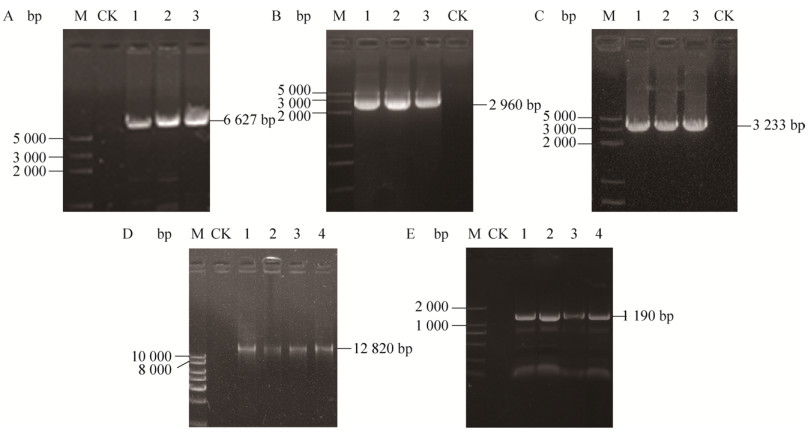 |
| 图 2 SMV-SXBX侵染性克隆构建电泳图 Fig. 2 Electropherogram of construction full-length infectious cDNA clone of SMV-SXBX. (A) PCR product of pGreen SMV1F and SMV1R. (B) PCR product of SMV2F and pGreen SMV2R, SMV2F and pGreen SMV2R′. (C) pGr35S-SMVF and pGr35S-SMVR, pGr35S-SMVF′ and pGr35S-SMVR. M: DL 5000TM DNA marker. (D) Plasmid extraction result. (E) PCR product of colony PCR. Lane 1-4: infected plant; CK: healthy plant. |
| 图选项 |
2.2 SMV-SXBX侵染性克隆鉴定将3个纯化的重叠片段Ⅰ、Ⅱ和载体在Gibson反应中进一步组装,重组产物冻融转化进农杆菌GV3101 (pSoup)感受态细胞,并挑取单克隆菌落,抽提质粒进行阳性克隆鉴定,结果表明,挑取的4个菌落为阳性(图 2D),通过用特异引物SMV3F、SMV3R进行菌落PCR鉴定,得到预期大小的清晰条带(图 2E),经测序分析确定为SMV。由于农杆菌质粒提取拷贝数低,故以农杆菌菌液为模板,采用分片段PCR鉴定全长cDNA侵染性克隆。
2.3 SMV-SXBX侵染性克隆活性检测2.3.1 体内转录SMV-SXBX侵染性克隆注射接种半夏的病症表现体内转录SMV-SXBX侵染性克隆农杆菌注射接种半夏的结果表明:SMV-SXBX(56A)侵染性克隆接种半夏植株的症状表现与野生SMV引起的症状相同,接种7 d时,同株珠芽新生非接种叶片在7 d时,具有典型症状,花叶、叶脉开始褪绿及黄化(图 3A),12 d时,随着症状的严重,会出现叶片完全退化整个植株出现坏死,受侵染的植株在14 d时死亡。而SMV-SXBX(28A)侵染性克隆农杆菌接种半夏植株、转化空载体pGreenⅡ-35S的农杆菌阴性对照组在接种半夏植株7 d后仍未表现出任何病症。
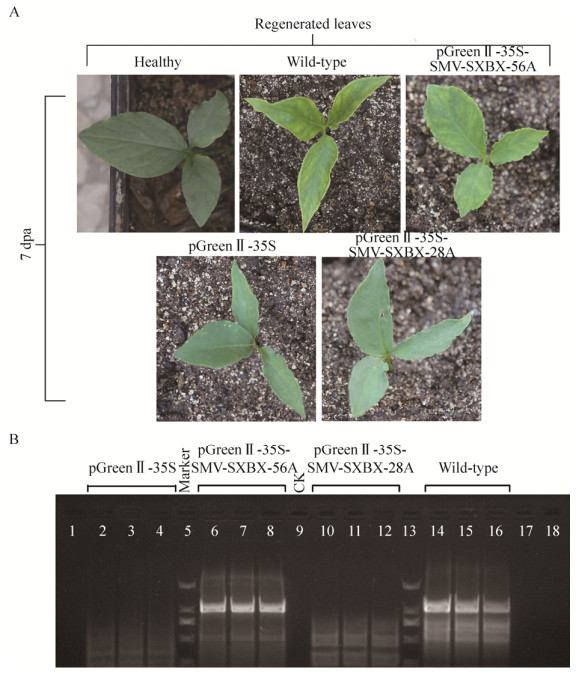 |
| 图 3 不同长度A尾SMV-SXBX全长cDNA克隆接种半夏再生叶7 d后的症状示意图(A)及RT-PCR检测SMV-SXBX侵染性克隆农杆菌注射接种半夏CP基因电泳图(B) Fig. 3 Schematic representation symptoms of different lengths poly(A) SMV-SXBX full-length cDNA clones inoculated after 7 days of regeneration in Pinellia ternata and RT-PCR analysis of Pinellia ternata agroinoculated with full-length infectious cDNA clone of SMV-SXBX to detect the CP-coding regions. (A) Pinellia ternata plants systemically infected with the poly(28A), poly(56A) or pGeenⅡ-35S SMV-SXBX infectious clone and were photographed at 7 days post infiltration. Healthy; infected plant. (B) RT-PCR using primer pairs of SMV 4F/SMV 4R to detect the partial of CP-coding regions. lane 2-4: Pinellia ternata inoculated with Agrobacterium carrying the empty vector pGreenII-35S for PCR; lane 6-8: Pinellia ternata inoculated with Agrobacterium carrying the clone of pGreenⅡ-35S-SXBX-56A for PCR; lane 10-12: Pinellia ternata inoculated with Agrobacterium carrying the clone of pGreenⅡ-35S-SXBX-28A for PCR; lane 14-16: SMV of wild-type for PCR; lane 5, 13: DNA marker DL 2 000; lane 1: 9, 17, 18: CK: healthy plant. |
| 图选项 |
