徐善恒1, 辛瑜1, 刘建民2, 石贵阳1, 丁重阳1, 顾正华1, 李由然1, 张梁1


1. 江南大学粮食发酵工艺与技术国家工程实验室, 江苏 无锡 214122;
2. 山东惠仕莱生物科技有限公司, 山东 济南 250101
收稿日期:2020-12-29;修回日期:2021-03-07;网络出版日期:2021-08-27
基金项目:江苏省自然科学基金(BE2018055)
*通信作者:张梁.Tel: +86-510-85910799; E-mail: zhangl@jiangnan.edu.cn.
摘要:[目的] 基于信号肽和信号肽酶在分泌系统中的重要作用,探索短小芽孢杆菌来源中性β-1,4-内切木聚糖酶在Bacillus subtilis中的重组分泌表达与优化。[方法] 首先,从短小芽孢杆菌基因组DNA中扩增β-1,4-内切木聚糖酶全长基因,连接到pWB980载体P43启动子下游,转化B.subtilis WB800构建重组菌NZ-X。之后,构建信号肽筛选载体,对23个从B.subtilis 168基因组DNA中扩增得到的信号肽进行筛选。最后,以B.subtilis WB800的xynA基因为整合位点,分别整合过表达SipS和SipT两个主要信号肽酶,考察其对融合不同信号肽异源蛋白分泌的影响。[结果] 重组菌NZ-X成功实现β-1,4-内切木聚糖酶的分泌表达,摇瓶发酵上清液酶活为5.33 U/mL,信号肽筛选结果发现YlaE、YfhK、EglS、YqxI、YpjP信号肽与β-1,4-内切木聚糖酶契合度较高,对应酶活依次为7.15、6.69、6.36、6.32、6.18 U/mL,其中SipS信号肽酶对融合YfhK信号肽的β-1,4-内切木聚糖酶的分泌促进作用最大,摇瓶发酵上清液酶活提高到10.64 U/mL,为NZ-X的1.99倍。[结论] 信号肽优化与信号肽酶过表达联用可有效提高B.subtilis中异源蛋白的分泌表达量。
关键词:枯草芽孢杆菌中性β-1, 4-内切木聚糖酶信号肽信号肽酶
Optimization of secretion of heterologous endo-β-1, 4-xylanase in Bacillus subtilis
Shanheng Xu1, Yu Xin1, Jianmin Liu2, Guiyang Shi1, Chongyang Ding1, Zhenghua Gu1, Youran Li1, Liang Zhang1


1. National Engineering Laboratory for Cereal Fermentation Technology, Jiangnan University, Wuxi 214122, Jiangsu Province, China;
2. Shandong Huishilai Biotechnology Co, Ltd, Jinan 250101, Shandong Province, China
Received: 29 December 2020; Revised: 7 March 2021; Published online: 27 August 2021
*Corresponding author: Liang Zhang.Tel: +86-510-85910799; E-mail: zhangl@jiangnan.edu.cn.
Foundation item: Supported by the Natural Science Foundation of Jiangsu Province (BE2018055)
Abstract: [Objective] We aimed to perform and optimize the recombinant secretory expression of neutral endo-β-1, 4-xylanase derived from Bacillus pumilus in Bacillus subtilis WB800 on the basis of important role of signal peptide and signal peptidase. [Methods] We amplified the full-length neutral endo-β-1, 4-xylanase gene from B. pumilus genomic DNA and then ligated to the downstream of the P43 promoter in the pWB980 vector. Thereafter, the recombinant vector was transformed into B. subtilis WB800 to construct the recombinant strain NZ-X. Signal peptides were screened among 23 signal peptides that were amplified from B. subtilis 168 genomic DNA. On this basis, we constructed two strains that overexpress two signal peptidases (SipS and SipT), respectively. And the effect of these two signal peptidases on the secretion of endo-β-1, 4-xylanase was investigated. [Results] Neutral endo-β-1, 4-xylanase was successfully secreted from the recombinant strain NZ-X and the enzyme activity in supernatant was 5.33 U/mL via shake flask fermentation. The results of signal peptide screening indicated that five signal peptides (YlaE, YfhK, EglS, YqxI, YpjP) were effective, and the enzyme activities were 7.15, 6.69, 6.36, 6.32, 6.18 U/mL, respectively. Among these signal peptides, the secretion of endo-β-1, 4-xylanase was promoted mostly by SipS signal peptidase when fusion with YfhK signal peptide. The enzyme activity could be increased to 10.64 U/mL, which was 1.99 times than that of NZ-X. [Conclusion] The secretion of heterologous proteins in B. subtilis could be improved effectively through signal peptide optimization and signal peptidase overexpression.
