蔺凌云, 潘晓艺, 姚嘉赟, 尹文林, 曹铮, 刘忆瀚, 沈锦玉


浙江省淡水水产研究所, 浙江省鱼类健康与营养重点实验室, 浙江 湖州 313001
收稿日期:2020-12-11;修回日期:2021-02-07;网络出版日期:2021-08-30
基金项目:浙江省重点研发计划(2019C02058);浙江省省属科研院所扶持专项(2021YSZX001);湖州市公益性应用研究重点项目(2019GZ16)
*通信作者:沈锦玉, E-mail: sjinyu@126.com.
摘要:[目的] 为查明浙江养殖光唇鱼大量死亡的病原,了解病原的遗传特征。[方法] 本工作对患病光唇鱼进行病原分离,结合形态特征、生理生化特性和16S rRNA基因序列同源性,对分离菌株进行鉴定;采用人工回感试验确定其病原性,并对分离株的血清型、多位点序列分型(multilocus sequence typing,MLST)、毒力基因型和表面蛋白抗原基因型等遗传特征进行分析;此外,还测试了菌株的药敏特性。[结果] 从患病光唇鱼体中分离得到优势菌株ACRO-0708,为革兰氏阳性球菌,不溶血,分子与生化鉴定为无乳链球菌(Streptococcus agalactiae);人工感染试验证实其对光唇鱼有较强的致病性,LD50为6.47×103 CFU/g,属于血清型Ⅰb和MLST型ST261,毒力基因型为sip+bibA+cfb+hylB+iagA+fbsA+fbsB+bac-bca-cylE-scpB-lmb-,不携带所检测的6种表面蛋白基因。药敏试验结果显示,对青霉素、氨苄西林等8种药物较敏感,对氯霉素、复方新诺明等7种药物耐药。[结论] 引起浙江养殖光唇鱼死亡的病原菌为无乳链球菌,其分子特征与水产动物主要流行的无乳链球菌株具有显著差异,生产中可选用氨苄西林、氟苯尼考等药物进行防治。
关键词:光唇鱼无乳链球菌分子血清型MLST毒力基因
Identification and genetic characterization of Streptococcus agalactiae isolated from Acrossocheilus fasciatus cultured in Zhejiang
Lingyun Lin, Xiaoyi Pan, Jiayun Yao, Wenlin Yin, Zheng Cao, Yihan Liu, Jinyu Shen


Key Laboratory of Fish Health and Nutrition of Zhejiang Province, Zhejiang Institute of Freshwater Fisheries, Huzhou 313001, Zhejiang Province, China
Received: 11 December 2020; Revised: 7 February 2021; Published online: 30 August 2021
*Corresponding author: Jinyu Shen, E-mail: sjinyu@126.com.
Foundation item: Supported by the Zhejiang Province Key Research and Development Plan (2019C02058), by the Zhejiang Provincial Research Institutes Support Special Projects (2021YSZX001) and by the Huzhou Public Welfare Application Research Key Project (2019GZ16)
Abstract: [Objective] This study aimed to investigate pathogenesis of diseased Acrossocheilus fasciatus cultured in Zhejiang province and analyze genetic characteristics of the pathogen. [Methods] We isolated and purified pathogens from diseased A. fasciatus and identified the pathogenic bacteria by analyzing the physiology biochemistry characteristics and 16S rRNA gene sequence. We also carried out artificial infection experiments to verify the pathogenicity of isolated strain to A. fasciatus. Then the genetic characteristics of the pathogen were analyzed by molecular serotyping, multilocus sequence typing (MLST) and PCR screening of surface protein antigen genes and virulence-related genes. Meanwhile, we tested its antimicrobial susceptibility by disc diffusion method. [Results] One predominant strain named ACRO-0708 was isolated from diseased A. fasciatus, which was gram-positive, chain-forming coccus and nonhemolytic, and was identified as Streptococcus agalactiae through the morphological characteristics, phenotypic characteristics and 16S rRNA sequence analysis. In Challenge trials, the strain ACRO-0708 exhibited high virulence in A. fasciatus with an intra-peritoneal LD50 value of 6.47×103 CFU/g. The strain ACRO-0708 was type of Ib-ST261, and the virulence-related genotype was sip+bibA+cfb+hylB+iagA+fbsA+fbsB+bac-bca-cylE-scpB-lmb-. However, it did not carry any of the six surface protein antigen genes which were detected. The antimicrobial susceptibility results showed strain ACRO-0708 was susceptible to 8 agents including penicillin, ampicillin and other antibiotics. Meanwhile, it showed resistance to chloroamphenicol, trimethoprim-sulfamethoxazole and other 5 antibiotics. [Conclusion] Our results demonstrated that S. agalactiae with molecular characteristics obviously different from the main epidemic strains of S. agalactiae in aquatic animals was the causative agent of the enzootic, and the disease could be prevented by using drugs such as penicillin and florfenicol in fisheries. To our knowledge, this is the first report of S. agalactiae as a pathogen of A. fasciatus.
