赵龙飞1

 , 徐亚军1, 邓振山2, 赖心河1, 周金源1, 马素珍1, 符可1, 周璞1, 朱艳芳1
, 徐亚军1, 邓振山2, 赖心河1, 周金源1, 马素珍1, 符可1, 周璞1, 朱艳芳1 1. 河南省特色微生物资源开发与应用工程研究中心, 商丘师范学院生物与食品学院, 河南 商丘 476000;
2. 延安大学生命科学学院, 陕西 延安 716000
收稿日期:2020-08-02;修回日期:2020-11-01;网络出版日期:2020-11-13
基金项目:国家自然科学基金(U1204301);河南省自然科学基金(182300410069);河南省重点研发与推广专项(科技攻关)(212102310223);国家级大学生创新创业训练计划(202010483004)
*通信作者:赵龙飞, Tel/Fax: +86-370-3057892;E-mail: hnzhaolongfei@163.com.
摘要:[目的] 为了筛选棉花枯萎病菌拮抗性菌株资源,从药用植物地黄根块中分离内生细菌,分析优良菌株的促植物生长特性和耐盐碱特性,发掘优良菌株资源,为研发棉花枯萎病生防菌剂提供参考价值。[方法] 采用平板对峙法对分离内生细菌进行棉花枯萎病菌拮抗性试验,荧光显微镜观察法研究病原菌菌丝的变化、分光光度计法测定吲哚乙酸(IAA)含量和1-氨基环丙烷-1-羧酸(ACC)脱氨酶活性、平板培养法测定溶磷特性、分隔培养法测定产生挥发性物质抑菌性、比浊法测定内生菌的耐盐碱特性、通过测定代表菌株理化特性、16S rDNA序列并分析系统发育地位、盆栽接种试验验证防病效果。[结果] 地黄内生细菌对棉花枯萎病菌具有拮抗性,其中菌株DH9、DH66、DH92拮抗作用较强。与对照组菌丝对比,处理组菌丝出现打结、弯曲和断裂现象,菌丝末端分枝明显增多,多数边缘菌丝呈珊瑚状分枝,存在明显被菌体包埋等现象。菌株DH83、DH66、DH92、DH9、DH56产生挥发性物质对棉花枯萎病菌均有抑制作用,但抑制效果不明显。产生IAA含量均大于1.32 mg/L的有7株(DH92、DH30、DH71、DH83、DH93、DH9、DH56),其中DH92产量最高,为34.696 mg/L。菌株DH92和DH30产生ACC脱氨酶活力分别为118.612μmol/(mg·h)和103.795 μmol/(mg·h)。菌株DH92无机磷溶磷能力最强,溶磷圈直径/菌落直径(D/d)为1.51;菌株DH71溶解有机磷能力最强,D/d为4.50。菌株DH9和DH56分别能够耐受在7%、3% NaCl盐浓度,在pH 8–10均能生长,具有一定耐盐碱性。结合菌株培养特征、生理生化特性和16S rDNA测序及系统发育分析,结果表明DH30最相似菌株为沙福芽孢杆菌(Bacillus safensis),DH9最相似菌株为枯草芽孢杆菌(Bacillus subtilis),DH92最相似菌株为菠萝泛菌(Pantoea ananatis)。DH92处理组防治效果达77.29%,其他防效在63%以上,可作为棉花枯萎病的生防菌株资源。[结论] 药用植物地黄根块中存在棉花枯萎病菌拮抗性菌株资源,其中菠萝泛菌DH92从地黄根块中分离未曾见报道。优良地黄内生细菌具有促进植物生长特性和一定耐盐碱性,为防治棉花枯萎病和研发生物防治菌剂提供了基础。
关键词:地黄内生细菌棉花枯萎病菌IAAACC脱氨酶溶磷活性抗逆性16S rDNA序列
Screening, identification and growth-promoting of antagonistic endophytic bacteria associated with Rehmannia glutinosa against Fusarium oxysporum f. sp. vasinfectum
Longfei Zhao1

 , Yajun Xu1, Zhenshan Deng2, Xinhe Lai1, Jinyuan Zhou1, Suzhen Ma1, Ke Fu1, Pu Zhou1, Yanfang Zhu1
, Yajun Xu1, Zhenshan Deng2, Xinhe Lai1, Jinyuan Zhou1, Suzhen Ma1, Ke Fu1, Pu Zhou1, Yanfang Zhu1 1. College of Biology and Food, Shangqiu Normal University, Engineering Research Center of Development and Application of Characteristic Microbial Resources in Henan, Shangqiu 476000, Henan Province, China;
2. College of Life Science, Yan'an University, Yan'an 716000, Shaanxi Province, China
Received: 2 August 2020; Revised: 1 November 2020; Published online: 13 November 2020
*Corresponding author: Longfei Zhao, Tel: +86-370-3057892; Fax: +86-370-3057892; E-mail: hnzhaolongfei@163.com.
Foundation item: Supported by the National Natural Science Foundation of China (U1204301), Natural Science Foundation of Henan Province of China (182300410069), Key Specialized Research and Development program of Henan Province (212102310223) and National Innovation Experiment Program for University Students (202010483004)
Abstract: [Objective] To screen antagonistic strain resources of Cotton Fusarium Wilt (Fusarium oxysporum f. sp. vasinfectum), endophytic bacteria were isolated from the root of Rehmannia glutinosa, a medical herb, and the excellent strains' characteristics of plant growth promotion and salt and alkali tolerance were analyzed, so as to explore the excellent strain resources and provide reference value for the research and development of biological control agents of F. oxysporum f. sp. vasinfectum. [Methods] The antagonistic test of F. oxysporum f. sp. vasinfectum was performed by plate confrontation method, and the changes in mycelia were studied through fluorescence microscopy. Such indexes as indoleacetic acid (IAA) production and 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase activity were determined by spectrophotometry. Phosphate solubilization was assayed by plate culture method. Bacteriostasis of volatile substances was examined by separation culture method. Salt and alkali tolerance was measured by turbidimetric method. The physiological and biochemical characteristics of the strains were determined, 16S rRNA gene were sequenced and analyzed for phylogenetic status analysis, and disease-control effects were demonstrated by greenhouse inoculation test. [Results] The endophytic bacteria of R. glutinosa exerted antagonistic effects on F. oxysporum f. sp. vasinfectum, among which the strains DH9, DH66 and DH92 exhibited obvious antagonistic effects. Compared with the control group, the hyphae in the treatment group showed knotting, bending and breaking, the end branches of hyphae increased significantly, and most of the edges were coral branches, and were obviously embedded by the bacteria. The volatile substances produced by strains DH83, DH66, DH92, DH9 and DH56 exerted inhibitory effects on F. oxysporum f. sp. vasinfectum, but such effects were not salient. IAA contents of seven strains (DH92, DH30, DH71, DH83, DH93, DH9 and DH56) were higher than 1.32mg/L.DH92 had the highest yield, which amounted to 34.696 mg/L. The activities of ACC deaminase produced by DH92 and DH30 were 118.612 μmol/(mg·h) and 103.795μmol/(mg·h), respectively. DH92 had the highest ability to dissolve inorganic phosphorus, with diameter of dissolving circle/colony (D/d) being 1.51, DH71 had the highest ability to dissolve organic phosphorus, and D/d was 4.50. The strains DH9 and DH56 could tolerate 7% and 3% NaCl concentration respectively, and could grow at pH 8–10, with certain resistance. Combined with the culture characteristics, physiological and biochemical characteristics and 16S rDNA sequencing and phylogenetic analysis, results showed that DH30 exhibited the highest similarity with Bacillus safensis, DH9 showed the highest similarity with Bacillus subtilis, and DH92 had the highest similarity with pantoa ananatis. Control effect of treatment group with DH92 was 77.29%, and those of others were above 63%. Therefore, these strains can be used as biocontrol resources against F. oxysporum f. sp. vasinfectum. [Conclusion] There are antagonistic strains of F. oxysporum f. sp. vasinfectum in the root of R. glutinosa, among them. The isolation of Pantoa ananatis DH92 from R. glutinosa root has not been reported before. The excellent endophytic bacteria of R. glutinosa have the characteristics of plant growth promotion and certain salt and alkali tolerance, which provide theoretical basis for the control of F. oxysporum f. sp. vasinfectum and the development of biological control agents.