Keywords: Bacillus subtilisneutral endo-β-1, 4-xylanasesignal peptidesignal peptidase
木聚糖是一种半纤维素,为自然界第二丰富的多聚糖[1],β-1, 4-内切木聚糖酶是木聚糖酶系中起主要作用的酶,它通过切割木聚糖主链的β-1, 4-糖苷键来降低其聚合度[2],在果蔬饮料、酿造、焙烤等领域应用广泛[3]。但高产野生菌筛选困难且酶系复杂难以纯化,故利用基因工程技术构建重组菌株来表达β-1, 4-内切木聚糖酶[4-5]逐渐成为研究热点。枯草芽孢杆菌作为一种应用广泛的工业微生物,生长速度快,发酵周期短,可直接将蛋白分泌到胞外培养基中,被FDA (Food and Drug Administration)认证为GRAS (generally regarded as safe)菌株[6],成为首选表达宿主。但枯草芽孢杆菌高效分泌的往往是其本身的内源蛋白,对异源蛋白的分泌量不高。
分泌蛋白通常以前体蛋白的形式合成,N-端包含一段信号肽序列来引导其完成分泌,信号肽在分泌过程中被位于细胞膜上的信号肽酶切除,之后分泌蛋白从膜上释放并折叠为成熟蛋白,Brockmeier等[7]通过融合枯草芽孢杆菌内源信号肽的方式,对Fusarium solani pisi来源的角质酶和宏基因组来源的一种酯酶进行了信号肽优化,使其胞外酶活显著提升,但分析显示两者对应的最佳信号肽并不一致。耐碱性木聚糖酶[8]、α-淀粉酶[9]以及枯草杆菌蛋白酶BPN′[10]信号肽优化的结果也表明,不同蛋白的最适信号肽往往也不相同,且未发现明确规律来预测特定蛋白的最佳信号肽。因此,进行信号肽筛选是目前寻找异源蛋白合适信号肽、提高其分泌表达量的有效方法。枯草芽孢杆菌分泌途径已被初步阐明[11],其中有许多潜在因素有待优化。Van Dijl等[12-13]在大肠杆菌中过表达信号肽酶后,观察到前体蛋白的处理效率得到提高,且在细胞周质中检测到更多目的蛋白的积累。Bolhuis等和Bron等[14-15]研究发现,SipS和SipT是枯草芽孢杆菌中最主要的两个信号肽酶,但直到指数生长后期才有较高表达水平,且两者对信号肽的处理效率存在偏好性。
本实验室保藏有一株野生型短小芽孢杆菌可分泌中性β-1, 4-内切木聚糖酶,在食品工业中有较大应用潜力,本研究选择Bacillus subtilis WB800作为表达宿主,探索短小芽孢杆菌来源中性β-1, 4-内切木聚糖酶在枯草芽孢杆菌中的重组分泌表达。通过信号肽筛选初步优化其分泌表达量,并进一步考察过表达SipS和SipT信号肽酶对融合不同信号肽β-1, 4-内切木聚糖酶分泌的影响。
1 材料和方法 1.1 材料
1.1.1 菌株、质粒与引物: 本文所用菌株和质粒见表 1,所用引物为生工生物工程(上海)股份有限公司合成,见表 2。
表 1. 本文所用菌株和质粒 Table 1. The strains and plasmids used in this study
| Strains and plasmids | Relevant characteristics | Sources |
| Strains | ||
| ??JM109 | Escherichia coli, used for gene cloning | This lab |
| ??WB800 | B. subtilis, used for initial expression host | This lab |
| ??168 | B. subtilis, wild type | This lab |
| ??NZ-X | WB800 harboring pWB980-X plasmid | This study |
| ??NZ-C | WB800 harboring pWB980 plasmid | This study |
| ??ZTS | WB800, ΔxynA: : Ps09-sipS-Tet, Tetr | This study |
| ??ZTT | WB800, ΔxynA: : Ps09-sipT-Tet, Tetr | This study |
| ??ZS | WB800, ΔxynA: : Ps09-sipS | This study |
| ??ZT | WB800, ΔxynA: : Ps09-sipT | This study |
| ??NZ-YlaE-X | WB800 harboring pWB980-YlaE-X plasmid | This study |
| ??NZ-YfhK-X | WB800 harboring pWB980-YfhK-X plasmid | This study |
| ??NZ-EglS-X | WB800 harboring pWB980-EglS-X plasmid | This study |
| ??NZ-YqxI-X | WB800 harboring pWB980-YqxI-X plasmid | This study |
| ??NZ-YpjP-X | WB800 harboring pWB980-YpjP-X plasmid | This study |
| ??ZS-YfhK-X | ZS harboring pWB980-YfhK-X plasmid | This study |
| Plasmids | ||
| ??pWB980 | B. subtilis secrete expression vector, Kanar | This lab |
| ??Cloned-T-FTF | pMD19-T based vector with FRT sequence and Tet expression cassette, Ampr | This lab |
| ??pNZT1-Kana-FLP | Temperature sensitive plasmid with flp expression cassette, Kanar | This lab |
| ??pWB980-X | pWB980 based vector with endo-β-1, 4-xylanase gene, Kanar | This study |
| ??pWB980-P43 | pWB980 based vector, Kanar | This study |