Keywords: Acrossocheilus fasciatusStreptococcus agalactiaemolecular serotypingMLSTvirulence-related genes
光唇鱼(Acrossocheilus fasciatus)俗称淡水石斑鱼,属鲤形目、鲤科、鲃亚科、光唇鱼属[1]。主要分布于我国南方的溪流中,其肉质鲜美,体色鲜艳,具有较高的营养价值和观赏价值,社会经济效益显著,目前光唇鱼已成为浙江山区重要的特色经济养殖品种[2-3]。光唇鱼生活的水质条件通常比较好,暴发疾病的情况并不多见。2020年7–8月份,浙江多家光唇鱼养殖场先后发生了急性死亡,病鱼眼角膜白浊,眼球突出甚至脱落,水中狂游或打转,身体逐渐失去平衡,部分病鱼背脊、尾部及尾柄等多处的表皮开始溃烂,肾脏肿大,个别病鱼腹腔较多积水。目前光唇鱼养殖过程中未见该病的研究报道。
无乳链球菌是鱼类链球菌病的主要病原之一,该菌可引起多种水产动物如罗非鱼(Oreochromis sp.)[4-5]、虹鳟(Oncorhynchus mykiss)[6-7]、红尾皇冠鱼(Aequidens rivulatus)[8]、宝石鲈(Scortum barcoo)[9]、黄河裸裂尻鱼(Schizopygopsis pylzovi Kessler)[10]、齐口裂腹鱼(Schizothorax prenanti)[11]和牛蛙(Bullfrog,Rana catesbeiana)[12]等的败血症和脑膜炎,给水产养殖业造成了巨大经济损失。无乳链球菌常用的分型方法主要有分子血清型、MLST (multilocus sequence typing)、MLVA (multiple-locus variant-repeat analysis)、毒力基因型及表面蛋白基因型等[13-16],这些分型方法具有良好的稳定性和可重复性,已被多个实验室使用。国内已报道的水产动物源无乳链球菌主要为β溶血,Ⅰa血清型,MLST分型为ST7[13, 16-17],而非溶血-Ⅰb血清型-ST261型的无乳链球菌较少发现,仅在鲮鱼[18]、梭鲈[19]、罗非鱼[20]上有过报道。
本研究拟对患病光唇鱼进行病原分离鉴定和人工感染试验,以查明本次暴发病的病原。通过MLST、分子血清型和毒力相关基因检测等对分离菌株进行遗传特征分析,同时进行药物敏感性试验,以期为光唇鱼的病原研究和病害防治提供参考,为了解光唇鱼源无乳链球菌的分子流行病学特征、探究其起源及今后流行菌株的遗传变异提供基础数据。
1 材料和方法 1.1 实验材料 发病光唇鱼(5尾)采自浙江某光唇鱼养殖场;健康光唇鱼由浙江省淡水水产研究所八里店养殖基地提供,每尾10–12 g;BHI培养基、血平板购自青岛海博生物技术有限公司;API 20 Strep细菌鉴定试剂条购自法国梅里埃公司;细菌基因组DNA提取试剂盒、PCR相关试剂等购自宝生物(大连)有限公司;药敏纸片购自杭州微生物试剂有限公司。
1.2 镜检及细菌分离纯化 取病鱼鳃丝、鳍条部位和体表粘液于载玻片上,显微镜下进行真菌和寄生虫检查;发病光唇鱼体表用70%乙醇擦拭后,进行无菌解剖,分别对肝、脾、肾及脑进行细菌分离,于BHI和血平板上进行细菌培养。平板于28 ℃培养2 d后,挑取优势单菌落进行纯化培养,多次纯化后,对获得的菌株进行保藏。
1.3 菌株的分类鉴定
1.3.1 菌株的形态特征: 12株纯化菌株分别在BHI及血平板上划线培养,观察菌落形态及溶血情况。挑取单菌落,经涂片、固定、革兰氏染色,于显微镜下观察菌体形态。
1.3.2 菌株的生化鉴定: 根据染色结果和菌体形态,选用API 20 Strep链球菌快速鉴定试剂条对12株纯化菌株进行生理生化鉴定。鉴于12株菌株的菌体形态和生理生化特性的完全一致性,为方便试验开展,本研究选取其中一株分离自肾脏组织的纯化菌株ACRO-0708作为代表菌株进行后续试验。
1.3.3 菌株的分子鉴定: 过夜培养(16–18 h)的ACRO-0708新鲜菌液,使用细菌基因组DNA提取试剂盒抽提DNA,以细菌16S rRNA通用引物27F和1492R[21]进行PCR扩增。PCR反应体系:2×premix Taq 25 μL,上下游引物(浓度为10 μmol/L)各2 μL,DNA模板1 μL (20–100 ng),ddH2O补足至50 μL。PCR扩增程序:94 ℃ 5 min;94 ℃变性30 s,55 ℃退火30 s,72 ℃延伸1.5 min;35个循环后72 ℃温育10 min。PCR产物用1%琼脂糖凝胶电泳检测后,对PCR产物进行测序分析。菌株16S rRNA序列与GenBank中序列进行同源性比对,使用MEGA X软件对从GenBank数据库中获得的序列相似性较高的菌株序列进行多序列比对,采用最大似然法(maximum likelihood method)的Jukes-Cantor模型构建系统发育树,并通过Boot-strap法(1000次重复)检验。
1.4 人工感染试验 健康光唇鱼在循环水养殖系统暂养一周后,随机分为5组,每组20尾。攻毒菌株ACRO-0708接种于BHI培养基,28 ℃恒温振荡(180 r/min)培养48 h,菌液5000 r/min离心5 min,沉淀用无菌PBS 10倍梯度稀释,平板计数法测定菌液浓度。采用腹腔注射方式进行攻毒,4个试验组的注射剂量分别为1.50×107、1.50×106、1.50×105和1.5×104 CFU/尾。对照组注射等体积的PBS (0.1 mL/尾)。养殖水温28 ℃,每天记录发病症状及死亡情况,连续观察7 d,根据Reed-Muench方法[22]计算攻毒菌株的LD50。对感染发病光唇鱼进行细菌分离鉴定。
1.5 分子血清型、MLST分型测定 分子血清型的鉴定参考Poyart等[23]依据无乳链球菌荚膜多糖差异建立的多重PCR检测方法。多位点序列分型(MLST)参考Jones等[24]的方法,分别对adhP、pheS、atr、glnA、sdhA、glcK和tkt等7个看家基因进行PCR扩增和测序。然后将这7个基因的序列与网上(http://pubmlst.org/sagalactiae/)相应等位基因序列进行比对分析, 获得各等位基因数值序列号,再通过该组数值序列号查找得到相应的ST序列型。
1.6 表面蛋白基因和毒力相关基因检测 参考Creti等[25]的方法进行表面蛋白(alpha-C protein,Alp2,Alp3,Rib,epsilon protein)基因的检测。通过PCR扩增检测分离菌株的以下毒力相关基因[4, 26-30]:sip (表面免疫原性蛋白)、bac (β C蛋白)、bca (α C蛋白)、bibA (细菌免疫原性黏附素)、cfb (CAMP因子)、cylE (β-溶血素/溶细胞素)、hylB (透明质酸酶)、iagA (侵袭相关基因)、lmb (层黏连蛋白结合蛋白)、scpB (C5a肽酶)、fbsA (纤维蛋白结合蛋白A)、fbsB (纤维蛋白结合蛋白B),其引物序列和退火温度见表 1。PCR产物采用1.5%琼脂糖凝胶电泳检测。
表 1. 毒力相关基因的PCR扩增引物 Table 1. Primers used for virulence associated genes identification
| Gene | Primer sequence (5′→3′) | Amplicon size/bp | Temperature of annealing/℃ | Time of extension/s | References |
| scpB | scpBF: CCTGCTAAAACTGCTGATAC scpBR: CATAAGCATAGTCGTAAGCC | 853 | 50 | 60 | [26] |
| hylB | hylBF: CACCAATCCCCACTCTACTA hylBR: GTGTCAAACCATCTATCAG | 444 | 50 | 45 | [26] |
| bca | bcaF: CTACAATTCCAGGGAGTGCA bcaR: ACTTTCTTCCGTCCACTTAG | 376 | 50 | 30 | [26] |
| bac | bacF: AAGCAACTAGAAGAGGAAGC bacR: TTCTGCTCTGGTGTTTTAGG | 479 | 52 | 45 | [26] |