Keywords: endophytic bacteria associated with Rehmannia glutinosa LiboschFusarium oxysporum f. sp. vasinfectumIAAACC deaminasephosphate solubilizationstress resistance16S rDNA sequence
尖孢镰刀菌萎蔫专化型(Fusarium oxysporum f. sp. vasinfectum)的一种病原真菌,是专化性较强、寄主范围较窄的病菌,引起的棉花枯萎病是生产中最严重的病害之一[1]。发病轻微棉株虽能存活,但植株生育受阻,导致过早落叶,蕾铃脱落;在幼苗期发病,严重时造成大量死苗、缺苗,导致棉花产量降低,品质变劣。目前,对于棉花枯萎病防治主要采用抗病品种种植[2]和喷施化学农药,较为理想的途径是采用生物防治法,从生态系统中筛选有针对性的微生物资源[3],利用微生物与植物之间的协同作用[4],抑制病原真菌的发生和发展,既有利于保护生态环境,又利于促进农作物的生长并提高其抗性。有研究已证实可利用内生细菌抑制棉花枯萎病的发生和蔓延[3-4],这为利用植物内生细菌资源拓展绿色环保生物防治措施提供了新思路。
植物内生菌指在一定阶段或全部阶段生活于健康植物的组织和器官内部的真菌或细菌类群[5]。植物丰富的内生菌资源是天然微生物资源库[2],具有生物固氮、促进植物生长、协助植物修复环境[5]和增强宿主植物抗逆性[6]等多种生物功能,是进行生物防治很有潜力的资源。目前,科研工作者已对许多植物内生菌进行研究,发现香蕉内生细菌促植物生长[7],人参内生菌促进人参皂基的积累[8],野生开心果内生菌具有促植物生长活性[9],杜仲内生菌抑制玉米叶枯病[10],辣椒内生菌减少辣椒种子脱落[11],油菜[2]、金银花[12]和大豆根瘤[13]等内生细菌具有抑制植物病原菌等相关功能的报道。因此,植物相关微生物研究已经成为植物科学研究的前沿和焦点[14],从中发掘优良菌株资源用于生物防治也成为当前微生物学、生态学和植物保护学等领域的研究热点。
地黄(Rehmannia glutinosa Libosch)属于玄参科(Scrpophulariaceae)地黄属(Rehmannia)多年生草本植物,其根部为传统中药材之一,具有清热凉血、补益、抗肿瘤等功能。地黄具有耐贫瘠、耐寒、耐干旱等特性,分布较为广泛,产区主要集中在河南、山西两地,其他产区包括河北、陕西、山东、安徽及甘肃等地。目前,对地黄内生菌的研究主要集中在抗细菌、抗真菌[15]、抗肿瘤活性菌株的筛选[16],产梓醇内生菌分离鉴定[17]和遗传稳定性[18],以及地黄膨大根茎内生真菌研究[19],但对地黄内生细菌拮抗植物病原菌的研究还鲜为少见。基于此,本研究拟以地黄组织块状根部为研究材料,以其分离的内生细菌为研究对象,通过对棉花枯萎病菌拮抗性内生菌的筛选,研究优良菌株促植物生长特性、抗逆性和系统发育地位等,初步探索其抑制病原菌的机理,以资生物防治研究及药用植物内生菌活性成分的研究。
1 材料和方法 1.1 实验材料和培养基
1.1.1 样品来源: 地黄样品来源于3个地点:山东菏泽市曹县田间(N34°51’,E115°20’,海拔52.1 m)、河南省商丘市虞城县田间(N34°19’,E115°43’,海拔43 m)、河南省商丘市应天公园(N34°22’,E115°40’,海拔55 m)。在植株分布较多区域,采集株高在10 cm以上地黄植株,植株间距在20 m以上,将整株挖出,抖掉根系土壤,置于无菌采样袋并标记,置入4 ℃车载冰柜。
1.1.2 植物病原菌: 棉花枯萎病菌(Fusarium oxysporum f. sp. vasinfectum),由西北农林科技大学生命科学学院提供,采用马铃薯葡萄糖琼脂培养基(PDA),以菌核形式保藏在试管斜面培养基。
1.1.3 棉花种子: “百朵大桃懒汉王”采购于河南省商丘市棉办农业技术服务部。
1.1.4 改良YM培养基制备(g/L): 甘露醇5,K2HPO4 0.25,NaCl 0.05,酵母膏1.5,色氨酸0.05,pH 7.0。
1.1.5 牛肉膏蛋白胨培养基(g/L): 牛肉膏3,蛋白胨10,NaCl 5,琼脂20,pH 7.2–7.4。
1.1.6 无氮培养基(g/L): MnSO4?7H2O 0.2,CaCO31.0,蔗糖10,K2HPO4?3H2O 0.05,NaCl 0.12,pH 7.2。
1.1.7 DF盐培养基: MnSO4?7H2O 0.2 g,K2HPO4 4.0 g,Na2HPO4 6.0 g,柠檬酸2.0 g,葡萄糖2.0 g,葡萄糖酸钠2.0 g。组分一与组分二溶液各取0.1 mL。组分一:100 mL蒸馏水,CuSO4?5H2O 78.22 mg,MoO3 10 mg,H3BO3 10 mg,ZnSO4?7H2O 124 mg,MnSO4?H2O 11.9 mg。组分二:FeSO4?7H2O 100 mg溶于已经灭过菌的蒸馏水中。组分一与组分二均置于–4 ℃的冰箱里保存使用。
1.1.8 ADF培养基: 把ACC溶于超纯水中,用细菌过滤器过滤灭菌,加入到不含有(NH4)2SO4且预先灭过菌的DF盐溶液中,此培养基的pH为7.2,ACC添加最终浓度为3.0 mmol/L。
1.1.9 比色液配方: 0.5 mol/L FeCl3 1.5 mL,H2SO4 30 mL蒸馏水50 mL。
1.1.10 蒙金娜培养基(g/L): 葡萄糖10,(NH4)2SO4 0.5,NaCl 3,KCl 0.3,FeSO4?7H2O 0.03,MnSO4?4H2O 0.03,CaCO3 5,酵母膏0.4,卵磷脂0.2,琼脂20,pH 7.0–7.2。
1.1.11 PKO培养基(g/L): 葡萄糖10,Ca3(PO4)2 5.0,(NH4)2SO4 0.5,NaCl 0.2,KCl 0.2,FeSO4 0.03,MgSO4?7H2O 0.03,MnSO4 0.03,酵母膏20,琼脂20,pH 6.8–7.0。
1.2 内生菌的分离、纯化和筛选 对地黄根块状组织表面消毒[12],内生菌分离和纯化采用平板涂布法[4],对平板上长出的单菌落进行染色、镜检,保藏于试管斜面中备用。
1.3 内生菌对棉花枯萎病菌的拮抗性初筛 采用对峙平板培养法[20]。用无菌打孔器和镊子将活化的棉花枯萎病菌饼置于PDA平板中央,30 ℃恒温培养2 d,观察棉花枯萎病菌生长。在距平板边缘2.5 cm处,接种5个筛选菌株,以不接种内生菌的病原菌菌落为对照组(CK),置于30 ℃恒温培养箱倒置培养,观察并测量培养48、96、144 h时受抑制棉花枯萎病菌落直径和对照菌落直径,计算抑菌率。抑菌率(%)=(对照菌落直径–处理菌落直径)×100%/(对照菌落直径)。
1.4 内生菌对棉花枯萎病菌拮抗性复筛 复筛方法基本如初筛方法,将初筛抑菌率较高菌株制成菌悬液[1],用移液器在培养皿病原菌落周围接种同一内生菌株3次。培养48 h、96 h、144 h时观察和拍照和记录数据。将其与对照组病菌对比,计算出3株内生菌的平均抑菌率[20]。抑菌率(%)=(对照菌落直径–处理菌落直径)/(对照菌落直径)×100%。
1.5 分泌生长素IAA菌株筛选和含量测定 采用分光光度计法[21]。将制备改良YM培养基注入试管进行灭菌。在无菌条件下把每一个试管中接上待测菌株。置于28 ℃摇床在135 r/min条件下培养96 h。离心保留上清液,与比色液混合,在室温下培养30 min,有粉红色出现,说明有IAA产生。在波长为530 nm处测量OD值,代入IAA标准曲线(IAA标准曲线的制作:配置浓度梯度为5、20、40、60、80、100、120 mg/L的溶液,用分光光度计测量OD值,制作标准曲线),计算内生菌产IAA的含量。
1.6 具ACC脱氨酶活性内生菌的筛选及酶活力测定 采用分光光度计法[13]。具体步骤:将所筛选的内生菌接入5 mL的无氮培养基中,在30 ℃下振荡培养24 h;吸取培养菌液2 mL接种到60 mL的无氮培养基中,30 ℃、130 r/min培养24-48 h,12000 r/min离心10 min收集菌体;用不含(NH4)2SO4的DF液体培养基洗涤2次;将菌体悬于ADF培养基中30 ℃振荡培养24 h,每管加入1 mL ADF培养基,然后收集菌体并称重;用pH 7.6的0.1 mol/L Tris-HCl缓冲液,在12000 r/min条件下离心5 min,洗涤2次(–20 ℃下储存);将菌体重悬于1 mL pH 8.5的0.1 mol/L Tris-HCl缓冲液中,加入30 μL甲苯迅速振荡30 s以破碎细胞,取200 μL粗酶液用于测定α-丁酮酸的含量和酶活力,其余粗酶液用–4 ℃储存用于测定蛋白浓度。以吸光度值(OD540)为纵坐标,以α-丁酮酸的浓度(mmol/L)为横坐标绘制标准曲线。以反应体系中每毫克菌体蛋白酶每小时催化ACC脱氨生成α-丁酮酸的量表示ACC脱氨酶活力,其酶活力单位为α-丁酮酸μmol/(mg·h),酶活性测定设置空白对照。测定结果为3次重复值。
1.7 溶磷性内生菌的筛选 溶解有机磷能力测定[22]采用蒙金娜培养基。用75%酒精加热溶解,单独灭菌,温度降至70 ℃后与培养基混合用注射器从新鸡蛋中抽取蛋黄,按照等量加入生理盐水,然后加入灭菌后保温在50 ℃的培养基中,用力摇匀后置于培养皿中,冷凝备用;把所筛选的地黄内生菌接种该培养基中,每个皿中4个重复,28 ℃培养,观察有无透明圈的产生以及透明圈直径大小,使用溶磷圈直径/菌落直径(D/d)表示溶磷能力大小。
溶解无机磷能力测定[23],采用PKO培养基,其中Ca3(PO4)2过筛并单独灭菌后与培养基混合,冷凝使用;并且将所筛选的地黄内生菌接在PKO培养基中,每个培养皿中做4个重复,28 ℃培养,观察有无透明圈的产生以及透明圈直径大小,使用溶磷圈直径/菌落直径(D/d)表示溶磷能力大小。
1.8 内生菌产生挥发性物质对病原真菌抑制性检测 采用分隔培养法[24]。将配置好灭菌PDA培养基和牛肉膏培养基分别倒入无菌分隔培养皿隔板两侧皿中,待培养基凝固后,进行放置菌饼与涂布操作。将接种菌株的培养皿放入培养箱中培养,在48、96、144 h时分别进行记录、拍照。
1.9 内生菌抑制棉花枯萎病菌菌丝的显微观察 用复筛内生菌抑制棉花枯萎病菌,在PDA培养基上培养观察,待其生长到一定时间,将其放置在荧光显微镜与倒置显微镜下进行观察,记录地黄内生菌对棉花枯萎病菌的抑制情况[20]。
1.10 筛选内生菌抗盐碱性测试 采用比浊法测定[13]内生菌在盐碱环境下生长规律。配置YM培养基,将配置好的培养基分装2个锥形瓶,每瓶125 mL培养基,进行灭菌处理,将菌接入两个锥形瓶中,放入摇床在30℃、135 r/min条件下培养48 h。将菌悬液倒入50 mL离心管4 ℃离心(10000 r/min)10 min,弃上清,用蒸馏水冲洗3–5次,加入适量无菌水摇匀,倒入锥形瓶中用于制备菌悬液。从锥形瓶中取1 mL菌液到比色皿中,加入2 mL无菌水,在波长600 nm处测OD值,调整菌悬液OD≈1.0,置于冰箱备用。