| ??pWB980-SC | pWB980-P43 based vector, Kanar | This study |
| ??T-ΔxynA: : Ps09-sipS | pMD19-T based vector with the knock-in cassette of sipS, Ampr | This study |
| ??T-ΔxynA: : Ps09-sipT | pMD19-T based vector with the knock-in cassette of sipT, Ampr | This study |
| ??T-ΔxynA: : Ps09-sipS-Tet | T-ΔxynA: : Ps09-sipS based vector, Ampr | This study |
| ??T-ΔxynA: : Ps09-sipT-Tet | T-ΔxynA: : Ps09-sipT based vector, Ampr | This study |
| ??pWB980-YlaE-X | pWB980-SC based vector, carrying YlaE signal peptide sequence, Kanar | This study |
| ??pWB980-YfhK-X | pWB980-SC based vector, carrying YfhK signal peptide sequence, Kanar | This study |
| ??pWB980-EglS-X | pWB980-SC based vector, carrying EglS signal peptide sequence, Kanar | This study |
| ??pWB980-YqxI-X | pWB980-SC based vector, carrying YqxI signal peptide sequence, Kanar | This study |
| ??pWB980-YpjP-X | pWB980-SC based vector, carrying YpjP signal peptide sequence, Kanar | This study |
表选项
表 2. 本文所用引物 Table 2. Primers used in this study
| Primers | Sequences (5′→3′) |
| For amplification of endo-β-1, 4-xylanase gene | |
| ??X-F | CGGGGTACCATAATTAAGGAGGATTCTTATGAATTTGAGAAAATTAAGACTGT |
| ??X-R | CGCGGATCCTTAGTGGTGGTGATGGTGATGATGATGGCCACCGTTGCCAATAAACAGCT |
| For construction of signal peptides screening vctors | |
| ??C1-F | CCGGAATTCGATATCaGCATTATTGAGTGGATGAT |
| ??C1-R | CTAGTCTAGAAATTACCGGAGCTCCGGTGGTACCGCTATCACTTTATATTTTAC |
| ??C2-F | AATTACCGGAGCTCAGAACCATTACGAATAATG |
| ??C2-R | CTAGTCTAGAGATCCTTAGTGGTGGTG |
| For amplification of signal peptides | |
| ??AbnA-F | CGGGGTACCATAATTAAGGAGGATTCTTATGAAAAAGAAAAAAACATGGAAACG |
| ??AbnA-R | AATTACCGGAGCTCAGCTGCCTCTGCGGGAGCAGCA |
| ??AfsK-F | CGGGGTACCATAATTAAGGAGGATTCTTATGGTCAAGTCATTTCG |
| ??AfsK-R | AATTACCGGAGCTCAGCCGCATAAGCAGCTGTCG |
| ??AmyE-F | CGGGGTACCATAATTAAGGAGGATTCTTATGTTTGCAAAACGATTCAAAAC |
| ??AmyE-R | AATTACCGGAGCTCAGCAGCACTCGCAGCCGCCG |
| ??BglS-F | CGGGGTACCATAATTAAGGAGGATTCTTATGCCTTATCTGAAACGAG |
| ??BglS-R | AATTACCGGAGCTCAGCAGCTGAGGCAGTAGCAG |
| ??CotC-F | CGGGGTACCATAATTAAGGAGGATTCTTATGAAAAATCGGCTCTTTAT |
| ??CotC-R | AATTACCGGAGCTCAGCAGATTTTGCGGCTTGG |
| ??Csn-F | CGGGGTACCATAATTAAGGAGGATTCTTATGAAAATCAGTATGCAAAAAG |
| ??Csn-R | AATTACCGGAGCTCAGCCGCAAAAACCGTTTCGC |
| ??DacF-F | CGGGGTACCATAATTAAGGAGGATTCTTATGAAACGTCTTTTATCCAC |
| ??DacF-R | AATTACCGGAGCTCAGCTGCAAATGCAGACGGTG |
| ??EglS-F | CGGGGTACCATAATTAAGGAGGATTCTTATGATGCGAAGGAGGAAAAG |
| ??EglS-R | AATTACCGGAGCTCAGCTGCTGATGCCGGCGAAG |
| ??MotB-F | CGGGGTACCATAATTAAGGAGGATTCTTATGGCGAGAAAAAAGAAGAAGAAGC |
| ??MotB-R | AATTACCGGAGCTCAGCGCTGCTCGCGTACAGC |
| ??Mpr-F | CGGGGTACCATAATTAAGGAGGATTCTTATGAAATTAGTTCCAAGATTCAG |
| ??Mpr-R | AATTACCGGAGCTCAGCCGCTTTTGCCGGTACGC |
| ??PhoB-F | CGGGGTACCATAATTAAGGAGGATTCTTATGAAAAAATTCCCGAAGAAATTACT |