| lmb | LmbF: CCGTCTGTAAATGATGTGGC LmbR: GAAATACCCGAGATACCAAG | 473 | 50 | 45 | [27] |
| cylE | cylEF: CATTGCGTAGTCACCTCCC cylER: GGGTTTCCACAGTTGCTTGA | 380 | 55 | 30 | [28] |
| fbsA | fbsAF: GAACCTTCTTGTCACACTTG fbsAR: TTGATCCTAGCACTCCCA | 556 | 50 | 45 | [28] |
| fbsB | fbsBF: GCGCAAACTTCTGTCCAA fbsBR: CCGATACGATTGTCCAAATG | 417 | 55 | 45 | [28] |
| iagA | iagAF: CGGGATTGATCTAAGTCGCT iagAR: CCATCAACATCAGTCGCTAA | 459 | 55 | 45 | [28] |
| cfb | cfbF: CGACAGCATCACACGAAAAATACA cfbR: TGACGACCTTTTGGACAAGTAGTAA | 900 | 55 | 60 | [4] |
| bibA | bibAF: AACCAGAAGCCAAGCCAGCAACC bibAR: AGTGGACTTGCGGCTTCACCC | 127 | 55 | 30 | [29] |
| sip | sipF: GTTAAACCAACTCAGACGTCAG sipR: TTCAGGATGTGCAGCTACTGC | 339 | 55 | 30 | [30] |
表选项
1.7 药物敏感性试验 采用琼脂扩散(纸片)法进行药敏试验。挑取血平板上过夜培养的ACRO-0708单菌落,用生理盐水配制成0.5麦氏浓度,以涂布法接种含5%脱纤维羊血的MH琼脂平板。贴上药敏纸片后28 ℃培养48 h,测量抑菌圈直径。参照CLSI动物源细菌抗菌药物敏感性试验纸片法与稀释法执行标准[31]中肺炎链球菌之外其他链球菌的抑菌圈直径解释标准判定敏感、中介和耐药,对部分缺乏判定标准的药物其浓度折点值参考人源的解释标准[32]。
2 结果和分析 2.1 菌株鉴定
2.1.1 菌株的形态特征: 发病光唇鱼经镜检未发现真菌及寄生虫感染。从患病光唇鱼的肝、脾、肾及脑组织中分离到了大量菌落形态一致的优势菌落。分离菌株生长缓慢,在BHI平板上形成无色、圆形、微隆起的细小菌落,在血平板上的生长情况好于BHI平板,菌落稍大,灰白色、圆形、表面湿润、粘稠,不溶血。通过纯化,从该养殖场5条发病光唇鱼上共获得12个细菌分离物(肾脏4株,脑4株,肝脏2株,脾脏2株),均为革兰氏阳性,菌体呈球形、链状排列。
2.1.2 菌株的生理生化特征: API 20 Strep链球菌快速鉴定的检测结果(表 2)显示,12株菌株的生理生化特征完全一致,与鱼源非溶血Ⅰb血清型无乳链球菌标准株LMG15977的符合度最高,其中仍有3项生理生化指标存在差异:光唇鱼分离菌株的精氨酸双水解酶为阳性、核糖和糖原发酵均为阴性,而对照菌株LMG15977的精氨酸双水解酶为阴性、核糖和糖原发酵均为阳性。因此,菌株的种属鉴定还需要利用分子生物学的方法进一步确定。
表 2. 菌株的生理生化鉴定结果 Table 2. Results on physiological and biochemical identification of A. fasciatus's isolates and reference strain
| Biochemical item | Result | |
| Twelve A. fasciatus’s isolates* | S. agalactiae LMG15977[7] | |
| V-P test | + | + |
| HIP | + | + |
| AES | – | – |
| PYRA | – | – |
| α-GAL | – | – |
| β-GUR | + | + |
| β-GAL | – | – |
| PAL | + | + |
| LAP | + | + |
| ADH | + | – |
| Haemolysis | – | – |
| RIB | – | +s |
| ARA | – | – |
| MAN | – | – |
| SOR | – | – |
| LAC | – | – |
| TRE | – | – |
| INU | – | – |
| RAF | – | – |
| AMD | – | – |
| GLYG | – | + |
| * The same biochemical results for all twelve isolates. “+”: positive; “–”: negative; “s”: slow reaction. | ||
表选项
2.1.3 菌株的分子鉴定: PCR扩增代表菌株ACRO-0708的16S rRNA基因获得1500 bp左右的目的片段,将测序结果提交至NCBI,获得序列号NW073926。BLAST比对分析结果显示,该序列与GenBank上已公布无乳链球菌(Streptococcus agalactiae) 16S rRNA序列(GenBank ID:FO393392.1,KT001225.1,KP294526.1)的同源性最高,相似性均为100%。基于16S rRNA序列构建的系统发育树表明(图 1),分离菌株ACRO-0708与S. agalactiae聚为一簇。综合分离菌株的表型特征、理化特性及16S rRNA序列同源性和进化树分析,确定菌株ACRO-0708为无乳链球菌。
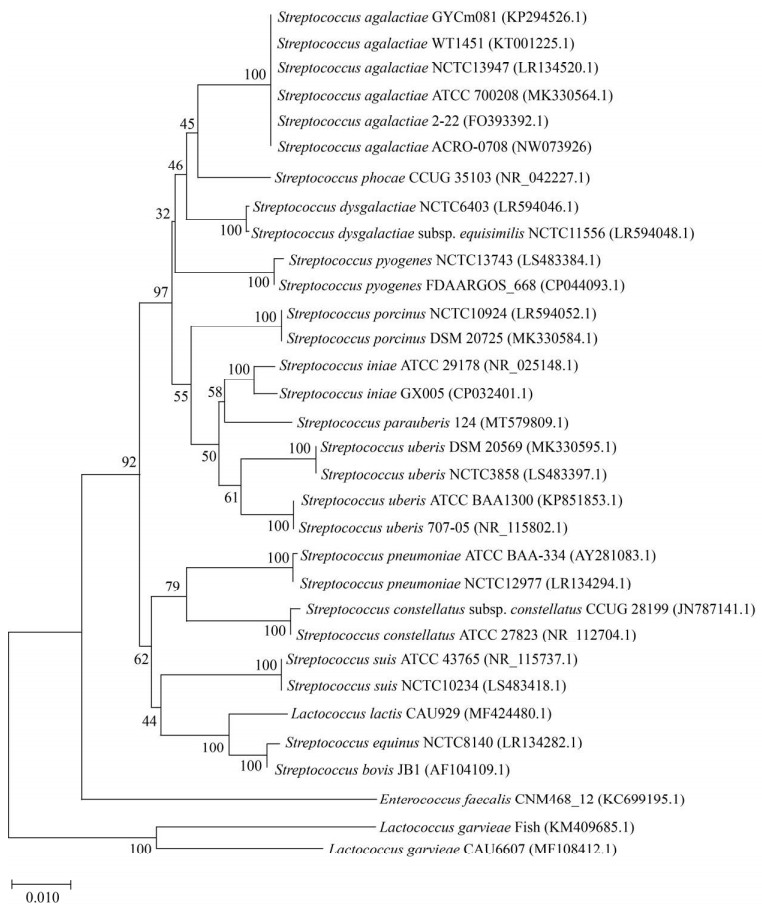 |
| 图 1 基于16S rRNA基因序列的系统发育进化树 Figure 1 Phylogenetic tree based on the sequences of the 16S rRNA genes. Numbers at notes indicate the levels of bootstrap support based on data for 1000 replicates; Numbers in parenthesis represented GenBank accession No.; the scale bar represents 0.010 nucleotide substitutions per site. |
| 图选项 |
2.2 菌株致病性 采用菌株ACRO-0708感染健康光唇鱼的发病症状及死亡情况见图 2和图 3。光唇鱼在感染后1–5 d内陆续发病死亡,高浓度试验组在感染第2天即发生急性死亡。急性死亡的鱼体表病症不明显,解剖可见肾脏肿大。第3天起,光唇鱼的发病症状逐渐显现(图 2),表现为眼球浑浊发白或脱落,身体平衡能力降低,或水中打转,或身体侧偏游动,逐渐沉入水底而死亡。第3–4天为发病死亡高峰期,5 d后基本稳定,未再发生死亡现象。感染7 d后,从高到低各浓度组的死亡率分别为100%、90%、60%、30%。菌株ACRO-0708对光唇鱼的LD50为6.47×103 CFU/g。对照组光唇鱼在7 d观察期内没有出现发病或死亡。从感染死亡鱼中分离细菌,结果显示再分离的菌株与攻毒菌株的形态特征、理化特性及16S rRNA基因序列均一致。
 |
| 图 2 人工感染光唇鱼的发病症状 Figure 2 The clinical signs of diseased Acrossocheilus fasciatus by artificial infection. |
| 图选项 |
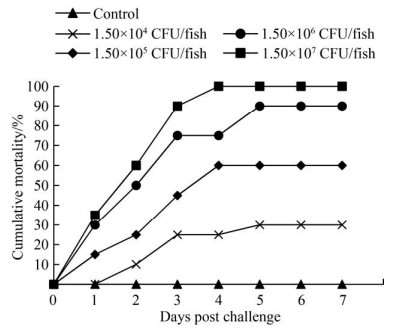 |
| 图 3 菌株ACRO-0708以不同剂量腹腔注射感染光唇鱼的累计死亡率 Figure 3 Cumulative mortality of Acrossocheilus challenged with different doses of the bacteria isolate ACRO-0708 via intraperitoneal injection. |
| 图选项 |