2.3.2 机械接种检测侵染性为了验证侵染性cDNA克隆产生后代病毒的生物学活性,将病毒接种液摩擦接种半夏健康植株。10 d机械接种植物也出现了同样的症状,证明该接种方法效果稳定,可以此为标准应用于后续实验。证明了病毒的后代在植物体内有系统地运动,表明构建侵染性克隆具有稳定性和侵染性。
2.3.3 RT-PCR检测接种一周后从接种半夏珠芽新生非接种叶中抽提RNA,分别用引物SMV4F、SMV4R进行RT-PCR检测,检测结果显示以待测半夏cDNA作为模板的泳道在相应位置均出现单一明亮条带(图 3B),证明SMV-SXBX(56A)接种半夏均被SMV-SXBX成功侵染,而转化空载体pGreenⅡ-35S、SMV-SXBX(28A)的农杆菌注射接种的阴性对照组未检测到,表明注射接种含空载体、SMV-SXBX(28A)的农杆菌不能侵染半夏植株,同时RT-PCR产物测序结果显示,均为SMV-SXBX病毒基因组序列。经机械接种摩擦接种、病症观察及RT-PCR检测均表明本实验中利用Gibson系统构建的适用农杆菌注射接种的体内转录侵染性克隆具有侵染性,能成功侵染半夏,对RT-PCR产物进行测序,并确认SMV感染,说明SMV-SXBX侵染性克隆构建成功。
3 讨论构建全长侵染性克隆对于反向遗传学研究病毒生物学、病理学和病毒载体构建必不可少,由于RNA基因组难以保存和操作,而克隆可用于稳定地维持RNA病毒分子特征[11],所以构建cDNA侵染性克隆对于研究RNA病毒分子的生物学特性及其基因功能具有重要意义。2009年Gibson等描述的装配法因其不需要酶或共同的限制位点故迄今为止最受欢迎,该装配法是基于将重叠的PCR扩增的DNA片段在一个等温步骤中组装而成[12],简化了侵染性克隆的装配,从而减少了植物病毒学的主要技术限制。Gibson组装已被成功应用于构建Tymovirus、Carlavirus、Comovirus、Potyvirus、Polerovirus、Benyvirus成员侵染性克隆[13]。然而在先前的研究报道中,许多病毒全长克隆在细菌繁殖过程中可能会发生序列改变,如点突变和缺失[14],尤其是基因组较大的PVY属RNA病毒全长cDNA克隆在利用大肠杆菌构建时由于其自身编码的病毒蛋白会对宿主菌产生很强的毒性作用[15-17],从而导致非特异性重组,难以或甚至不可能在大肠杆菌中进行繁殖。为了克服病毒基因序列在大肠杆菌中的不稳定性,故构建侵染性克隆时通常采用低拷贝载体、降低培养温度、接种前进行体外连接、插入内含子等方式减轻质粒不稳定性而获得稳定的侵染性克隆[18],近年来更高效、更方便的策略是构建穿梭质粒,利用放射杆菌对植物病毒侵染性克隆进行克隆和接种,绕过了大肠杆菌克隆的要求,避免了不稳定性[1]。
Poly(A)尾在真核生物mRNA翻译以及从细胞核到细胞质的运动中起着重要作用[19],许多病毒的RNA具有3′ poly(A)尾,对于阳性RNA病毒,poly(A)尾与RNA的稳定、翻译和复制有关[20]。据报道,在一些病毒中poly(A)尾对病毒侵染性具有重要作用,poly(A)尾的长度也可能影响病毒的复制[21],poly(A)长度因病毒而异,病毒poly(A)尾存在一个阈值,在低于阈值时会严重影响病毒的侵染性,尤其是对于PVY属病毒,这种现象更为明显,如TVMV需要37个或96个A[22]、PPV需要100个A[23],ZYMV需要66个A[24],TEV需要70-75个A[25]。几种病毒
poly(A)尾长度与病毒侵染性呈正相关,包括竹花叶病毒(Bamboo mosaic virus,BaMV)、白三叶草花叶病毒(White clover mosaic virus,WCLMV)、豇豆花叶病毒(Cowpea mosaic virus,CPMV)等。BaMV中一条足够长的poly(A)与上游二级结构元素相互作用形成假结结构,这种相互作用减少了病毒的积累,此外BaMV等几种阳性RNA病毒的3′ poly(A)尾序列与病毒RNA复制所需的宿主蛋白之间会发生相互作用[26]。其他植物病毒如马铃薯Y病毒的3′末端poly(A)尾,也通过与许多宿主蛋白相互作用发挥重要作用[27],木槿潜伏新加坡病毒(Hibiscus latent singapore virus,HLSV) poly(A)尾长度为78-96 nt,当poly(A)尾长度小于24 nt时,HLSV不能复制,因此24 nt poly(A)尾是HLSV在烟草中进行复制所需的最小长度,其长度影响着HLSV的病毒复制效率[28]。故本研究推测28 nt poly(A)还没有达到SMV复制在半夏中所需的最小长度,而56 nt poly(A)达到或已超过SMV复制在半夏中所需的最小长度,具体SMV复制在半夏中所需的最小长度还有待于进一步研究,本文为SMV侵染性克隆构建研究奠定理论基础。