配置4瓶YM培养基,每瓶200 mL共800 mL。用pH仪(定位6.86,斜率4.0)将YM培养基的pH调到7、8、9、10、11 5个梯度,共2000 mL,每个梯度两瓶,每瓶200 mL,分成两组;配置盐浓度为0%、3%、5%、7%、9%,共2000 mL,每个梯度两瓶,每瓶200 mL,分成两组。将配好的4800 mL培养基分装后进行灭菌,每瓶接种1 mL菌悬液。把接种的培养基放入摇床中,每隔2 h测一次菌株生长OD值,未接种菌株的培养基为对照,一天测6次,连测3 d,并记录数据和现象。测得OD600值用于制作生长曲线[4],分析筛选内生菌抗盐碱情况。
1.11 筛选菌株培养特征及生理生化测试 对筛选菌株进行菌落培养特征观察和生理生化特性测试[20]。
1.12 内生菌16S rDNA序列测定及系统发育分析 提取内生菌DNA,PCR扩增获得扩增产物进行16S rDNA序列测定,方法见文献[13, 25],将获得序列提交到GenBank,使用ClustalX 1.81软件进行序列比对,在DNAstar和Bioedit 4.8.4上进行手工纠正,采用TRENCONW软件以Neighbor-Joining方法构建系统发育树,用Bootstrap重复1000次进行可信度评估,使用DNAMAN6.0软件进行相似性分析。
1.13 接种菌株对棉花枯萎病的防治效果 试验设置处理组(接种内生细菌+病原真菌)、空白对照组CK0(不接菌)和阴性对照组CK1(只接种病原真菌)。每个处理6盆,设置3次重复。选择籽粒饱满、无破损、无霉变的棉花种子,表面消毒后按每盆5粒播种于灭菌蛭石中,置于人工气候箱中保持一定温湿度。待棉花苗长至2–3叶时(约7–8 cm),活化内生细菌并制成菌悬液(浓度6×108 CFU/mL),每株1 mL浇灌幼苗根部。每株菌重复3次,每重复处理5株幼苗,置于22–32 ℃保湿培养。接种内生细菌2 d后,采用切根灌注接种法[20]接种处理组,病原菌悬液孢子浓度为1×106个/mL,置于24–30 ℃保湿培养。第一次接种3周后每7 d统计植株的发病率、发病严重程度和病情指数。严重程度分级标准:0级,无病症,不致病,叶色油绿;1级,1–2片子叶边缘发病,病斑微泛黄;2级,子叶及一片真叶局部发黄;3级,两片真叶发病或脱落仅剩心叶,叶色泛黄色枯萎症状明显;4级,植株生长点或全株枯萎,出现落叶。发病率(%)=∑病株数/调查总株数×100;病情指数=∑(该级植株数×病害等级)/(调查植株数×最严重等级)×100;防治效果(%)=[(对照病情指数-处理病情指数)/对照病情指数]×100。
1.14 数据统计与分析 用SPSS 21软件对复筛结果、分光光度计测得的OD值、地黄内生菌产IAA含量、ACC脱氨酶活性进行统计分析。采用单因素分析和显著差异法分析,显著性水平设为α=0.05。
2 结果和分析 2.1 内生菌对棉花枯萎病菌抑制效果初筛 将采集的地黄植株样品(根块)进行涂布分离,共获得94株内生细菌。其中,分离自山东曹县28株,河南虞城县30株、河南省商丘市睢阳区应天公园36株。对这94株内生细菌采用平板对峙培养法进行初筛,初筛得到10株对棉花枯萎病菌有明显抑制现象的内生菌,分别为DH89、DH30、DH83、DH93、DH71、DH84、DH92、DH56、DH9、DH66。在培养96 h后发现其抑菌率均在20%以上,其中菌株DH9抑制效果最为明显。
2.2 内生菌对棉花枯萎病菌抑制效果复筛 把这10株筛选的内生细菌采用接种菌悬液和培养皿对峙培养法进行复筛实验,内生菌生长48 h,抑菌率在10%以上有8株,分别为DH30、DH83、DH93、DH84、DH92、DH56、DH9、DH66,其中DH9抑制效果最为明显,DH92次之;在内生菌生长96 h,抑菌率在25%以上有8株,分别是DH89、DH30、DH83、DH71、DH92、DH56、DH9、DH66,其中DH9抑制效果最为明显,DH66次之;在内生菌生长144 h,抑菌率在30%以上有4株,分别是DH83、DH92、DH9、DH66,其中DH9抑制效果最为明显。
在复筛过程中,对照组(图 1-A)棉花枯萎病菌在不受地黄内生菌影响下正常生长,随着时间延长,病原菌菌丝遍布整个培养平皿。而处理组菌株DH83(图 1-B)、DH92(图 1-C)、DH9(图 1-D)、DH66(图 1-E)、DH56(图 1-F)对于棉花枯萎病菌的抑制情况可知,棉花枯萎病菌生长在接触内生菌时菌丝生长受到阻碍,其中菌株DH83(图 1-B)、DH9 (图 1-C)随着生长时间延长,菌丝生长愈受内生菌的抑制,可能是菌株在生长过程中产生代谢物质对棉花枯萎病菌有抑制作用。菌株DH56 (图 1-F)随时间延长,内生菌生长并无明显变化,但其抑制情况却十分明显,这可能与内生菌本身生理特性有关。
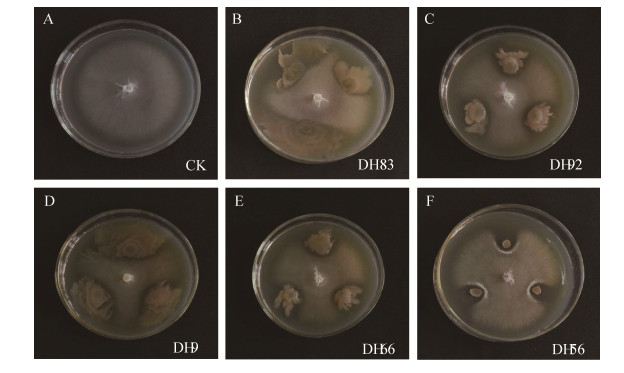 |
| 图 1 菌株DH83、DH92、DH9、DH66、DH56对棉花枯萎病菌的抑制作用 Figure 1 Inhibition of endophytic strains on F. oxysporum f. sp. vasinfectum by DH83, DH92, DH9, DH66 and DH56. |
| 图选项 |
由表 1知,地黄内生细菌对棉花枯萎病菌抑菌率(96 h)均达到21.33%以上有10个菌株,其中对棉花枯萎病菌抑制效果最明显菌株为DH9,抑菌率为28.44%,DH66次之,抑菌率为27.11%。而培养144 h地黄内生菌株对棉花枯萎病菌抑菌率均达到26.89%以上有7个菌株,其中对棉花枯萎病菌抑制作用最明显菌株为DH92,抑菌率为32.22%;DH9次之,抑菌率为31.82%。由此可见,地黄内生菌株对棉花枯萎病菌的抑制作用,随培养时间延长抑菌效果发生变化。
表 1. 地黄内生细菌对棉花枯萎病菌FO101的抑制作用复筛结果 Table 1. Antagonistic effects of endophytic bacteria associated with Rehmannia glutinosa against to F. oxysporum f. sp. vasinfectum
| Strains | Colony diameter of pathogen1/cm | Inhibition rate of endophytic bacteria/% | Colony diameter of pathogen2/cm | Inhibition rate of endophytic bacteria/% | Colony diameter of pathogen3/cm | Inhibition rate of endophytic bacteria/% |
| CK | 4.50 | – | 7.5 | – | 8.8 | – |
| DH92 | 3.77±0.06 | 16.30±1.28ab | 5.50±0.00 | 26.67±0.00a | 5.97±0.21 | 32.20±2.78a |
| DH9 | 3.73±0.23 | 17.04±5.13a | 5.37±0.15 | 28.44±2.04a | 6.00±0.10 | 31.82±1.33a |
| DH66 | 3.97±0.15 | 11.85±3.39abcde | 5.47±0.15 | 27.11±2.04a | 6.03±0.06 | 31.44±0.77ab |
| DH83 | 4.00±0.10 | 11.11±2.22bcde | 5.53±0.15 | 26.22±2.04ab | 6.13±0.06 | 30.30±0.77abc |
| DH93 | 4.00±0.20 | 11.11±4.44bcde | 5.87±0.06 | 21.78±0.77bc | 6.23±0.12 | 29.17±1.54bcd |
| DH56 | 3.90±0.10 | 13.33±2.22abcd | 5.53±0.06 | 26.22±0.77ab | 6.27±0.15 | 28.79±2.04cd |
| DH71 | 4.13±0.12 | 8.15±2.57de | 5.53±0.46 | 26.22±6.12ab | 6.43±0.06 | 26.89±0.77d |
| DH89 | 4.17±0.12 | 7.41±2.57e | 5.53±0.06 | 26.22±0.78ab | – | – |
| DH30 | 4.03±0.06 | 10.37±1.28cde | 5.57±0.12 | 25.78±1.54ab | – | – |
| DH84 | 3.83±0.06 | 14.81±1.28abc | 5.90±0.10 | 21.33±1.33c | – | – |
| CK: no inoculation group; 1 means 48 hours, 2 means 96 hours, 3 means 144 hours. Data are mean±SD in table, different letters behind data in the same column show significant difference at P < 0.05 level. | ||||||
表选项
2.3 受抑制病原菌菌丝的显微分析 由图 2可知,对照组(图 2-A)棉花枯萎病菌菌丝长势健壮、挺直、粗细均一。而处理组菌丝有明显不同,菌株DH83菌丝扭曲呈束状分布(图 2-A);菌株DH92菌丝发生分支,菌丝局部细胞壁溶解、断裂(图 2-B);菌株DH9菌丝末端发生膨大,呈球状(图 2-C);菌株DH66的菌丝粗细不一、畸形(图 2-D),发生波形弯曲(图 2-F),菌丝末端膨大成球状(图 2-E),菌株DH56菌丝发生分支弯曲(图 2-G),由于地黄内生菌产生代谢物质使菌丝发生包埋现象(图 2-H)。以上结果表明:棉花枯萎病菌菌丝在生长过程中,受地黄内生菌株的作用,使病原菌菌丝发生断裂、弯曲、膨大、畸形、包埋等现象,阻碍了病原菌菌丝的正常生长。
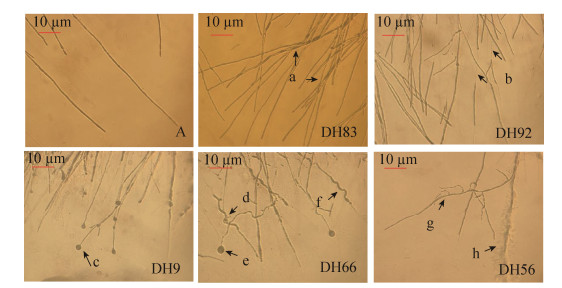 |
| 图 2 菌株DH83、DH92、DH9、DH66、DH56对棉花枯萎病菌抑制作用显微观察 Figure 2 Microscopic observation on the inhibitory effect of control, DH83, DH92, DH9, DH66 and DH56 against F. oxysporum f. sp. vasinfectum. |
| 图选项 |
2.4 产生IAA内生菌株的筛选和含量测定 结果(表 2)表明,所筛菌株多数可产生IAA,但产生IAA的量有所不同。在供试菌株培养96 h,IAA产量在1.322 mg/L以上有7个菌株,即DH92、DH56、DH30、DH71、DH83、DH93、DH9。因其产生IAA的量不同颜色深浅不一(图 3),菌株DH92产生IAA的量最高,为34.696 mg/L,呈紫色;菌株DH56产生IAA含量次之,为6.812 mg/L,呈粉红色;菌株DH9产生IAA的量最低,为1.322 mg/L,液体颜色微显红色。可见,不同菌株产生IAA的量有所不同,在反应颜色上存在差异,且差异显著(P < 0.05)。
表 2. 地黄内生菌在改良YM培养基培养96 h生长素IAA的积累 Table 2. Content accumulation of auxin IAA produced by endophytes from R. glutinosa cultured for 96 h.