2.3 分子血清型、MLST分型 采用多重PCR方法检测菌株的分子血清型,结果显示菌株PCR扩增出1条约770 bp的目的条带(图 4),表明其血清型为Ⅰb型,Blast对比分析表明,该序列与Ⅰb血清型无乳链球菌的荚膜多糖生物合成基因(GenBank ID:LT671984.1)同源性为99.06%。同时PCR扩增用于MLST分型的7个管家基因获得了大小为550-780 bp的目的片段(图 4),将adhP、pheS、atr、glnA、sdhA、glcK和tkt的测序结果分别上传GenBank数据库,登录号为NW073918–NW073924,并与网站上(http://pubmlst.org/sagalactiae/)相应等位基因序列进行比对分析,获得这7个等位基因类型分别为54、17、31、4、26、25、19,根据此数值序列号查找得到该菌株的MLST分型为ST261型。
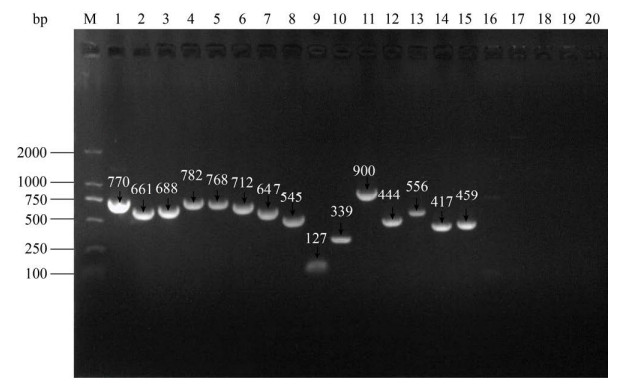 |
| 图 4 分子血清型、MLST分型基因及毒力相关基因的PCR检测结果 Figure 4 PCR for detection of molecular serotype, MLST genotype and virulence-related genes. M-DNA marker (Trans 2K); 1: molecular serotype; 2–8: seven housekeeping genes for MLST, 2-tkt, 3-sdhA, 4-atr, 5-pheS, 6-glnA, 7-glcK, 8-adhP; 9–20: 12 virulence related genes, 9-bibA, 10-sip, 11-cfb, 12-hylB, 13-fbsA, 14-fbsB, 15-iagA, 16-bca, 17-bac, 18-scpB, 19-cylE, 20-lmb. |
| 图选项 |
2.4 表面蛋白抗原基因型和毒力基因型 菌株表面蛋白抗原的PCR反应没有扩增产物,即不存在所检测的6个已知类型的表面蛋白基因,其表面蛋白抗原基因类型未知。如图 4,所检测的12个毒力相关基因中sip、bibA、cfb、hylB、iagA、fbsA、fbsB等7个基因均扩增出了特异性目的条带,毒力基因的阳性携带率为58.3%,而bac、bca、cylE、scpB、lmb等均无目的片段。
2.5 药物敏感性试验 药敏结果(表 3)显示,菌株对青霉素、氨苄西林、头孢曲松、万古霉素、红霉素、克林霉素、壮观霉素及氟苯尼考等8种药物敏感,对氯霉素、左氧氟沙星、庆大霉素、复方新诺明、恩诺沙星、磺胺异噁唑等7种药物耐药,对利福平和四环素中介。
表 3. 菌株ACRO-0708的药物敏感性试验结果 Table 3. Antibiotic sensitivities of strain ACRO-0708
| Drug and contents/(μg or U/piece) | Breakpoint/mm | ACRO-0708 | |||
| Sensibility | Resistance | Zone diameter/mm | Susceptibility | ||
| Penicillin 10 U | ≥24 | — | 28 | S | |
| Ampicillin 10 μg | ≥24 | — | 24 | S | |
| Ceftriaxone 30 μg | ≥24 | — | 26 | S | |
| Vancomycin 30 μg | ≥17 | — | 22 | S | |
| Erythromycin 15 μg | ≥21 | ≤15 | 25 | S | |
| Tetracycline 30 μg | ≥23 | ≤18 | 20 | I | |
| Chloroamphenicol 30 μg | ≥21 | ≤17 | 0 | R | |
| clindamycin 2 μg | ≥19 | ≤15 | 30 | S | |
| levofloxacin 5 μg | ≥17 | ≤13 | 0 | R | |
| Gentamicin 10 μg | ≥15 | ≤12 | 0 | R | |
| Trimethoprim-sulfamethoxazole (SMZ/TMP) 23.75 μg/1.25 μg | ≥19 | ≤15 | 0 | R | |
| Enrofloxacin 10 μg | ≥23 | ≤16 | 0 | R | |
| Sulfafurazole 300 μg | ≥17 | ≤12 | 0 | R | |
| Spectinomycin 100 μg | ≥14 | ≤10 | 20 | S | |
| Florfenicol 30 μg | ≥22 | ≤18 | 24 | S | |
| Rifampicin 5 μg | ≥20 | ≤16 | 17 | I | |
表选项
3 讨论 3.1 光唇鱼源无乳链球菌的鉴定 本研究从发病光唇鱼上分离到12株形态一致的优势菌株,其生化特征与鱼源Ⅰb血清型无乳链球菌LMG15799的匹配度较高,但在精氨酸水解、核糖和糖原发酵等3项生化特性仍存在差异。参考国内已有的文献报道,光唇鱼分离菌株与张德锋等[20]报道的罗非鱼源新型无乳链球菌株WT1451及刘礼辉等[18]报道的鲮鱼源无乳链球菌株GYCm081的生化反应结果一致;与赵长臣等[19]报道的梭鲈源无乳链球菌SL1701株,除VP试验外,其余生理生化结果均相同。16S rRNA基因序列的同源性比对结果表明,代表菌株ACRO-0708与GenBank上登录无乳链球菌(GenBank ID:FO393392.1,KT001225.1,KP294526.1)的同源性为100%。同时ACRO-0708感染健康光唇鱼复制出了自然发病症状,且再次分离的菌株与攻毒菌株相同,证实该菌株即为本次光唇鱼暴发病的病原。因此,本研究基于分离菌株的形态及生理生化特性、16S rRNA基因序列同源性和人工感染试验结果,鉴定该光唇鱼暴发病的病原为无乳链球菌(Streptococcus agalactiae)。这是国内外首次发现无乳链球菌感染养殖光唇鱼的报道。
3.2 光唇鱼源无乳链球菌的毒力相关基因 本研究检测的12个毒力基因分别参与无乳链球菌的黏附(bibA、lmb、scpB、fbsA和fbsB)、侵袭(iagA、lmb和scpB)、组织损伤(cfb、cylE和hylB)和免疫逃避(bac和bca)等致病机制[33-34]。菌株ACRO-0708的毒力基因型为sip+bibA+cfb+hylB+iagA+fbsA+fbsB+bac–bca–cylE–scpB–lmb–,该毒力基因型与张德锋等[20]报道的罗非鱼源新型无乳链球菌WT1451完全相同。此外,张德锋等[35]检测了7种不同水生动物源无乳链球菌的毒力基因,除虎纹蛙源菌株携带scpB和lmb基因外,其他鱼源(罗非鱼、鳙、宝石鲈、红尾皇冠鱼、鲮鱼及卵形鲳鲹)菌株均缺失scpB和lmb基因。Rosinski- Chupin等[36]的研究发现,Ⅰb-ST261型的无乳链球菌在基因组水平上缺失两个可移动遗传元件:(1)编码表面抗原蛋白Alpha样蛋白或Rib蛋白的顺式可移动元件;(2) 编码C5a多肽酶(ScpB)和层黏连蛋白结合蛋白(Lmb)的复合转座子。因此,ACRO-0708菌株很可能在基因组水平上就缺失了lmb、scpB、bac和bca基因。Delannoy等[37]的研究亦表明,Ⅰb血清型的鱼源无乳链球菌均不携带bac和bca基因。ACRO-0708还缺失编码β-溶血素/溶细胞素的cylE基因,Rosinski-Chupin等[36]的研究还发现,ST260-261型无乳链球菌的cyl位点已经失活,这与菌株不具有溶血活性的表型相一致,但从攻毒试验的结果来看,该基因的缺失并未影响菌株的致病性。值得注意的是,ST260-261型无乳链球菌虽然cfb基因呈阳性,其CAMP反应却为阴性[38],这是由于ST261型中编码CAMP因子的基因已经被破坏,而ST260型则可能是基因表达水平过低所致[36]。