| ??PhoB-R | AATTACCGGAGCTCAGCGGCGCTGGCTTCAGGC |
| ??YdjM-F | CGGGGTACCATAATTAAGGAGGATTCTTATGTTGAAGAAAGTCATTTTAG |
| ??YdjM-R | AATTACCGGAGCTCAGCCGCACTGGCATCTGATG |
| ??XynC-F | CGGGGTACCATAATTAAGGAGGATTCTTATGATTCCACGCATAAAAAAAACAAT |
| ??XynC-R | AATTACCGGAGCTCAGCTGCCAAAACTTCAGTAGCG |
| ??YfhK-F | CGGGGTACCATAATTAAGGAGGATTCTTATGAAAAAGAAACAAGTAATGC |
| ??YfhK-R | AATTACCGGAGCTCAGCAGCTTTTGCTGCGGGAG |
| ??YhfM-F | CGGGGTACCATAATTAAGGAGGATTCTTATGAAAAAAATAGTGGCAGC |
| ??YhfM-R | AATTACCGGAGCTCAGCCGCGTCTACCGATTGATAC |
| ??YlaE-F | CGGGGTACCATAATTAAGGAGGATTCTTATGAAGAAAACATTTGTAAAAAAAGC |
| ??YlaE-R | AATTACCGGAGCTCAGCTGCGCTGGCTGCATCAG |
| ??YncM-F | CGGGGTACCATAATTAAGGAGGATTCTTATGGCGAAACCACTATC |
| ??YncM-R | AATTACCGGAGCTCAGCAGCGTCTGCCGCGGGT |
| ??YolA-F | CGGGGTACCATAATTAAGGAGGATTCTTATGAAGAAGAGAATTACATATTCAC |
| ??YolA-R | AATTACCGGAGCTCAGCCGCTTTTGCTTTTGATG |
| ??YpjP-F | CGGGGTACCATAATTAAGGAGGATTCTTATGAAATTGTGGATGAGAAAGAC |
| ??YpjP-R | AATTACCGGAGCTCAGCCGCCATAAGGGCAGCCG |
| ??YqfZ-F | CGGGGTACCATAATTAAGGAGGATTCTTATGAAGCGTCTCACCTT |
| ??YqfZ-R | AATTACCGGAGCTCAGCTGCTGATGCTTCATAGACAG |
| ??Yqxl-F | CGGGGTACCATAATTAAGGAGGATTCTTATGTTTAAGAAATTACTTTTAGCAAC |
| ??Yqxl-R | AATTACCGGAGCTCAGCAGCTTTGGCATGTCCAT |
| ??YqxM-F | CGGGGTACCATAATTAAGGAGGATTCTTATGTTTCGATTGTTTCACAATCAGC |
| ??YqxM-R | AATTACCGGAGCTCAGCAGCGCTTGTATCATCGG |
| ??Yurl-F | CGGGGTACCATAATTAAGGAGGATTCTTATGACAAAAAAAGCATGGTT |
| ??Yurl-R | AATTACCGGAGCTCAGCCGCGCTTGCTGAAGCTG |
| For the construction of expression cassette of SipS and SipT | |
| ??S1-F | AATTACCGGAGCTCAGCGGCTCGGTTACATCCCACAGTTAC |
| ??S1-R | ATGTTACCTCCTATAATATTTTTTCCGAC |
| ??S2-F | GTCGGAAAAAATATTATAGGAGGTAACATGATCGTCACAATGCGCCATC |
| ??S2-R | CATGGATCCCACTTTATGGACGC |
| ??S3-F | AGCTAGCTAACAGATCATCCTTAATCAGGG |
| ??S3-R | CGGGGTACCCCGCTACGTAGTGTGATTATATCACCG |
| ??S4-F | CGTCCATAAAGTGGGATCCATGTTGAAATCAGAAAATGTTTCGAAG |
| ??S4-R | ATTAAGGATGATCTGTTAGCTAGCTAGGGAAGATCTTTAATTTGTTTTGCGCATTTCG |
| ??S5-F | CGTCCATAAAGTGGGATCCATGTTGACCGAGGAAAAAAATAC |
| ??S5-R | ATTAAGGATGATCTGTTAGCTAGCTAGGGAAGATCTTTATTTTGTTTGACGCATTTCG |
| For qRT-PCR | |
| ??gyrB-F | AACTCAGAAGCACGGACGATC |
| ??gyrB-R | CTCTTGCCGCCATTAAACCTT |
| ??sipS-F | TATTTTTGCGCCGTATGTCGTTG |
| ??sipS-R | CTCCTCTATCAAACTCGCCGATG |
表选项
1.1.2 主要试剂: 2×Rapid Taq Master Mix、Phanta Max Super-Fidelity DNA Polymerase、FastPure Plasmid Mini Kit、FastPure Gel DNA Extraction Mini Kit、HiScript Ⅲ 1st Strand cDNA Synthesis Kit (+gDNA wiper)、ChamQ Universal SYBR qPCR Master Mix均购自南京诺唯赞生物科技股份有限公司,T-Vector pMD-19 (Simple)、T4 DNA连接酶、限制性内切酶购自TaKaRa公司,氨苄青霉素、卡那霉素、四环素购自Sigma公司,Amicon? Ultra-15 3K离心过滤器购自默克密理博公司,榉木木聚糖购自源叶生物科技有限公司,Biospin Bacteria Genomic DNA Extraction Kit、Simply P Total RNA Extraction Kit购自杭州博日科技股份有限公司,胰蛋白胨、酵母粉购自OXOID公司,其他化学与生化试剂均购自国药集团化学试剂有限公司。