| Strains | OD530 value | IAA production/(mg/L) |
| DH92 | 0.575±0.031 | 34.696±1.845a |
| DH56 | 0.108±0.010 | 6.812±0.569b |
| DH30 | 0.067±0.006 | 4.345±0.329c |
| DH71 | 0.065±0.045 | 4.246±2.693c |
| DH83 | 0.058±0.007 | 3.808±0.397c |
| DH93 | 0.057±0.003 | 3.769±0.158c |
| DH9 | 0.016±0.007 | 1.322±0.430d |
| Data are mean±SD in table, different letters behind data in the same column show significant difference at P < 0.05 level. | ||
表选项
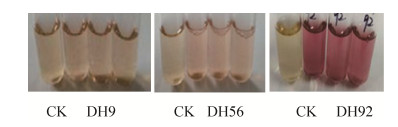 |
| 图 3 内生菌DH9、DH56和DH92产生IAA量不同在颜色上的差异 Figure 3 Color difference on the amount of IAA produced by endophytic bacteria DH9, DH56 and DH92. |
| 图选项 |
2.5 地黄内生菌产生ACC脱氨酶活性检测 ACC脱氨酶活力测定结果(表 3)表明,能够产生ACC脱氨酶的菌株有22株,其中,菌株DH92产生ACC脱氨酶活性最高,为118.612 μmol/(mg·h),菌株DH30的ACC脱氨酶活性次之,为103.795 μmol/(mg·h),菌株DH50酶活性最小,为29.304 μmol/(mg·h)。由表可知,酶活性大于50 μmol/(mg·h)有14株,酶活性在100 μmol/(mg·h)以上有2株(DH92和DH30)。由此可见,内生菌株间所产生的ACC脱氨酶活性存在差异,这与菌株本身的生物特性或分离宿主植物的生长环境有关。
表 3. 地黄内生菌产生ACC脱氨酶活性检测 Table 3. Detection on ACC deaminase activity of endophytic bacteria isolated from R. glutinosa
| Strains | ACC deaminase activity/(U/mg) |
| DH92 | 118.612±0.004A |
| DH30 | 103.795±0.006B |
| DH5 | 85.563±0.004C |
| DH48 | 78.064±0.003D |
| DH38 | 75.535±0.002E |
| DH1 | 74.787±0.001F |
| DH83 | 74.246±0.001G |
| DH4 | 69.400±0.100H |
| DH71 | 65.041±0.003I |
| DH36 | 64.466±0.002J |
| DH6 | 55.633±0.003K |
| DH3 | 54.386±0.002L |
| DH64 | 52.763±0.002M |
| DH9 | 50.568±0.002N |
| DH51 | 49.149±0.003O |
| DH93 | 48.609±0.003P |
| DH12 | 47.108±0.003Q |
| DH53 | 46.652±0.001R |
| DH56 | 43.411±0.003S |
| DH40 | 43.229±0.003T |
| DH19 | 38.574±0.002U |
| DH50 | 29.304±0.003V |
| Data are mean±SD in table, different letters behind data in the same column show significant difference at P < 0.01 level. | |
表选项
2.6 菌株产生溶磷性测定 由表 4发现,对无机磷溶磷性较明显的内生菌有3个菌株,其中DH92和DH36产生溶磷圈,溶解无机磷能力较为明显。菌株DH92溶磷能力最强,D/d为1.51;DH36次之,D/d为1.32。
表 4. 地黄内生菌DH92、DH36、DH30溶解无机磷测定结果 Table 4. Detection results of inorganic phosphorus produced by endophytic bacteria DH92, DH36 and DH30 isolated from R. glutinosa
| Strains | Diameter of dissolved phosphorus halo(D)/cm | Colony diameter(d)/cm | Ratio(D/d) |
| DH92 | 1.20±0.10 | 0.8±0.10 | 1.51±0.16a |
| DH36 | 0.83±0.06 | 0.63±0.06 | 1.32±0.03b |
| DH30 | 0.83±0.06 | 0.73±0.06 | 1.14±0.01b |
| data are mean ±SD in table, different letters behind data in the same column show significant difference at P < 0.05 level. | |||
表选项
有机磷检测结果(图 4、表 5)表明,菌株DH56、DH71和DH30溶磷圈明显,溶解有机磷能力较强。由表 5可知,菌株DH71产生溶磷圈直径最大,溶磷能力最强的菌株为DH71,D/d为4.50;DH56次之,D/d为4.05。可见,不同菌株形成溶磷圈大小不同,表明溶磷能力不同。
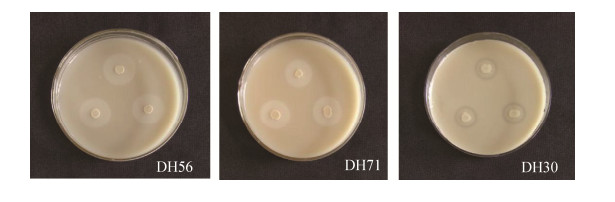 |
| 图 4 地黄内生菌溶解有机磷测试结果 Figure 4 Detection results of organophosphorus dissolution by endophytic bacteria from R. glutinosa. |
| 图选项 |
表 5. 地黄内生菌株DH71、DH56、DH30溶解有机磷测试结果 Table 5. Detection results of organophosphorus dissolution by endophytic bacteria DH71, DH56 and DH30 from R. glutinosa
| Strains | Diameter of dissolved phosphorus halo(D)/cm | Colony diameter(d)/cm | Ratio(D/d) |
| DH71 | 2.67±0.15 | 0.60±0.10 | 4.50±0.50a |
| DH56 | 2.80±0.10 | 0.73±0.06 | 4.05±0.08a |
| DH30 | 1.70±0.10 | 0.87±0.06 | 1.96±0.06b |
| Data are mean±SD in table, different letters behind data in the same column show significant difference at P < 0.05 level. | |||
表选项
2.7 内生菌产生挥发性气体对棉花枯萎病菌抑制作用的检测 对复筛中5株菌(DH83、DH92、DH9、DH66、DH56)进行挥发性气体抑制病原菌检测。结果表明,处理组棉花枯萎病菌在内生菌作用下,菌丝生长情况与对照组相比,菌丝扩展面积明显缩小,DH83和DH66在培养72 h时抑菌率为4.44 %,DH9、DH56、DH92抑菌率为2.22%,抑菌效果不明显(图 5),表明地黄内生菌产生挥发性气体对棉花枯萎病菌有抑制作用。
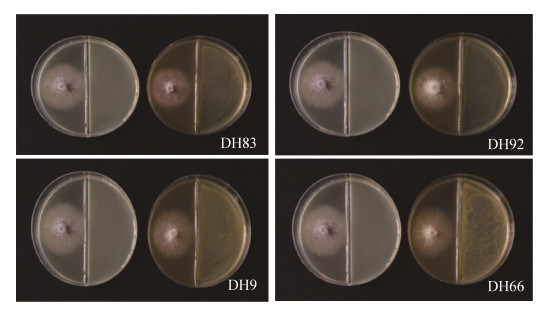 |
| 图 5 内生细菌产生挥发性气体对棉花枯萎病菌抑制作用检测 Figure 5 Inhibitory effect of volatile gas produced by endophytic bacteria on F. oxysporum f. sp. vasinfectum. |
| 图选项 |
2.8 筛选菌株的耐盐碱性 根据复筛结果结合地黄内生细菌理化特性,测定菌株DH9、DH56抗盐碱能力,绘制菌株生长曲线。
DH9抗盐生长规律曲线(图 6)表明,随着盐浓度升高,菌株生长潜伏期不断增长,在盐浓度为0时延滞期最短(14 h),盐浓度为7%时延滞期最长(36 h);在起始盐浓度为0%–5%,DH9都可正常生长;到达平稳期OD值随盐浓度升高逐渐减小。菌株在7%盐浓度下能生长,平稳期较短很快进入衰亡期,具有一定抗性。在盐浓度9%时OD值接近0,菌株不能生长。DH9菌株在3%–5% NaCl盐浓度抗性最为明显。菌株DH56抗盐生长规律曲线(图 6)表明,随着盐浓度升高,菌株生长潜伏期不断增长,在盐浓度为0时,延滞期最短(14 h),盐浓度为3%时延滞期为24 h;在起始盐浓度为0%–3%时,DH56可生长,生长速率随盐浓度升高逐渐减小;菌株在5%–9%盐浓度下不能生长。因此,DH56在3% NaCl盐浓度表现一定耐盐性。
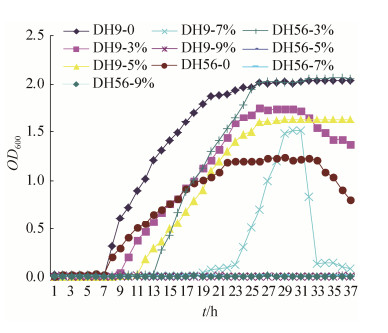 |
| 图 6 地黄内生细菌DH9和DH56在不同盐浓度下生长曲线 Figure 6 The growth curve of DH9 and DH56 from R. glutinosa under different NaCl concentration. |
| 图选项 |
菌株DH9抗碱生长规律曲线如图 7所示,在pH 7-9下菌株延滞期最短(6 h),在pH8时,菌株在58 h时提前进入衰亡期。在pH 10时菌株延滞期为30 h,在60 h时进入平稳期;在pH 7-10范围内,DH9可正常生长,进入对数期时间不同;在pH 11时OD值接近0,菌株不能生长。可见,该菌株在一定碱性环境,内生菌可调节细胞自身适应环境生长。因此,菌株DH9具有一定耐碱性,尤其在pH 8–10表现最为显著。DH56抗碱生长规律曲线(图 7)表明,在pH 7时菌株延滞期最短(4 h),在pH 9时菌株延滞期为14 h;在pH 10时菌株延滞期最长,为32 h。对数期,在起始pH 7–10范围内,DH56可正常生长,且生长速率较快;在pH 7–10条件下,菌株呈缓慢增长,随pH值增大延迟期变长,生长状态良好。可见,在一定碱性环境中,内生菌可调节自身适应生长碱性环境。因此,菌株DH56具有一定耐碱性,可耐受较宽的pH范围。
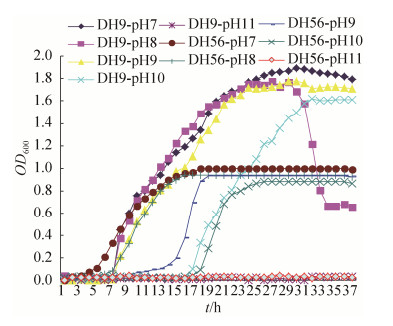 |
| 图 7 地黄内生菌DH9和DH56在不同pH下生长曲线 Figure 7 The growth curve of endophyte DH9 and DH56 from R. glutinosa under different pH. |
| 图选项 |