3.3 鱼源无乳链球菌的MLST分型分析 无乳链球菌是危害鱼类健康的重要病原之一,可感染多种海淡水鱼类,特别是罗非鱼,每年由无乳链球菌感染造成的损失达数亿元。目前,已报道的鱼源无乳链球菌有3种血清型:Ⅰa、Ⅰb和Ⅲ型[13-14, 16-17, 28-29, 35, 37-39];而MLST分型相对丰富,包括ST-7、ST-260、ST-261、ST-257、ST-258、ST-259、ST-103、ST-552、ST-553、ST-19、ST-891、ST-6、ST-500、ST-283、ST-491等[13-14, 20, 28, 35-38, 40-41]。根据相同等位基因的数量,这些STs可被聚类为不同的克隆群(clonal complexes,CCs)。ST7、ST6和ST500同属CC7克隆群,为β溶血,血清型Ⅰa、Ⅰb,主要分布于亚洲地区,美国和巴西等国家也有流行记录。Ⅰa-ST7是东南亚地区鱼源无乳链球菌的主要流行株,也是CC7克隆群的优势序列型,它的宿主广泛,目前在人、鱼、蛙、海豚及兔等动物上均有发现,被认为是人兽共患致病性无乳链球菌[37-38]。ST283和ST491同属CC283克隆群,ST491是ST283序列型单一位点突变而来,两者仅在tkt位点存在差异。CC283为β溶血,血清型为Ⅲ型,主要流行于东南亚地区。由于Ⅲ-ST283及其单位点突变型(single locus variant,SLV) ST-11也是人源致病性无乳链球菌的分型,CC283克隆群在人和鱼之间存在交叉感染的可能性[37, 42]。CC552是鱼类及两栖动物感染相关的无乳链球菌克隆群,包括ST-552、ST-553、ST-257、ST-259和ST-260,这些STs具有6个相同的等位基因。ST261、ST-891及ST258与CC552克隆群有4–5个相同的等位基因,也可认为是CC552的成员。CC552的宿主均为变温动物,无表面蛋白基因,不携带可移动遗传元件(mobile genetic elements,MGEs),与人类中已发现的无乳链球菌CCs亲缘关系较远。除ST-891为β溶血的Ⅰa血清型,ST-257和ST-259中部分菌株为Ⅰa血清型外,CC552成员均为不溶血的Ⅰb血清型。其中,ST260和ST261是该群的优势序列型,ST260是美洲地区的主要流行菌株,ST261主要分布在亚洲,澳大利亚、比利时和洪都拉斯等也有报道。ST-19仅见于四川省齐口裂腹鱼感染,β溶血,血清型为Ⅲ型。ST-19是孕妇中常见的携带菌株,侵袭能力较弱,通常不致病[43]。ST-103为β溶血,Ⅰa血清型,其感染宿主包括人、牛、鱼、猫、豚鼠等,中国、法国、巴西等地均有分布,该型无乳链球菌也被认为存在人兽共患传染的风险[28]。总体而言,鱼源无乳链球菌的遗传多样性明显低于人、牛等恒温哺乳动物源。
Ⅰa-ST7型和Ⅰb-ST261/ST260型是世界范围内水产动物的流行株,在我国Ⅰa-ST7型是主要的流行株,Ⅰb-ST261在罗非鱼、鲮鱼、梭鲈上有报道,流行范围较小,而Ⅰb-ST260未见报道。本研究中光唇鱼源无乳链球菌株ACRO-0708为无溶血活性的Ⅰb-ST261型,研究表明该型无乳链球菌与水生动物源Ⅰa-ST7型及人源无乳链球菌的亲缘关系均较远,为独立的一个分支,其基因组容量比常见的ST7型缩减了10%–25%[36]。Ⅰb-ST261/ST260型无乳链球菌在适应鱼类及其他变温动物的过程中,伴随大量非必需功能基因的失活、缺失及代谢和调控网络的深度改变,并最终过渡到专性病原体的生活方式,与此同时,我们可以观察到其相应的表型变化,包括碳源利用能力的降低、热敏感性、缺乏溶血活性和CAMP因子等[36]。这也解释了国内已报道的Ⅰb-ST261型无乳链球菌均无溶血活性且发病水温都不高的原因,梭鲈的发病水温为25 ℃,鲮鱼的发病水温为25–28 ℃,本研究光唇鱼的发病水温26–28 ℃,而危害罗非鱼的主要流行株Ⅰa-ST7型通常在30 ℃以上才大面积感染发病[44]。虽然Ⅰb-ST261/ST260在世界范围内具有广泛的流行性并具有较强的致病性,但迄今为止未发现其感染人类的报道[37],这可能与其寄主专化的进化方式相关。在表面蛋白抗原基因型的检测中,本分离株ACRO-0708未检测到已知的基因型,同样,Delannoy等[37]检测了34株不同水生动物来源无乳链球菌的表面蛋白抗原基因,发现所有非溶血性的Ⅰb-ST261/ST260分离株均不携带任何表面蛋白基因,推测可能与该型菌株基因组中大量基因的失活或缺失有关。
综上可知,本次光唇鱼暴发病的病原为无乳链球菌,与我国水产动物主要流行株的β溶血- Ⅰa-ST7型不同,该分离菌株ACRO-0708为非溶血-Ⅰb-ST261型,这是一种鱼类及变温动物专化的病原菌,毒性比Ⅰa-ST7型更强[35]。虽然该型菌株同人源无乳链球菌的亲缘关系较远,且未见感染人类的报道,但是鉴于其在世界范围内水产动物中的广泛流行,该型无乳链球菌的流行病学应引起注意。
References
| [1] | 伍献文. 中国鲤科鱼类志(下卷). 上海: 上海科学技术出版社, 1977: 296-298. |
| [2] | Ji DW, Li MY, Shi YH, Zhou JB, Ren P, Zhang YM. The nutritional composition and evaluation in muscle of Acrossocheilus fasciatus. Acta Nutrimenta Sinica, 2009, 31(3): 298-300, 303. (in Chinese) 冀德伟, 李明云, 史雨红, 周健博, 任凭, 张玉明. 光唇鱼的肌肉营养组成与评价. 营养学报, 2009, 31(3): 298-300, 303. |
| [3] | Wu YA, Pan TR. Experiments on domestication and artificial propagation of Acrossocheilus fasciatus. Journal of Aquaculture, 2009, 30(11): 10-11. (in Chinese) 巫一安, 潘坛仁. 光唇鱼的驯养与人工繁殖试验. 水产养殖, 2009, 30(11): 10-11. DOI:10.3969/j.issn.1004-2091.2009.11.005 |
| [4] | Ye X, Li J, Lu MX, Deng GC, Jiang XY, Tian YY, Quan YC, Jian Q. Identification and molecular typing of Streptococcus agalactiae isolated from pond-cultured tilapia in China. Fisheries Science, 2011, 77(4): 623-632. DOI:10.1007/s12562-011-0365-4 |
| [5] | Tan JJ, Chen CF, Gao Y, Liu ZX, Wang MZ, Li GL. Identification, pathogenicity and drug sensitivity of Streptococcus agalactiae from hybrid tilapia, Oreochromis niloticus×Oreochromis aureus. Journal of Huazhong Agricultural University, 2010, 29(6): 745-751. (in Chinese) 谭晶晶, 陈昌福, 高宇, 刘振兴, 王美珍, 李革雷. 奥尼罗非鱼无乳链球菌的鉴定、致病性及药物敏感性研究. 华中农业大学学报, 2010, 29(6): 745-751. |
| [6] | Eldar A, Bejerano Y, Bercovier H. Streptococcus shiloi and Streptococcus difficile: Two new streptococcal species causing a meningoencephalitis in fish. Current Microbiology, 1994, 28(3): 139-143. DOI:10.1007/BF01571054 |
| [7] | Vandamme P, Devriese LA, Pot B, Kersters K, Melin P. Streptococcus difficile is a nonhemolytic group B, type Ib Streptococcus. International Journal of Systematic Bacteriology, 1997, 47(1): 81-85. DOI:10.1099/00207713-47-1-81 |