发酵培养基:胰蛋白胨12 g/L、酵母粉24 g/L、甘油4 mL/L、KH2PO4 2.53 g/L、Na2HPO4 5.79 g/L、MgSO4 0.5 g/L、(NH4)2SO4 0.5 g/L、CaCl2 0.5 g/L,灭菌前将pH调节至7.5。
1.2 表达载体的构建 以短小芽孢杆菌基因组为模板,以X-F、X-R为引物(X-F 5′端添加Kpn Ⅰ酶切位点、RBS及间隔序列,X-R 3′端添加6×His及BamH Ⅰ酶切位点)扩增得全长β-1, 4-内切木聚糖酶基因片段,与pWB980载体分别用Kpn I、BamH Ⅰ双酶切后连接,得表达载体pWB980-X。将pWB980-X和原pWB980分别转化至B. subtilis WB800,获得重组菌NZ-X和对照菌NZ-C。
1.3 胞外蛋白SDS-PAGE分析 发酵液于12000 r/min离心2 min后,上清于Amicon? Ultra-15 3K离心过滤器中以4500×g转速离心超滤40 min,浓缩液进行SDS-PAGE分析。浓缩胶浓度5%,电泳电压80 V;分离胶浓度15%,电泳电压120 V。
1.4 β-1, 4-内切木聚糖酶酶活的测定 采用3, 5-二硝基水杨酸(3, 5-dinitrosalicylic acid,DNS)定糖法[16],以0.067 mol/L pH 7.5的磷酸盐溶液为缓冲液,以D-木糖作标准曲线。向1 mL适当稀释的预热酶液中加入1 mL预热的10 mg/mL木聚糖溶液,45 ℃反应10 min后迅速加入2 mL DNS试剂混匀,沸水浴15 min,流动自来水下冷却至室温,定容至10 mL,在575 nm波长处测定吸光值。
酶活定义:每分钟从浓度为5 mg/mL的木聚糖溶液中释放1 μmol还原糖所需要的酶量为一个酶活力单位U。
1.5 信号肽筛选表达载体的构建 以pWB980-X为模板,分别以C1-F、C1-R为引物扩增P43启动子(删除原质粒P43启动子前的Sac Ⅰ酶切位点),以C2-F、C2-R为引物扩增无信号肽β-1, 4-内切木聚糖酶基因片段。将pWB980与上述扩增得到的P43启动子分别用EcoR I、Xba Ⅰ双酶切并连接,获得中间载体pWB980-P43。将pWB980-P43与上述无信号肽β-1, 4-内切木聚糖酶基因片段分别用Sac I、Xba Ⅰ双酶切并连接,获得信号肽筛选载体pWB980-SC,如图 1-A。以B. subtilis 168基因组为模板,用表 2中扩增信号肽引物(上游引物5′端添加Kpn Ⅰ酶切位点、RBS及间隔序列,下游引物3′端添加GCT三个碱基和Sac Ⅰ酶切位点)扩增得23个信号肽基因片段,如图 1-B。将信号肽基因片段与pWB980-SC分别用Kpn I、Sac Ⅰ双酶切并连接,转化至B. subtilis WB800获得23个携带融合了B. subtilis不同内源信号肽的短小芽孢杆菌β-1, 4-内切木聚糖酶基因的表达载体的重组菌。
 |
| 图 1 信号肽筛选载体及信号肽酶敲入表达盒的构建 Figure 1 Cloning strategy of signal peptide screening vector and signal peptidase gene knock-in cassette. A: schematic overview of the expression vector used in this study. The signal peptide secretion target fusion was Kpn I/Xab I cloned into the vector downstream of the P43 promoter. B: sequence details of the signal peptide secretion target fusion. All fusions contain the junction built from the amino acids Ala, Glu and Leu. The signal peptidase cleavage site is between -1 and +1 residue. C: the overview of signal peptidase gene knock-in expression cassette. Contain up- and downstream homologous arm, signal peptidase (SipS and SipT) controlled by Ps09 promoter, and tetracycline screening marker flanked by FRT site used for resistance rescue. |
| 图选项 |
1.6 信号肽酶整合过表达菌株的构建 利用FLP/FRT基因编辑系统[17],在B. subtilis WB800基因组xynA位点分别整合过表达SipS和SipT两个主要信号肽酶。
以B. subtilis 168基因组为模板,分别以S1-F/S1-R、S2-F/S2-R、S3-F/S3-R、S4-F/S4-R和S5-F/S5-R为引物扩增得上游同源臂armX-F、含armX-F 3′端同源片段的Ps09启动子、下游同源臂armX-R、含Ps09 3′端同源片段及armX-R 5′端同源片段的sipS和sipT信号肽酶基因。利用重叠延伸PCR技术[18],拼接为armX F-Ps09-sipS-armX R和armX F-Ps09-sipT-armX R,分别连接T-Vector pMD-19 (Simple),获得中间载体T-ΔxynA: : Ps09-sipS和T-ΔxynA: : Ps09-sipT。
Cloned-T-FTF质粒用BamH I、Nhe Ⅰ双酶切,电泳后胶回收其FRT-Tet-FRT片段,将T-ΔxynA: : Ps09-sipS和T-ΔxynA: : Ps09-sipT用Bgl II、Nhe Ⅰ双酶切后分别与FRT-Tet-FRT片段连接,获得载体T-ΔxynA: : Ps09-sipS-Tet和T-ΔxynA: : Ps09-sipT-Tet。