2.9 复筛菌株的培养特征及生理生化特性 在牛肉膏蛋白胨培养基上经过72 h培养,菌株DH9、DH30和DH92菌落表面湿润、不透明状、有光泽,菌落小而圆整,不凸起,DH9、DH92颜色为浅黄色,DH30为乳白色,DH30菌体大小为(0.5–0.7)μm×(1.0–1.2)μm,DH9菌体大小为(0.7–0.8)μm×(2.0–3.0)μm,DH92菌体大小为(7.0–8.0)μm×(18–20)μm。革兰氏染色结果表明,3株菌均为革兰氏阳性、有芽孢,其中DH9和DH30菌体呈短杆状,DH92菌体呈长杆状。理化试验结果(表 6)表明,3株菌对接触酶、D-甘露醇、木糖、肉汤、酪素分解、柠檬酸盐利用反应都呈阳性,对苯丙氨酸脱氢酶反应均为阴性;除DH30外V-P试验、D-葡萄糖产酸反应均为阳性;L-阿拉伯糖产酸反应DH9为阴性;淀粉酶水解反应DH9为阳性;卵黄磷脂酶反应DH30为阳性,硝酸还原酶、酪氨酸水解反应DH92反应阴性。结合培养特征和理化特性分析结果,查阅《常见细菌系统鉴定手册》发现:DH9和DH30符合芽孢杆菌属(Bacillus)的特征,DH92符合泛菌属(Pantoea)的特征。
表 6. 筛选菌株的理化特性 Table 6. Physiological and biochemical characteristics of screened strains
| Physiological and biochemical characteristics | DH30 | DH9 | DH92 |
| Catalase test | + | + | + |
| V-P test | – | + | + |
| D-glucose acid yield | – | + | + |
| L-Arab sugar acid yield | + | – | + |
| D-mannitol | + | + | + |
| Xylose | + | + | + |
| Broth (pH5.0) | + | + | + |
| Amylase | – | + | – |
| Phenylalanine deaminase | – | – | – |
| Lecithinase | + | – | – |
| Nitrate reductase | + | + | – |
| Casein decomposition | + | + | + |
| Citrate utilization | + | + | + |
| Tyrosine hydrolysate | + | + | – |
| Morphology | Short rod | Short rod | Long rod |
| Strain size(μm) | (0.5-0.7)×(1.0-1.2) | (0.7-0.8)×(2.0-3.0) | (7.0-8.0)×(18-20) |
| Gram stain | G+ | G+ | G+ |
| Spore forming | + | + | + |
| +: positive; –: negative. | |||
表选项
2.10 内生菌16S rDNA序列测定及系统发育分析 代表性菌株DH30、DH9和DH92,经16S rDNA序列测定,序列片段大小均为1.5 kb,序列号分别为MN365895、MN365896、MN365890。由图 8-9分析可知,这3株菌分别属于2属3种,菌株DH9、DH30与参比菌株形成分支Ⅰ芽孢杆菌属(Bacillus)(图 8),其中DH30最相似菌株为模式菌株B. safensis kp9T(MK483703),相似率为100%;DH9的最相似菌株为模式菌株B. subtilis TT207T (MH819693),相似率为99.86%。菌株DH92与参比菌株形成泛菌属(Pantoea)(图 9),最相似菌株为模式菌株P. ananatis SAD2-6T (HQ236020),相似性为99.59%。综合菌株培养特征、生理生化特性和16S rDNA序列分析结果,DH9最相似菌株为枯草芽孢杆菌(Bacillus subtilis),DH30最相似菌株为沙福芽孢杆菌(Bacillus safensis),DH92最相似菌株为菠萝泛菌(Pantoea ananatis)。
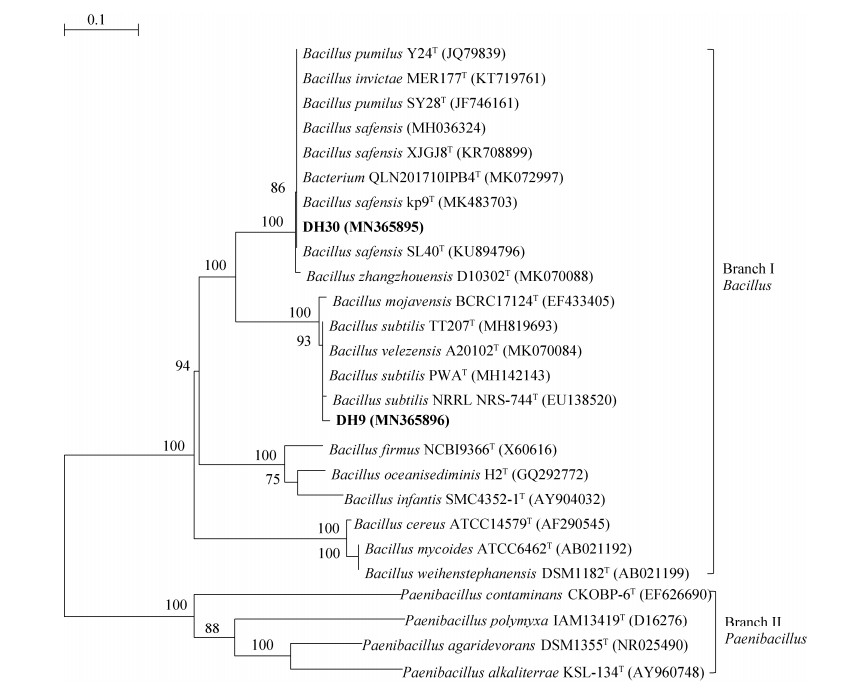 |
| 图 8 基于地黄内生菌代表菌株DH9和DH30的16S rDNA序列构建的系统发育树 Figure 8 Phylogenetic tree generated by the neighbor-joining method based on 16S rDNA sequences of selected representative strains DH9 and DH30 from R. glutinosa. Numbers in bracket represent the sequences accession number in GenBank. The number at each branch points is the percentage supported by bootstrap. Bar, 0.1 sequence divergence. |
| 图选项 |
 |
| 图 9 基于地黄内生菌代表菌株DH92的16S rDNA序列构建的系统发育树 Figure 9 Phylogenetic tree generated by the neighbor-joining method based on 16S rDNA sequences of selected representative strain DH92 from R. glutinosa. Numbers in bracket represent the sequences accession number in GenBank. The number at each branch points is the percentage supported by bootstrap. Bar, 0.02 sequence divergence. |
| 图选项 |
2.12 接种拮抗性内生细菌对棉花枯萎病的防病效果 盆栽试验结果(图 10)表明,接种8周时对照组(CK1)出现明显病状,叶色大面积发灰绿、泛黄,叶片边缘出现不规则干枯,沿叶脉向叶片内部延伸,枯萎面积扩大,严重者叶片脱落,发病率为85.36%。而内生细菌处理组,部分叶片个别部位变黄,叶片大部分仍为绿色,发病程度相对CK1较轻,而CK0组叶片微黄,无感染症状。由表 7可知,菌株DH92和DH9处理植株整体上发病率和病情指数均降低,植株发病率均小于32%,防治效果均在75%以上。另外,DH30处理植株叶片绿色,病斑面积不再扩大,生长趋于正常;DH66处理植株部分叶片局部发黄,病斑处出现枯死。整体上,拮抗性内生细菌处理组病情指数和防治效果与对照相比达极显著(P < 0.01),因此,可作为棉花枯萎病的生防菌株资源。
 |
| 图 10 接种拮抗性内生菌8周后处理组和对照组棉花幼苗生长状况 Figure 10 Growth conditions of cotton seedlings in treatment group and control group after inoculation of antagonistic endophytes for 8 weeks. |
| 图选项 |
表 7. 拮抗性内生细菌在苗期对棉花枯萎病防治效果 Table 7. The control efficiency of antagonistic endophyic bacteria against F. oxysporum f. sp. vasinfectum
| Strains | Incidence/% | Disease index | Control efficacy/% |
| CK | 85.36±0.01A | 65.32±0.01A | / |
| DH92 | 26.75±0.01B | 15.35±0.01B | 77.29±0.01A |
| DH9 | 31.52±0.01C | 17.96±0.01C | 75.52±0.01B |
| DH30 | 36.61±0.01D | 18.61±0.01D | 66.28±0.01C |
| DH66 | 38.73±0.01E | 23.53±0.01E | 63.87±0.01D |
| The data are mean±SD. Different uppercase letters in the same column show significant difference at P < 0.01 level. | |||
表选项
3 讨论 3.1 地黄内生菌对棉花枯萎病菌的抑制作用探讨 植物与微生物关系紧密,通过构建根际微生物区系,植物根部分泌的代谢物可趋使植物生长和发生防御反应[26],而细菌基因组特性决定其对宿主的适应性[27]。近年来利用植物内生菌来抑制植物病原微生物进行生物防治的研究在国内外已展开[20, 28-31],这些研究为棉花枯萎病的生物防治提供了新的思路和借鉴。本研究从地黄中分离出94株内生细菌,经初筛复筛测试,地黄内生菌DH9、DH66、DH92对棉花枯萎病菌具较强拮抗作用,抑菌率均在31%以上,其中DH92抑制率为32.20%,DH9抑制率为31.82%。盆栽防治效果最佳菌株DH92达77.29%,其余3株防治效果均在63%以上,这与徐亚军等[28]研究基本一致。系统发育分析结果表明,DH92最相近菌株为菠萝泛菌(Pantoea ananatis),有研究[32]表明,Pantoea ananatis CcSh-1存生于宿主植物香茅草体内,可产生萜烯类和柠檬醛挥发性物质,与宿主香茅草的代谢有关,具有拮抗病原真菌活性和促进植物生长的特性,有力支持了本实验结果。地黄内生细菌对病原菌的抑菌特性可能与宿主植物地黄药用部位块根的代谢有关,内生菌长期与宿主形成的协同效应,能有效抑制病原菌菌丝的生长和发育[33]。本研究从药用植物地黄根块中分离出菠萝泛菌Pantoea ananatis DH92还未见报道。
地黄内生菌接种处理棉花枯萎病菌,其菌丝出现粗细不均一、扭曲、断裂、末端膨大、菌丝溶解和被菌体包埋等现象。可能机制是内生菌与病原菌对峙过程中产生化感物质及胞外代谢产物[34],如螯合型铁载体、杀菌性挥发物、解毒酶、水解酶类和生物碱等。这些代谢产物不断释放出来,抑菌机制大多为干扰细胞壁合成、损害细胞膜机能、抑制核酸合成或干扰能量代谢等方式[35],导致病原真菌细胞壁变薄,因细胞壁受力不均出现局部菌丝畸形,出现菌丝断裂[36]。易龙等[37]研究支持了这个解释,烟草内生细菌Itb162对烟草赤星病原菌丝生长有明显抑制作用,主要是拮抗菌使病原菌丝形成膨胀泡,破裂、消解,致使菌丝停止生长。内生菌DH56作用病原真菌,使菌丝发生弯曲并被包埋,内生菌沿菌丝迅速生长,大量利用培养基养分,导致病原菌因营养缺乏而生长受阻,出现菌丝局部萎缩、弯曲现象[34]。Miller等[35]研究结果从另外一个角度解释了这个现象,内生菌可能产生抗生素类物质如寡霉素A、卡那霉素水解物、双效菌素A等对病原菌菌丝发生作用,如菌丝新生细胞壁合成受到抑制,导致菌丝出现局部萎缩等现象。徐亚军等[28]对野生艾蒿内生菌的研究结果支持了本实验结果,内生菌产生的挥发性气体[24]或者自身分泌的化学物质[34]对病原菌菌丝有明显抑制作用。本文仅对内生菌抑菌病原菌丝进行初步探索,对内生菌产生抑菌物质的具体机制还需进一步探索。
3.2 内生菌促植物生长特性和溶磷作用 植物生长素IAA在植物细胞生长发育中发挥重要作用,内生菌产生IAA可促进植株生长[21]。本实验筛选菌株大多数可产生IAA,但分泌IAA的量存在差异。本实验菌株DH92最相似菌株为菠萝泛菌Pantoea ananatis,产生IAA量最高,达34.696±1.845 mg/L。而沙棘根瘤内生细菌SR308产生植物生长激素IAA最高达23.17±0.17 mg/L[36],春兰根中内生菌9株分泌IAA产量高于50 mg/L[37],这些研究与本实验结果基本一致,表明宿主植物内定殖大量产生IAA的内生菌资源[38]。Walitang等[39]研究表明来自野生水稻的6株内生细菌可产生IAA,其中泛菌属菌株Pantoea hericii LTE3产量最高,达47.06± 1.99 mg/L,有力支持了本研究结果。如果给宿主植物接种产生IAA菌株,可引起根的形态建成,刺激根毛形成,增加侧根和主根数量及长度,增加根分泌物和扩大表面积,促进对矿物质的吸收[40]。盆栽试验结果表明,DH92防治效果最好,达77.29%。