4 结论本研究基于Gibson体外组装策略快速高效构建具有生物活性的SMV-SXBX农杆菌侵染性克隆,由侵染性克隆产生的病毒汁液接种半夏引起了典型的SMV感染症状,并被稳定地机械传播给后代。我们发现SMV侵染性与其3′末端poly(A)尾长度相关,构建了28 nt和56 nt poly(A)尾的SMV-SXBX侵染性克隆,结果表明,SMV-SXBX(28)侵染植株无症状而SMV-SXBX (56)具有侵染性,接种叶片坏死,致死症状严重。本文高效便捷的构建方法及poly(A)尾对病毒侵染性的影响也为构建其他PVY属病毒或基因组较大的RNA病毒提供了新的借鉴和思路。
参考文献
| [1] | Tuo DC, Fu LL, Shen WT, et al. Generation of stable infectious clones of plant viruses by using Rhizobium radiobacter for both cloning and inoculation. Virology, 2017, 510: 99-103. DOI:10.1016/j.virol.2017.07.012 |
| [2] | Hajimorad MR, Domier LL, Tolin SA, et al. Soybean mosaic virus:a successful potyvirus with a wide distribution but restricted natural host range. Mol Plant Pathol, 2018, 19(7): 1563-1579. DOI:10.1111/mpp.12644 |
| [3] | Cui XY.Functional characterization of potyvirus-encoded membrance-associated proteins[D].Yangling: Northwest A & F University, 2010(in Chinese). 崔晓艳.马铃薯Y病毒属编码的膜相关蛋白的功能研究[D].杨凌: 西北农林科技大学, 2010. |
| [4] | Tuo DC.Identification, generation of infectious full-length cDNA clones of Papaya leaf distortion mosaic virus from Hainan (PLDMV-DF) and its use for GFP expression[D].Haikou: Hainan University, 2015(in Chinese). 庹德财.番木瓜畸形花叶病毒检测鉴定及侵染性克隆构建与应用[D].海口: 海南大学, 2015. |
| [5] | Boissinot S, Pichon E, Sorin C, et al. Systemic propagation of a fluorescent infectious clone of a polerovirus following inoculation by agrobacteria and aphids. Viruses, 2017, 9(7): 166. DOI:10.3390/v9070166 |
| [6] | Cui TT, Yan JH, Bin Y, et al. Construction of Citrus leaf blotch virus infectious cDNA clone by yeast homologous recombination system. Sci Agric Sin, 2018, 51(9): 1695-1705 (in Chinese). 崔甜甜, 晏建红, 宾羽, 等. 利用酵母同源重组系统快速构建柑橘叶斑驳病毒的侵染性克隆. 中国农业科学, 2018, 51(9): 1695-1705. |
| [7] | Wang W, Wei YY, Zhai SH, et al. Construction of recombinant infectious cDNA clone of SRV9 Rabies virus by Gibson assembly. Prog Vet Med, 2017, 38(11): 11-17 (in Chinese). 王伟, 魏玉圆, 翟少华, 等. 应用Gibson Assembly方法构建狂犬病病毒SRV9株重组感染性cDNA克隆. 动物医学进展, 2017, 38(11): 11-17. DOI:10.3969/j.issn.1007-5038.2017.11.003 |
| [8] | Yang FY, Yang YP, Huo YX. Restriction enzyme-free molecular cloning strategies in synthetic biology. Chin J Biochem Mol Biol, 2018, 34(4): 364-370 (in Chinese). 杨发誉, 杨云彭, 霍毅欣. 合成生物学中不依赖限制性内切酶的分子克隆策略. 中国生物化学与分子生物学报, 2018, 34(4): 364-370. |