将T-ΔxynA: : Ps09-sipS-Tet和T-ΔxynA: : Ps09-sipT-Tet用Sac I、Kpn Ⅰ双酶切,电泳后胶回收armX F- Ps09-sipS-FRT-Tet-FRT-armX R和armX F-Ps09-sipT- FRT-Tet-FRT-armX R敲入表达盒片段,如图 1-C。将2个敲入表达盒片段分别转化至B. subtilis WB800,获得整合表达盒的双交换菌株ZTS和ZTT。将pNZT1-Kana-FLP质粒分别转化至ZTS和ZTT,于卡那霉素抗性LB培养基、30 ℃条件下培养4代后,以四环素抗性平板负筛得抗性丢失菌株。转接至无抗LB培养基,于42 ℃条件下培养2代,以卡那霉素抗性平板负筛得质粒丢失菌株,最终得到ZS和ZT两株分别整合过表达SipS和SipT信号肽酶的重组菌株。
1.7 统计学分析 使用IBM SPSS Statistics 26软件进行统计学分析,实验数据以x±s表示,采用独立样本t检验(双尾法)比较两组间差异性,P < 0.05时认为两组间存在显著性差异,用*表示,P≥0.05则认为不存在显著性差异。
1.8 qRT-PCR分析 使用实时荧光定量PCR技术,分析整合过表达信号肽酶菌株信号肽酶基因的相对转录水平。提取样品总RNA,进行cDNA第一链的扩增,随后进行qRT-PCR扩增检测Ct值。总RNA提取、cDNA第一链的扩增以及qPCR步骤均按试剂盒说明操作。所用参比基因为gyrB[19],扩增参比基因和sipS基因的引物序列见表 2。分别测定对照菌和目标菌信号肽酶基因及参比基因的Ct值,按照2-??Ct法[20],计算信号肽酶基因的相对转录水平。
2 结果和分析 2.1 β-1, 4-内切木聚糖酶的分泌表达 将重组菌NZ-X和对照菌NZ-C分别以1%接种量接种至装50 mL LB的250 mL摇瓶中,于30 ℃、200 r/min条件下培养36 h。发酵液12000 r/min离心5 min后取上清液检测酶活。重组菌检测到酶活5.33 U/mL,而对照菌没有检测到酶活,发酵液上清SDS-PAGE分析(图 2)也证明短小芽孢杆菌来源的中性β-1, 4-内切木聚糖酶成功分泌表达。
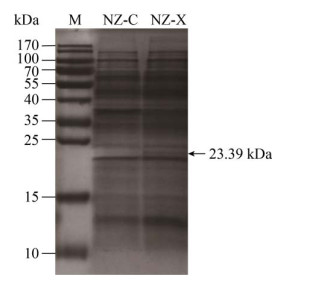 |
| 图 2 胞外蛋白SDS-PAGE分析 Figure 2 SDS-PAGE analysis of the extracellular proteins. 15% SDS-PAGE analysis of the fermentation supernatant after concentrated by ultrafiltration. M: molecular weight marker; NZ-C: B. subtilis harboring the pWB980 vector as control; NZ-X: B. subtilis harboring the expression vector containing full-length endo-β-1, 4-xylanase gene. |
| 图选项 |
2.2 信号肽筛选 将重组菌NZ-X和23个携带融合了B. subtilis不同内源信号肽的β-1, 4-内切木聚糖酶表达载体的重组菌分别以1%接种量接种至24孔板中,每孔装2 mL LB培养基,于30 ℃、200 r/min条件下培养36 h,测定发酵液上清的木聚糖酶酶活,结果如图 3。原始信号肽表现仅排在第11位,表现最好的5个分别为融合了YlaE、YfhK、EglS、YqxI、YpjP信号肽的β-1, 4-内切木聚糖酶,酶活依次为7.15、6.69、6.36、6.32、6.18 U/mL。
 |
| 图 3 各重组菌酶活的测定 Figure 3 Determination of enzyme activity of recombinant bacteria. Comparison of signal peptides used for export of heterologous endo-β-1, 4-xylanase in B. subtilis WB800. The "original" represent the signal peptide derived from endo-β-1, 4-xylanase itself. |
| 图选项 |
2.3 信号肽与信号肽酶的匹配 将pWB980-YlaE-X、pWB980-YfhK-X、pWB980- EglS-X、pWB980-YqxI-X、pWB980-YpjP-X分别转化ZS和ZT。同样以1%接种量接种至24孔板中,每孔装2 mL LB培养基,于30 ℃、200 r/min条件下培养36 h。测定发酵液上清酶活,结果如图 4。总体来看,过表达信号肽酶对蛋白分泌有一定的促进作用,其中SipS对融合了YfhK信号肽的β-1, 4-内切木聚糖酶分泌促进作用最明显,酶活达到10.64 U/mL,为未过表达信号肽酶时的1.59倍,为信号肽优化前的1.99倍。
 |
| 图 4 信号肽与信号肽酶的匹配 Figure 4 Match signal peptides with signal peptidases. Comparison of the enzyme activity of the strains that harboring endo-β-1, 4-xylanase express vector fused different signal peptide that overexpress different signal peptidase. ZS: the strain overexpress SipS; ZT: the strain overexpress SipT; *: P < 0.05. |
| 图选项 |
统计学分析发现,过表达SipS对融合Yfhk信号肽蛋白的分泌促进作用显著大于SipT,过表达SipT对融合YqxI信号肽蛋白的分泌促进作用显著大于SipS,这体现了信号肽酶对信号肽处理效率的偏好性,但对融合另外3个信号肽蛋白的分泌影响却没有表现出明显偏好性,可能SipS和SipT对这3个信号肽的处理效率相似,体现了不同信号肽酶也具有一定通用性[15]。
2.4 qRT-PCR分析sipS转录水平及发酵曲线的测定 以NZ-YfhK-X为对照,分别将NZ-YfhK-X和ZS-YfhK-X按1%接种量接种至装有50 mL发酵培养基的250 mL摇瓶中,于30 ℃、200 r/min条件下培养,从12 h开始,每6 h取样至60 h。