众多研究表明[27],具有ACC脱氨酶活性的内生细菌对宿主植物生长发育起着重要作用,主要表现在提高植物抗旱涝、耐盐、耐高低温及抗重金属等特性[13],对宿主植物有促生长作用。具ACC脱氨酶活性的耐盐菌可拮抗玉米轮斑病菌(Gloeocercospora sorghi D.Bain.et Edgerton)和弯孢叶斑病菌(Curvularia lunata)[41]及病原细菌[42],可用于拮抗病原菌[43]。本研究筛选棉花枯萎病菌拮抗性内生细菌中,DH92具ACC脱氨酶最高活性,为118.612 μmol/(mg·h),其次DH30酶活力达到103.795 μmol/(mg·h),其余菌株酶活力均为100 μmol/(mg·h)以下。盆栽结果表明,ACC脱氨酶高产菌株DH92对植株病原菌防治效果最好。而本课题组前期从大豆根瘤中分离的具ACC脱氨酶活性菌株DD132,其酶活力最高为15.71 μmol/(mg·h),DD248最低为0.93 μmol/(mg·h)[13]。可见,地黄内生菌产生ACC脱氨酶活力比大豆根瘤内生菌高出6.6倍,明显高于国外报道的5–10 μmol/(mg·h)[44],远远高于分离自碱蓬的内生细菌LP11产生的最高酶活力(0.576 μmol/mg·h)[45],与贺字典[43]等研究结果基本一致,而嗜麦芽寡养单胞菌CRG-2产生的ACC脱氨酶活性为324.3 μmol/(mg·h)。可见,不同宿主植物内生菌产生ACC脱氨酶活力大小不同,而同一种宿主内生菌也存在差异。
溶磷微生物是土壤磷循环中重要成员,能将无效磷转化为有效磷,促进植物对磷的吸收。溶磷菌的活动不仅能为植物提供足够有效磷,还能分泌生长素等植物生长调节剂促进植物的生长,提高植物抗逆性[46]。菌株DH92在无机磷培养基上产生溶磷圈直径最大(1.2 cm),D/d为1.51;菌株DH30溶磷圈直径为0.83 cm,D/d为1.14。这与朱颖等[47]从红三叶根际分离的溶磷菌株溶解无机磷能力基本一致,D/d值为1.13–1.62。而乔志伟等[22]从石灰性土壤中分离30株溶解无机磷细菌,D/d值为1.25–5.1,支持了本实验结果。有机磷的矿化溶解主要依靠酶解作用,在有机磷培养基上产生溶磷圈,溶磷圈直径为2.67±0.15 cm,菌株DH71溶磷效果最好,D/d为4.50±0.50;其次为DH30,溶磷圈直径为1.70±0.10 cm,D/d为1.96±0.08。祁娟等[48]表明从紫花苜蓿种子中分离的内生菌对有机磷溶解,溶磷性最强菌株SL01,D/d为2.567;朱颖等[47]从红三叶草根际分离溶解有机磷菌株,D/d为1.07–1.83。可见,这些菌株对有机磷溶解性不如菌株DH71效果好,且菌株DH30对无机磷和有机磷都有溶解能力,这可能与宿主植物种类有关。可见,溶磷菌对有机化肥和土壤中有机磷农药残留具有降解潜能。但溶磷菌的溶磷过程十分复杂,其潜在机制也因菌种不同而各不相同,从筛选菌株中分离并克隆溶磷相关基因,了解其表达调控机制正在研究中。
References
| [1] | Shapulatov UM, Buriev ZT, Ulloa M, Saha S, Devor EJ, Ayubov MS, Norov TM, Shermatov SE, Abdukarimov A, Jenkins JN, Abdurakhmonov IY. Characterization of small RNAs and their targets from Fusarium oxysporum infected and noninfected cotton root tissues. Plant Molecular Biology Reporter, 2016, 34(3): 698-706. DOI:10.1007/s11105-015-0945-z |
| [2] | Cox KL, Meng FH, Wilkins KE, Li FJ, Wang P, Booher NJ, Carpenter SCD, Chen LQ, Zheng H, Gao XQ, Zheng Y, Fei ZJ, Yu JZ, Isakeit T, Wheeler T, Frommer WB, He P, Bogdanove AJ, Shan LB. TAL effector driven induction of a SWEET gene confers susceptibility to bacterial blight of cotton. Nature Communications, 2017, 8: 15588. DOI:10.1038/ncomms15588 |
| [3] | Pereg L, McMillan M. Scoping the potential uses of beneficial microorganisms for increasing productivity in cotton cropping systems. Soil Biology and Biochemistry, 2015, 80: 349-358. DOI:10.1016/j.soilbio.2014.10.020 |
| [4] | Delgado-Baquerizo M, Maestre FT, Reich PB, Jeffries TC, Gaitan JJ, Encinar D, Berdugo M, Campbell CD, Singh BK. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nature Communications, 2016, 7: 10541. DOI:10.1038/ncomms10541 |
| [5] | Santoyo G, Moreno-Hagelsieb G, del Carmen Orozco-Mosqueda M, Glick BR. Plant growth-promoting bacterial endophytes. Microbiological Research, 2016, 183: 92-99. DOI:10.1016/j.micres.2015.11.008 |
| [6] | Etesami H. Bacterial mediated alleviation of heavy metal stress and decreased accumulation of metals in plant tissues: mechanisms and future prospects. Ecotoxicology and Environmental Safety, 2018, 147: 175-191. DOI:10.1016/j.ecoenv.2017.08.032 |
| [7] | Andrade LF, de Souza GLOD, Nietsche S, Xavier AA, Costa MR, Cardoso AMS, Pereira MCT, Pereira DFGS. Analysis of the abilities of endophytic bacteria associated with banana tree roots to promote plant growth. Journal of Microbiology, 2014, 52(1): 27-34. DOI:10.1007/s12275-014-3019-2 |
| [8] | Song XL, Wu H, Yin ZH, Lian ML, Yin CR. Endophytic bacteria isolated from Panax ginseng improves ginsenoside accumulation in adventitious Ginseng root culture. Molecules, 2017, 22(6): 837. DOI:10.3390/molecules22060837 |
| [9] | Etminani F, Harighi B. Isolation and identification of endophytic bacteria with plant growth promoting activity and biocontrol potential from Wild Pistachio Trees. The Plant Pathology Journal, 2018, 34(3): 208-217. DOI:10.5423/PPJ.OA.07.2017.0158 |
| [10] | Ding T, Su B, Chen XJ, Xie SS, Gu SY, Wang Q, Huang DY, Jiang HY. An endophytic bacterial strain isolated from Eucommia ulmoides inhibits southern corn leaf blight. Frontiers in Microbiology, 2017, 8: 903. DOI:10.3389/fmicb.2017.00903 |
| [11] | Suryanto D, Wulandari SD, Nurtjahja K, Munir E, Bungsu A. An ability of endophytic bacterial isolated from chilli to reduce seedling-off caused by Fusarium oxysporum. Journal of Physics: Conference Series, 2018, 1116(5): 052067. |
| [12] | Zhao LF, Xu YJ, Lai XH, Shan CJ, Deng ZS, Ji YL. Screening and characterization of endophytic Bacillus and Paenibacillus strains from medicinal plant Lonicera japonica for use as potential plant growth promoters. Brazilian Journal of Microbiology, 2015, 46(4): 977-989. DOI:10.1590/S1517-838246420140024 |
| [13] | Zhao LF, Xu YJ, Chang JL, Li M, Zhang YL, Dang YJ, Wang MS, Cheng YW, Zhang BY. Screening, resistance and growth-promoting effect of endophytic bacteria with ACC deaminase activity isolated from soybean nodules. Acta Microbiologica Sinica, 2016, 56(6): 1009-1021. (in Chinese) 赵龙飞, 徐亚军, 常佳丽, 李敏, 张艳玲, 党永杰, 王梦思, 程亚稳, 张斌月. 具ACC脱氨酶活性大豆根瘤内生菌的筛选、抗性及促生作用. 微生物学报, 2016, 56(6): 1009-1021. |