| [8] | Yao XL, Xu XL, Li HM, Zhong WH, Zang L, Li H, Yang CJ. Biological characteristics of pathogenic Streptococcus agalactiae isolated from Aequidens rivulatus. Progress in Fishery Sciences, 2015, 36(2): 106-112. (in Chinese) 姚学良, 徐晓丽, 李贺密, 钟文慧, 臧莉, 李灏, 杨超敬. 红尾皇冠鱼(Aequidens rivulatus)病原无乳链球菌的分离、鉴定与特性分析. 渔业科学进展, 2015, 36(2): 106-112. |
| [9] | Liu L, Li YW, He RZ, Xiao XX, Zhang X, Su YL, Wang J, Li AX. Outbreak of Streptococcus agalactiae infection in barcoo grunter, Scortum barcoo (McCulloch & Waite), in an intensive fish farm in China. Journal of Fish Diseases, 2014, 37(12): 1067-1072. DOI:10.1111/jfd.12187 |
| [10] | Zhang YW, Geng Y, Yu ZH, Wang KY, Li YJ, Mou WH, Zeng YR, Ye MT. Identification of the pathogen Streptococcus agalactiae in Schizopygopsis pylzovi (Kessler) by multilocus sequence typing. Journal of Fishery Sciences of China, 2016, 23(5): 1217-1224. (in Chinese) 张雨薇, 耿毅, 余泽辉, 汪开毓, 李亚军, 牟维豪, 曾毅仁, 叶梦婷. 黄河裸裂尻鱼无乳链球菌的分离鉴定与多位点序列分型. 中国水产科学, 2016, 23(5): 1217-1224. |
| [11] | Geng Y, Wang KY, Huang XL, Chen DF, Li CW, Ren SY, Liao YT, Zhou ZY, Liu QF, Du ZJ, Lai WM. Streptococcus agalactiae, an emerging pathogen for cultured ya-fish, Schizothorax prenanti, in China. Transboundary and Emerging Diseases, 2012, 59(4): 369-375. DOI:10.1111/j.1865-1682.2011.01280.x |
| [12] | Hang XY, Zhou DR, Ye XP, Yu XP, Luo YZ, Shi WD. Isolation and identification on the pathogen of bullfrog streptoccus disease. Acta Hydrobiologica Sinica, 2012, 36(2): 361-364. (in Chinese) 杭小英, 周冬仁, 叶雪平, 余旭平, 罗毅志, 施伟达. 牛蛙无乳链球菌病病原的分离鉴定. 水生生物学报, 2012, 36(2): 361-364. |
| [13] | Zhang DF, Yuan W, Ke XL, Liu ZG, Cao JM, Lu MX, Wang M, Yi MM. Molecular characteristics and transmission of Streptococcus agalactiae in a major tilapia culturing area of China. Journal of Fishery Sciences of China, 2017, 24(3): 606-614. (in Chinese) 张德锋, 袁伟, 可小丽, 刘志刚, 曹建萌, 卢迈新, 王淼, 衣萌萌. 中国罗非鱼主养区无乳链球菌的分子流行特征及其传播方式. 中国水产科学, 2017, 24(3): 606-614. |
| [14] | Zhang YW, Geng Y, Yu ZH, Wang KY, Chen DF, Xiang ZG, Li YJ, Guo KN, Mou WH. Serotypes and molecular typing of Streptococcus agalactiae isolated from fish. Acta Hydrobiologica Sinica, 2017, 41(4): 800-806. (in Chinese) 张雨薇, 耿毅, 余泽辉, 汪开毓, 陈德芳, 向正刚, 李亚军, 郭坤宁, 牟维豪. 鱼源无乳链球菌的血清型及分子分型研究. 水生生物学报, 2017, 41(4): 800-806. |
| [15] | Radtke A, Bruheim T, Afset JE, Bergh K. Multiple-locus variant-repeat assay (MLVA) is a useful tool for molecular epidemiologic analysis of Streptococcus agalactiae strains causing bovine mastitis. Veterinary Microbiology, 2012, 157(3/4): 398-404. |
| [16] | Guo YJ, Zhang DF, Fan HP, Chen XN, Li TT, Li AH. Molecular epidemiology of Streptococcus agalactiae isolated from tilapia in Southern China. Journal of Fisheries of China, 2012, 36(3): 399-406. (in Chinese) 郭玉娟, 张德锋, 樊海平, 陈学年, 李彤彤, 李爱华. 中国南方地区罗非鱼无乳链球菌的分子流行病学研究. 水产学报, 2012, 36(3): 399-406. |
| [17] | Li LP, Wang R, Huang T, Liang WW, Lei AY, Li J, Huang WY, Tang JY, Shi JG, Gan X, Chen M. Serotype of Streptococcus agalactiae isolated from Nile tilapia Oreochromis niloticus in China from 2007 to 2012. Journal of Dalian Ocean University, 2014, 29(5): 469-475. (in Chinese) 李莉萍, 王瑞, 黄婷, 梁万文, 雷爱莹, 李健, 黄维义, 唐佳有, 施金谷, 甘西, 陈明. 2007-2012年中国罗非鱼无乳链球菌流行菌株血清型分析. 大连海洋大学学报, 2014, 29(5): 469-475. DOI:10.3969/J.ISSN.2095-1388.2014.05.009 |
| [18] | Liu LH, Zhang DF, Li NQ, Shi CB, Yan X, Fu XZ, Lin Q. Identification, serotype analysis and drug sensitivity test of Streptococcus agalactiae from Cirrhinus molitorella. Journal of Southern Agriculture, 2015, 46(11): 2053-2058. (in Chinese) 刘礼辉, 张德锋, 李宁求, 石存斌, 颜曦, 付小哲, 林强. 鲮鱼源无乳链球菌的鉴定、血清型分析及药敏试验. 南方农业学报, 2015, 46(11): 2053-2058. DOI:10.3969/j:issn.2095-1191.2015.11.2053 |