其中第12、18、24、36小时的样品进行qRT-PCR分析,结果见表 3。ZS-YfhK-X菌株的sipS基因转录水平高于NZ-YfhK-X的sipS基因,且随着培养时间的增长呈上升趋势,36 h后达到NZ-YfhK-X的14.52倍。
表 3. sipS基因的qRT-PCR转录分析 Table 3. The transcription analysis of sipS using qRT-PCR
| Cycle number | ZS-YfhK-X | ?Ct | NZ-YfhK-X | ?Ct | ??Ct | 2-??Ct | ||
| sipS | gyrB | sipS | gyrB | |||||
| 12 h (mean) | 20.48 | 21.33 | -0.85 | 22.34 | 22.09 | 0.25 | -1.10 | 2.14 |
| 18 h (mean) | 22.44 | 23.28 | -0.84 | 24.26 | 23.32 | 0.94 | -1.78 | 3.43 |
| 24 h (mean) | 24.27 | 25.38 | -1.11 | 26.13 | 24.79 | 1.34 | -2.45 | 5.46 |
| 36 h (mean) | 19.71 | 22.40 | -2.69 | 21.14 | 19.97 | 1.17 | -3.86 | 14.52 |
表选项
生长曲线及酶活测定结果见图 5,过表达信号肽酶对菌体生长并无明显影响,但两者的β-1, 4-内切木聚糖酶的产量却有差异,整个发酵过程中ZS-YfhK-X菌株的酶活一直高于NZ-YfhK-X,最终达到48.97 U/mL,为NZ-YfhK-X的1.29倍。
 |
| 图 5 生长曲线及酶活的测定 Figure 5 Determination of growth and enzyme activity curves. NZ-YfhK-X: B. subtilis WB800 harboring the expression vector that fused signal peptide YfhK; ZS-YfhK-X: ZS (The strain overexpress SipS) harboring the expression vector that fused signal peptide YfhK. |
| 图选项 |
3 讨论 虽然枯草芽孢杆菌在食品、医药、酶制剂等领域应用广泛,但因其分泌机制的限制,异源蛋白的分泌表达量往往不高,一系列的优化工作必不可少。
本研究以B. subtilis WB800为宿主,构建分泌表达载体,成功实现短小芽孢杆菌来源的中性β-1, 4-内切木聚糖酶的重组分泌表达,摇瓶发酵上清液酶活为5.33 U/mL。信号肽优化后,酶活提高到7.15 U/mL。结果表明,短小芽孢杆菌中的信号肽也可在B. subtilis中发挥作用,但并不是最合适的,将B. subtilis内源的不同信号肽与该酶基因进行组合优化可有效改善其分泌表达量。在信号肽优化的基础上,进一步考察了信号肽酶对融合不同信号肽异源蛋白分泌的影响。研究结果表明,过表达不同信号肽酶对融合不同信号肽的β-1, 4-内切木聚糖酶的分泌均有一定的促进作用,但幅度不一,说明不同信号肽酶在功能上既有一定广谱性又有一定的偏好性[15]。
信号肽系统非常复杂,不仅在蛋白的修饰、聚合及靶向运输中起到至关重要的作用,而且被切除的信号肽部分还可参与调节细胞的生理状态,在众多领域表现出巨大的应用潜力[21],但我们对信号肽序列所携带信息的解读能力仍十分有限。Von Heijne[22]综合统计了不同信号肽切割位点附近各氨基酸的出现频率,发现-3和-1位置(以信号肽酶切割位点为中心,向N-端方向为-1、-2、-3……,向C-端方向为+1、+2、+3……)偏好Ala、Gly、Cys和Ser等中性、小氨基酸,其中Ala出现频率远高于其他氨基酸,总结出Ala-X-Ala模式,极大提高信号肽切割位点预测的准确性。此外,Zalucki等[23]发现+2位置对Asp、Glu等酸性氨基酸存在显著偏好性,其中Glu出现频率最高,同时+1位置Ala出现频率远高于其他氨基酸,因此在信号肽下游引物设计时5′端添加了GCT三个碱基和Sac Ⅰ酶切位点,确保+1和+2位置为Ala和Glu。
通常认为信号肽酶识别信号肽-3到+1位置,于-1和+1位置之间切割,本研究所用信号肽皆按上述偏好性挑选和设计,2.3中YfhK和YqxI两个信号肽-3到+1位置均为Ala-Lys-Ala-Ala,但SipS和SipT对融合这2个信号肽的β-1, 4-内切木聚糖酶分泌的影响依然表现出偏好性。Duffaud等[24]对OmpA信号肽切割位点附近氨基酸进行突变,逐步降低其β-转角的程度,测得E. coli信号肽酶对它的切割效率也随之下降,说明类似的二级结构会影响信号肽酶的切割。随后,Chatterjee等[25]用E. coli信号肽酶分别与变性和复性后的Pro-OpmA-nuclease A蛋白反应,发现信号肽酶对复性后蛋白的亲和力远远大于变性蛋白,而且切割此前体蛋白的效率远远高于人工设计包含切割位点的多肽,推测信号肽酶可能对底物的构象存在偏好性。Choo等[26]对E. coli信号肽酶进行建模分析,模型显示在信号肽酶的催化过程中信号肽-7到+6位置的氨基酸残基均参与其中。因此,信号肽酶识别的可能不仅仅是切割位点附近-3到+1之间的位置,其更远端的位置、成熟蛋白N-末端的一段位置以及构象上的差异也可能起一定作用。
过表达信号肽酶后,指数生长中期sipS基因的转录水平提高了14倍之多,但对应时期胞外酶活并无明显提高,直到指数生长后期才观察到胞外酶活的较大提升。这或许与Whiteley等[27]描述的群体感应现象有关:群体感应系统调节胞外公共酶的生产,当群体达到一定规模、公共酶可以惠及足够多菌体时才开始大量分泌。在指数生长前期与中期,菌体对营养物质的利用主要用于菌体细胞生物量的增加,当细胞进入指数生长后期,分泌系统才逐渐完善并产生和分泌与生长无直接相关的蛋白物质,在这之前仅仅提高信号肽酶的表达量并不能弥补整个分泌系统的短板,直到指数生长后期信号肽酶成为限制因素时才能起到明显促进作用。
综上所述,信号肽优化之后过表达与之匹配的信号肽酶可以进一步提高胞外蛋白的产量,但若想实现大幅提高,则需要从分泌系统的全局角度考虑,优化某一关键因素的同时维持其他相关因素的平衡,或可利用全局调控策略[28],挖掘某些关键因子,实现对分泌系统整体的加强。