| [14] | Van Overbeek LS, Saikkonen K. Impact of bacterial-fungal interactions on the colonization of the endosphere. Trends in Plant Science, 2016, 21(3): 230-242. DOI:10.1016/j.tplants.2016.01.003 |
| [15] | Wang RF, Kang CX, Xu YY, Yang QX. Isolation, identification and antibacterial activity of endophytic bacteria from Rehmannia glutinosa. Jiangsu Agricultural Sciences, 2017, 45(13): 82-86. (in Chinese) 王瑞飞, 康春晓, 许圆圆, 杨清香. 怀地黄内生细菌的分离鉴定及抗菌活性. 江苏农业科学, 2017, 45(13): 82-86. |
| [16] | Yang QX, Xie YS, Zhang H, Li XM. Isolation, identification and antagonism on microorganisms and cancer cells by active endophytes from Rehmannia glutinosa libosch. Microbiology China, 2010, 37(10): 1467-1474. (in Chinese) 杨清香, 谢永生, 张昊, 李学梅. 怀地黄活性内生菌的分离鉴定及抗菌抗肿瘤活性. 微生物学通报, 2010, 37(10): 1467-1474. |
| [17] | Zhang TJ, Jiang J, Xu SX, Zhang SM, Wu K. Identification of endogenous bacteria in Radix rehmanniae with catalpol. Journal of Zhejiang Agricultural Sciences, 2011(2): 395-399. (in Chinese) 张庭静, 蒋瑾, 徐淑霞, 张世敏, 吴坤. 一株产梓醇的地黄内生细菌的鉴定. 浙江农业科学, 2011(2): 395-399. DOI:10.3969/j.issn.0528-9017.2011.02.061 |
| [18] | Zhao Y, Xu HY, Xin GQ, Wu XY, Gu W. Isolation and identification of endophytic bacteria produced catalpol from rehmannia and studies on genetic stability. Journal of Pharmaceutical Research, 2014, 33(10): 567-570, 577. (in Chinese) 赵影, 徐海燕, 辛国芹, 武香玉, 谷巍. 产梓醇地黄内生菌的分离鉴定及遗传稳定性研究. 药学研究, 2014, 33(10): 567-570, 577. |
| [19] | Li J, Wang M, Lin ZP, Yuan QJ, Yu RM, Huang LQ. Isolation and identification of endophytic fungi from different swollen root of Rehmannia glutinosa. China Journal of Chinese Materia Medica, 2010, 35(13): 1679-1683. |
| [20] | Zhao LF, Xu YJ, Chang JL, Li QF, Yang JY, Ou QF, Yang MR. Screening endophytic bacteria from soybean root nodules to inhibit Fusarium oxysporum f. sp. vasinfectum. Acta Microbiologica Sinica, 2017, 57(5): 710-723. (in Chinese) 赵龙飞, 徐亚军, 常佳丽, 李全芬, 杨静雅, 欧启凡, 杨梦冉. 大豆根瘤内抗棉花枯萎病菌株的筛选及其防病试验. 微生物学报, 2017, 57(5): 710-723. |
| [21] | Luo J, Zhou JJ, Zhang JZ. Aux/IAA gene family in plants: molecular structure, regulation, and function. International Journal of Molecular Sciences, 2018, 19(1): 259. DOI:10.3390/ijms19010259 |
| [22] | Qiao ZW, Hong JP. Selection and identify phosphate-solubilizing bacteria and research their phosphate-dissolving ability in lime soil of Shanxi province. Hans Journal of Soil Science, 2017(4): 918-919. (in Chinese) 乔志伟, 洪坚平. 山西石灰性土壤磷细菌的筛选鉴定与溶磷作用的研究. 土壤科学, 2017(4): 918-919. |
| [23] | Yang S, Yang T, Lin B, Liu XZ, Xiang MC. Isolation and evaluation of two phosphate-dissolving fungi. Acta Microbiologica Sinica, 2018, 58(2): 264-273. (in Chinese) 杨顺, 杨婷, 林斌, 刘杏忠, 向梅春. 两株溶磷真菌的筛选、鉴定及溶磷效果的评价. 微生物学报, 2018, 58(2): 264-273. |
| [24] | Fernando WGD, Ramarathnam R, Krishnamoorthy AS, Savchuk SC. Identification and use of potential bacterial organic antifungal volatiles in biocontrol. Soil Biology and Biochemistry, 2005, 37(5): 955-964. DOI:10.1016/j.soilbio.2004.10.021 |
| [25] | Zhu BC, Gu L, Li Z, Wu HJ, Gu Q, Wu LM, Gao XW. Isolation and identification of the Antarctic soil Bacillus and its biocontrol effect against brown stalk rot on maize. Journal of Nanjing Agricultural University, 2017, 40(4): 641-648. (in Chinese) 朱碧春, 顾丽, 李正, 伍辉军, 顾沁, 吴黎明, 高学文. 南极土壤芽孢杆菌的分离鉴定及其防治玉米细菌性褐腐病的研究. 南京农业大学学报, 2017, 40(4): 641-648. |
| [26] | Hu LF, Robert CAM, Cadot S, Zhang X, Ye M, Li BB, Manzo D, Chervet N, Steinger T, van der Heijden MGA, Schlaeppi K, Erb M. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota. Nature Communications, 2018, 9: 2738. DOI:10.1038/s41467-018-05122-7 |
| [27] | Levy A, Salas Gonzalez I, Mittelviefhaus M, Clingenpeel S, Herrera Paredes S, Miao JM, Wang KR, Devescovi G, Stillman K, Monteiro F, Rangel Alvarez B, Lundberg DS, Lu TY, Lebeis S, Jin Z, McDonald M, Klein AP, Feltcher ME, Rio TG, Grant SR, Doty SL, Ley RE, Zhao BY, Venturi V, Pelletier DA, Vorholt JA, Tringe SG, Woyke T, Dangl JL. Genomic features of bacterial adaptation to plants. Nature Genetics, 2017, 50(1): 138-150. |
| [28] | Xu YJ, Zhao LF, Chen P, Jiang XR, Wei GH. Isolation, screening and characterization of phytopathogen antagonistic endophytes from wild Artemisia argyi. Acta Ecologica Sinica, 2013, 33(12): 3697-3705. (in Chinese) 徐亚军, 赵龙飞, 陈普, 蒋晓瑞, 韦革宏. 植物病原菌拮抗性野生艾蒿内生菌的分离、筛选和鉴定. 生态学报, 2013, 33(12): 3697-3705. |
| [29] | Liu QL, Zhang JX, Xu RF, Duan CY. Studies on isolation of endophytic bacteria from citrus skins and screening of antagonistic strains against Penicillium italicum. Chinese Agricultural Science Bulletin, 2011, 27(28): 235-239. (in Chinese) 刘起丽, 张建新, 徐瑞富, 段长勇. 柑橘皮内生细菌分离及柑橘青霉病菌拮抗菌筛选研究. 中国农学通报, 2011, 27(28): 235-239. |
| [30] | Dou RM, Lei QQ, Cao KQ. Study on physiological and biochemical characters of endophytic bacterial strain TS-5 from densefruit pittany root-bark and its antibacterial activity to botrytis cinerea. Chinese Agricultural Science Bulletin, 2010, 26(13): 324-327. (in Chinese) 窦瑞木, 雷清泉, 曹克强. 中药白鲜皮内生细菌TS-5生化特性及对番茄灰霉病菌的抑制作用. 中国农学通报, 2010, 26(13): 324-327. |
| [31] | Zhao LF, Xu YJ, Peng DH, Zhao RY, Chen LL, Liu BB. Screening and inhibitory effect of antagonistic endophytic bacteria associated with soybean root nodules against Magnaporthe grisea. Microbiology China, 2016, 43(5): 998-1008. (in Chinese) 赵龙飞, 徐亚军, 彭顶华, 赵瑞英, 陈乐乐, 刘备备. 稻瘟病菌拮抗性大豆根瘤内生细菌的筛选及抑制效果. 微生物学通报, 2016, 43(5): 998-1008. |