| [19] | Zhao CC, Jiang XY, Liu CH, Zhang DF, Luo X, Zeng WW, Chen ZH, Huang ZB. Identification, characterizations and molecular subtyping analysis of a Streptococcus agalactiae isolated from Lucioperca lucioperca. Freshwater Fisheries, 2018, 48(3): 61-67. (in Chinese) 赵长臣, 江小燕, 刘春花, 张德峰, 罗霞, 曾伟伟, 陈总会, 黄志斌. 一株梭鲈源无乳链球菌的分离、鉴定及分子分型分析. 淡水渔业, 2018, 48(3): 61-67. DOI:10.3969/j.issn.1000-6907.2018.03.010 |
| [20] | Zhang DF, Liu LH, Ren Y, Li NQ, Lin Q, Pan HJ, Shi CB, Wu SQ. Isolation, identification, and molecular characteristics of a new genotype of Streptococcus agalactiae from cultured tilapia in China. Journal of Fishery Sciences of China, 2015, 22(5): 1044-1054. (in Chinese) 张德锋, 刘礼辉, 任燕, 李宁求, 林强, 潘厚军, 石存斌, 吴淑勤. 我国罗非鱼源新型无乳链球菌的分离、鉴定及其分子特征. 中国水产科学, 2015, 22(5): 1044-1054. |
| [21] | Weisburg WG, Barns SM, Pelletier DA, Lane DJ. 16S ribosomal DNA amplification for phylogenetic study. Journal of Bacteriology, 1991, 173(2): 697-703. DOI:10.1128/jb.173.2.697-703.1991 |
| [22] | Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. American Journal of Epidemiology, 1938, 27(3): 493-497. DOI:10.1093/oxfordjournals.aje.a118408 |
| [23] | Poyart C, Tazi A, Reglier-Poupet H, Billoet A, Tavares N, Raymond J, Trieu-Cuot P. Multiplex PCR assay for rapid and accurate capsular typing of group B streptococci. Journal of Clinical Microbiology, 2007, 45(6): 1985-1988. DOI:10.1128/JCM.00159-07 |
| [24] | Jones N, Bohnsack JF, Takahashi S, Oliver KA, Chan MS, Kunst F, Glaser P, Rusniok C, Crook DWM, Harding RM, Bisharat N, Spratt BG. Multilocus sequence typing system for group B Streptococcus. Journal of Clinical Microbiology, 2003, 41(6): 2530-2536. DOI:10.1128/JCM.41.6.2530-2536.2003 |
| [25] | Creti R, Fabretti F, Orefici G, von Hunolstein C. Multiplex PCR assay for direct identification of group B streptococcal alpha-protein-like protein genes. Journal of Clinical Microbiology, 2004, 42(3): 1326-1329. DOI:10.1128/JCM.42.3.1326-1329.2004 |
| [26] | Corrêa ABDA, Oliveira ICMD, Pinto TDCA, Mattos MCD, Benchetrit LC. Pulsed-field gel electrophoresis, virulence determinants and antimicrobial susceptibility profiles of type Ia group B streptococci isolated from humans in Brazil. Memorias Do Instituto Oswaldo Cruz, 2009, 104(4): 599-603. DOI:10.1590/S0074-02762009000400011 |
| [27] | Corrêa ABA, Américo MA, Oliveira ICM, Silva LG, de Mattos MC, Ferreira AMM, Couceiro JNSS, Fracalanzza SEL, Benchetrit LC. Virulence characteristics of genetically related isolates of group B streptococci from bovines and humans. Veterinary Microbiology, 2010, 143(2/3/4): 429-433. |
| [28] | Godoy DT, Carvalho-Castro GA, Leal CAG, Pereira UP, Leite RC, Figueiredo HCP. Genetic diversity and new genotyping scheme for fish pathogenic Streptococcus agalactiae. Letters in Applied Microbiology, 2013, 57(6): 476-483. DOI:10.1111/lam.12138 |
| [29] | Kayansamruaj P, Pirarat N, Katagiri T, Hirono I, Rodkhum C. Molecular characterization and virulence gene profiling of pathogenicStreptococcus agalactiaepopulations from tilapia (Oreochromissp.) farms in Thailand. Journal of Veterinary Diagnostic Investigation, 2014, 26(4): 488-495. DOI:10.1177/1040638714534237 |
| [30] | Zhang DF, Li AH, Guo YJ, Zhang QQ, Chen XN, Gong XN. Molecular characterization of Streptococcus agalactiae in diseased farmed tilapia in China. Aquaculture, 2013, 412/413: 64-69. DOI:10.1016/j.aquaculture.2013.07.014 |
| [31] | Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals (Approved Standard-Fourth Edition, CLSI document VET01-A4). Wayne, PA: Clinical and Laboratory Standards Institute, 2013: 12-15, 18, S4-S11. |