| [9] | Pan HB, Yan YQ, Zhang J, et al. Rapid construction of a replication-competent infectious clone of human adenovirus type 14 by Gibson assembly. Viruses, 2018, 10(10): 568. DOI:10.3390/v10100568 |
| [10] | Zarzyńska-Nowak A, Ferriol I, Falk BW, et al. Construction of Agrobacterium tumefaciens-mediated tomato black ring virus infectious cDNA clones. Virus Res, 2017, 230: 59-62. DOI:10.1016/j.virusres.2017.01.014 |
| [11] | Liu QL, Dan HL, Zhao XP, et al. Construction and characterization of an infectious cDNA clone of coxsackievirus A 10. Virol J, 2019, 16(1): 98. DOI:10.1186/s12985-019-1201-1 |
| [12] | Bordat A, Houvenaghel MC, German-Retana S. Gibson assembly:an easy way to clone potyviral full-length infectious cDNA clones expressing an ectopic VPg. Virol J, 2015, 12: 89. DOI:10.1186/s12985-015-0315-3 |
| [13] | Pasin F, Menzel W, Daròs JA. Harnessed viruses in the age of metagenomics and synthetic biology:an update on infectious clone assembly and biotechnologies of plant viruses. Plant Biotechnol J, 2019, 17(6): 1010-1026. DOI:10.1111/pbi.13084 |
| [14] | Boyer JC, Haenni AL. Infectious transcripts and cDNA clones of RNA viruses. Virology, 1994, 198(2): 415-426. |
| [15] | Gao R, Tian YP, Wang J, et al. Construction of an infectious cDNA clone and gene expression vector of Tobacco vein banding mosaic virus (genus Potyvirus). Virus Res, 2012, 169(1): 276-281. DOI:10.1016/j.virusres.2012.07.010 |
| [16] | Tuo DC, Shen WT, Yan P, et al. Rapid construction of stable infectious full-length cDNA clone of papaya leaf distortion mosaic virus using in-fusion cloning. Viruses, 2015, 7(12): 6241-6250. DOI:10.3390/v7122935 |
| [17] | Bukovinszki á, G?tz R, Johansen E, et al. The role of the coat protein region in symptom formation on Physalis floridana varies between PVY strains. Virus Res, 2007, 127: 122-125. DOI:10.1016/j.virusres.2007.03.023 |
| [18] | Tuo DC, Shen WT, Yan P, et al. A novel E.coli-free method for the rapid construction of full-length cDNA infectious clone of Papaya leaf distortion mosaic virus (PLDMV) by yeast homologous recombination system. Chin J Trop Crops, 2017, 38(8): 1492-1500 (in Chinese). 庹德财, 沈文涛, 言普, 等. 一种E.coli-Free的酵母同源重组系统稳定快速构建PLDMV侵染性克隆的新方法. 热带作物学报, 2017, 38(8): 1492-1500. DOI:10.3969/j.issn.1000-2561.2017.08.019 |