| [32] | Liu XM, Zhang X, Xu T, Li Y, Liao HD, Liu Q, Ma JS, Shi XW, Yuan SS, Zhu YH. Isolation and identification of an endophytic bacterium from Cymbopogon Citrates (DC.) stapf and its optimal flavor-producing conditions. Journal of Hunan University (Natural Sciences), 2017, 44(6): 112-118. (in Chinese) 刘选明, 张鑫, 徐婷, 李燕, 廖红东, 柳倩, 马江山, 施小伟, 袁珊珊, 朱咏华. 一株香茅草内生细菌Pantoea ananatis CcSh-1的分离、鉴定及产香条件优化研究. 湖南大学学报(自然科学版), 2017, 44(6): 112-118. |
| [33] | Yang Z, Cao J. Research progress of endophytic fungi and their secondary metabolites. Journal of Microbiology, 2016, 36(4): 1-6. (in Chinese) 杨镇, 曹君. 植物内生菌及其次级代谢产物的研究进展. 微生物学杂志, 2016, 36(4): 1-6. DOI:10.3969/j.issn.1005-7021.2016.04.001 |
| [34] | Zhao LF, Xu YJ, Lai XH, Kou TC, Yan JL, Zhou PP, Fan SS, Yan YF. Screening probiotic endophytic bacteria from medicinal plant Ilex cornuta and the phytopathogen-inhibiting effect. Chinese Journal of Applied Ecology, 2015, 26(5): 1553-1562. (in Chinese) 赵龙飞, 徐亚军, 赖心河, 寇田超, 闫俊利, 周佩佩, 范闪闪, 闫永峰. 枸骨益生菌的筛选及其抑菌作用. 应用生态学报, 2015, 26(5): 1553-1562. |
| [35] | Miller KI, Qing C, Sze DMY, Roufogalis BD, Neilan BA. Culturable endophytes of medicinal plants and the genetic basis for their bioactivity. Microbial Ecology, 2012, 64(2): 431-449. DOI:10.1007/s00248-012-0044-8 |
| [36] | Compant S, Duffy B, Nowak J, Clément C, Barka AE. Use of plant growth-promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Applied and Environmental Microbiology, 2005, 71(9): 4951-4959. |
| [37] | Yi L, Xiao CG, Ma GH, Wang WN, Long LK. Inhibition and screening of beneficial endophytic bacteria to control tobacco brown spot. Acta Microbiologica Sinica, 2004, 44(1): 19-22. (in Chinese) 易龙, 肖崇刚, 马冠华, 王万能, 龙良鲲. 防治烟草赤星病有益内生细菌的筛选及抑菌作用. 微生物学报, 2004, 44(1): 19-22. DOI:10.3321/j.issn:0001-6209.2004.01.004 |
| [38] | Zhang AM, Han XY, Zhang SH, Sun K, Wu JY, Yu J, Guo BM. Diversity of root nodule endophytic bacteria in Hippophae thibetana at different altitudes in Gannan area. Biotic Resources, 2018, 40(2): 114-119. (in Chinese) 张爱梅, 韩雪英, 张世虎, 孙坤, 吴菊艳, 于杰, 郭保民. 甘南高寒地区不同海拔西藏沙棘根瘤内生菌多样性. 生物资源, 2018, 40(2): 114-119. |
| [39] | Liu L, Sun L, Zhang RY, Yao N, Li LB. Diversity of IAA-producing endophytic bacteria isolated from the roots of Cymbidium goeringii. Biodiversity Science, 2010, 18(2): 182-187. (in Chinese) 刘琳, 孙磊, 张瑞英, 姚娜, 李潞滨. 春兰根中可分泌吲哚乙酸的内生细菌多样性. 生物多样性, 2010, 18(2): 182-187. |
| [40] | Kim YC, Leveau J, Gardener BBM, Pierson EA, Pierson III LS, Ryu CM. The multifactorial basis for plant health promotion by plant-associated bacteria. Applied and Environmental Microbiology, 2011, 77(5): 1548-1555. |
| [41] | Pragash MG, Narayanan KB, Naik PR, Sakthivel N. Characterization of Chryseobacterium aquaticum strain PUPC1 producing a novel antifungal protease from rice rhizosphere soil. Journal of Microbiology and Biotechnology, 2009, 19(1): 99-107. |
| [42] | Gong FJ, Borrathybay E, Zhang YF, Nazierbieke W. Isolation and antibacterial activity of ACC deaminase-containing endophytic bacteria from Eucommia ulmoides Oliver. Microbiology China, 2011, 38(10): 1526-1532. (in Chinese) 龚凤娟, 恩特马克·布拉提白, 张宇凤, 吾鲁木汗·那孜尔别克. 具有ACC脱氨酶活性的杜仲内生细菌的分离鉴定及其抗菌活性. 微生物学通报, 2011, 38(10): 1526-1532. |
| [43] | He ZD, Yan LY, Shi YX, Li BJ, Gao YF, Zhang MZ, Sun J. Bio-control of PGPR seed coating producing ACC deaminase to cucumber bacterial stem soft rot disease. Chinese Journal of Biological Control, 2017, 33(6): 817-825. (in Chinese) 贺字典, 闫立英, 石延霞, 李宝聚, 高玉峰, 张明珠, 孙健. 产生ACC脱氨酶的PGPR种衣剂对黄瓜细菌性茎软腐病的防治效果. 中国生物防治学报, 2017, 33(6): 817-825. |
| [44] | Belimov AA, Dodd IC, Safronova VI, Hontzeas N, Davies WJ. Pseudomonas brassicacearum strain Am3 containing 1-aminocyclopropane-1-carboxylate deaminase can show both pathogenic and growth-promoting properties in its interaction with tomato. Journal of Experimental Botany, 2007, 58(6): 1485-1495. DOI:10.1093/jxb/erm010 |
| [45] | Teng SS, Liu YP, Zhao L. Isolation, identification and characterization of ACC deaminase-containing endophytic bacteria from halophyte Suaeda salsa. Acta Microbiologica Sinica, 2010, 50(11): 1503-1509. (in Chinese) 滕松山, 刘艳萍, 赵蕾. 具ACC脱氨酶活性的碱蓬内生细菌的分离、鉴定及其生物学特性. 微生物学报, 2010, 50(11): 1503-1509. |
| [46] | Priya S, Panneerselvam T, Sivakumar T. Original research article evaluation of indole-3-acetic acid in phosphate solubilizing microbes isolated from rhizosphere soil. International Journal of Current Microbiology and Applied Sciences, 2013, 2(3): 29-36. |
| [47] | Zhu Y, Yao T, Li YE, Sun HY. Screening of phosphate-solubilizing bacteria and their acting mechanisms in the rhizosphere of red clover. Acta Agrestia Sinica, 2009, 17(2): 259-263. (in Chinese) 朱颖, 姚拓, 李玉娥, 孙红阳. 红三叶根际溶磷菌分离及其溶磷机制初探. 草地学报, 2009, 17(2): 259-263. |
| [48] | Qi J, Shi SL. Preliminary study on the ability of phosphorus-solubilizing and IAA-secreting of endogenous rhizobia in seeds of different alfalfa varieties. Grassland and Turf, 2006(5): 18-20, 25. (in Chinese) 祁娟, 师尚礼. 不同品种紫花苜蓿种子内生根瘤菌溶磷和分泌生长素能力. 草原与草坪, 2006(5): 18-20, 25. |