| [32] | Performance Standards for Antimicrobial Susceptibility Testing (Twenty-eighth Informational Supplement: M100-S28, CLSI document M100-S28). Wayne, PA: Clinical and Laboratory Standards Institute, 2018: 75-78. |
| [33] | Maisey HC, Doran KS, Nizet V. Recent advances in understanding the molecular basis of group B Streptococcus virulence-CORRIGENDUM. Expert Reviews in Molecular Medicine, 2008, 10: e31. DOI:10.1017/s1462399408000914 |
| [34] | Rajagopal L. Understanding the regulation of Group B Streptococcal virulence factors. Future Microbiology, 2009, 4(2): 201-221. DOI:10.2217/17460913.4.2.201 |
| [35] | Zhang DF, Ke XL, Liu ZG, Wang SF, Yuan W, Shi CB, Lu MX. Molecular characteristics and the pathogenicity to zebrafish of Streptococcus agalactiae strains isolated from seven aquatic animals in China. Journal of Fisheries of China, 2017, 41(11): 1788-1797. (in Chinese) 张德锋, 可小丽, 刘志刚, 王世锋, 袁伟, 石存斌, 卢迈新. 中国七种水生动物源无乳链球菌的分子特征及其对斑马鱼的致病性. 水产学报, 2017, 41(11): 1788-1797. |
| [36] | Rosinski-Chupin I, Sauvage E, Mairey B, Mangenot S, Ma L, Da Cunha V, Rusniok C, Bouchier C, Barbe V, Glaser P. Reductive evolution in Streptococcus agalactiae and the emergence of a host adapted lineage. BMC Genomics, 2013, 14(1): 1-15. DOI:10.1186/1471-2164-14-1 |
| [37] | Delannoy CM, Crumlish M, Fontaine MC, Pollock J, Foster G, Dagleish MP, Turnbull JF, Zadoks RN. Human Streptococcus agalactiae strains in aquatic mammals and fish. BMC Microbiology, 2013, 13(1): 1-9. DOI:10.1186/1471-2180-13-1 |
| [38] | Evans JJ, Bohnsack JF, Klesius PH, Whiting AA, Garcia JC, Shoemaker CA, Takahashi S. Phylogenetic relationships among Streptococcus agalactiae isolated from piscine, dolphin, bovine and human sources: a dolphin and piscine lineage associated with a fish epidemic in Kuwait is also associated with human neonatal infections in Japan. Journal of Medical Microbiology, 2008, 57(Pt 11): 1369-1376. |
| [39] | Dangwetngam M, Suanyuk N, Kong FR, Phromkunthong W. Serotype distribution and antimicrobial susceptibilities of Streptococcus agalactiae isolated from infected cultured tilapia (Oreochromis niloticus) in Thailand: Nine-year perspective. Journal of Medical Microbiology, 2016, 65(3): 247-254. DOI:10.1099/jmm.0.000213 |
| [40] | Elliott JA, Facklam RR, Richter CB. Whole-cell protein patterns of nonhemolytic group B, type Ib, streptococci isolated from humans, mice, cattle, frogs, and fish. Journal of Clinical Microbiology, 1990, 28(3): 628-630. DOI:10.1128/jcm.28.3.628-630.1990 |
| [41] | Lusiastuti AM, Textor M, Seeger H, Akineden ?, Zsch?ck M. The occurrence of Streptococcus agalactiae sequence type 261 from fish disease outbreaks of tilapia Oreochromis niloticus in Indonesia. Aquaculture Research, 2014, 45(7): 1260-1263. DOI:10.1111/are.12069 |
| [42] | Ip M, Cheuk ESC, Tsui MHY, Kong F, Leung TN, Gilbert GL. Identification of a Streptococcus agalactiae serotype III subtype 4 clone in association with adult invasive disease in Hong Kong. Journal of Clinical Microbiology, 2006, 44(11): 4252-4254. DOI:10.1128/JCM.01533-06 |
| [43] | Shabayek S, Spellerberg B. Group B streptococcal colonization, molecular characteristics, and epidemiology. Frontiers in Microbiology, 2018, 9: 437. DOI:10.3389/fmicb.2018.00437 |
| [44] | Liu ZG, Ke XL, Lu MX, Zhu HP, Gao FY. Effect of temperature on the virulence of Streptococcus agalactiae from Nile tilapia (Oreochromis niloticus). Journal of Fisheries of China, 2013, 37(11): 1733-1741. (in Chinese) 刘志刚, 可小丽, 卢迈新, 朱华平, 高风英. 温度对尼罗罗非鱼无乳链球菌毒力的影响. 水产学报, 2013, 37(11): 1733-1741. |