| [19] | Nicholson AL, Pasquinelli AE. Tales of detailed Poly (A) tails. Trends Cell Biol, 2019, 29(3): 191-200. DOI:10.1016/j.tcb.2018.11.002 |
| [20] | Li WM, Zhang YQ, Zhang C, et al. Presence of poly (A) and poly (A)-rich tails in a positive-strand RNA virus known to lack 3′ poly (A) tails. Virology, 2014, 454-455: 1-10. DOI:10.1016/j.virol.2014.02.002 |
| [21] | Guo S, Kierzek E, Chen G, et al. TMV mutants with poly (A) tracts of different lengths demonstrate structural variations in 3′UTR affecting viral RNAs accumulation and symptom expression. Sci Rep, 2015, 5: 18412. DOI:10.1038/srep18412 |
| [22] | Domier LL, Franklin KM, Hunt AG, et al. Infectious in vitro transcripts from cloned cDNA of a potyvirus, tobacco vein mottling virus. Proc Natl Acad Sci USA, 1989, 86(10): 3509-3513. DOI:10.1073/pnas.86.10.3509 |
| [23] | Riechmann J, Laín S, Garcia JA. Infectious in vitro transcripts from a plum pox potyvirus cDNA clone. Virology, 1990, 177(2): 710-716. DOI:10.1016/0042-6822(90)90537-2 |
| [24] | Gal-On A, Meiri E, Huet H, et al. Particle bombardment drastically increases the infectivity of cloned DNA of zucchini yellow mosaic potyvirus. J Gen Virol, 1995, 76(12): 3223-3227. DOI:10.1099/0022-1317-76-12-3223 |
| [25] | Dolja VV, McBride HJ, Carrington JC. Tagging of plant potyvirus replication and movement by insertion of beta-glucuronidase into the viral polyprotein. Proc Natl Acad Sci USA, 1992, 89(21): 10208-10212. DOI:10.1073/pnas.89.21.10208 |
| [26] | Chen IH, Cheng JH, Huang YW, et al. Characterization of the polyadenylation activity in a replicase complex from Bamboo mosaic virus-infected Nicotiana benthamiana plants. Virology, 2013, 444(1/2): 64-70. |
| [27] | Lima SA, Chipman LB, Nicholson AL, et al. Short poly (A) tails are a conserved feature of highly expressed genes. Nat Struct Mol Biol, 2017, 24(12): 1057-1063. DOI:10.1038/nsmb.3499 |
| [28] | Niu SN, Cao SS, Huang LJ, et al. The length of an internal poly (A) tract of hibiscus latent Singapore virus is crucial for its replication. Virology, 2015, 474: 52-64. DOI:10.1016/j.virol.2014.10.029 |