References
| [1] | Collins T, Gerday C, Feller G. Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiology Reviews, 2005, 29(1): 3-23. DOI:10.1016/j.femsre.2004.06.005 |
| [2] | Polizeli MLTM, Rizzatti ACS, Monti R, Terenzi HF, Jorge JA, Amorim DS. Xylanases from fungi: properties and industrial applications. Applied Microbiology and Biotechnology, 2005, 67(5): 577-591. DOI:10.1007/s00253-005-1904-7 |
| [3] | Teng C, Lu FZ, Fan GS, Li XT. Advances in xylanase and its application in food industry. Biotechnology & Business, 2019(4): 34-41. (in Chinese) 滕超, 鹿发展, 范光森, 李秀婷. 木聚糖酶的研究进展及其在食品领域的应用. 生物产业技术, 2019(4): 34-41. DOI:10.3969/j.issn.1674-0319.2019.04.005 |
| [4] | Li JP, Bao CJ, Chen G, Zhang ST. Research advances in heterologous expression of xylanase. China Biotechnology, 2019, 39(7): 91-99. (in Chinese) 李吉萍, 包昌杰, 陈光, 张斯童. 木聚糖酶异源表达的研究进展. 中国生物工程杂志, 2019, 39(7): 91-99. |
| [5] | Kalinina AN, Borshchevskaya LN, Gordeeva TL, Sineoky SP. Expression of the xylanase gene from pyromyces finnis in Pichia pastoris and characterization of the recombinant protein. Applied Biochemistry and Microbiology, 2020, 56(7): 787-793. DOI:10.1134/S0003683820070054 |
| [6] | Schallmey M, Singh A, Ward OP. Developments in the use of Bacillus species for industrial production. Canadian Journal of Microbiology, 2004, 50(1): 1-17. DOI:10.1139/w03-076 |
| [7] | Brockmeier U, Caspers M, Freudl R, Jockwer A, Noll T, Eggert T. Systematic screening of all signal peptides from Bacillus subtilis: a powerful strategy in optimizing heterologous protein secretion in gram-positive bacteria. Journal of Molecular Biology, 2006, 362(3): 393-402. DOI:10.1016/j.jmb.2006.07.034 |
| [8] | Zhang WW, Yang MM, Yang YD, Zhan J, Zhou YQ, Zhao X. Optimal secretion of alkali-tolerant xylanase in Bacillus subtilis by signal peptide screening. Applied Microbiology and Biotechnology, 2016, 100(20): 8745-8756. DOI:10.1007/s00253-016-7615-4 |
| [9] | Fu G, Liu JL, Li JS, Zhu BW, Zhang DW. Systematic screening of optimal signal peptides for secretory production of heterologous proteins in Bacillus subtilis. Journal of Agricultural and Food Chemistry, 2018, 66(50): 13141-13151. DOI:10.1021/acs.jafc.8b04183 |
| [10] | Degering C, Eggert T, Puls M, Bongaerts J, Evers S, Maurer KH, Jaeger KE. Optimization of protease secretion in Bacillus subtilis and Bacillus licheniformis by screening of homologous and heterologous signal peptides. Applied and Environmental Microbiology, 2010, 76(19): 6370-6376. DOI:10.1128/AEM.01146-10 |
| [11] | Tjalsma H, Bolhuis A, Jongbloed JDH, Bron S, van Dijl JM. Signal peptide-dependent protein transport in Bacillus subtilis: a genome-based survey of the secretome. Microbiology and Molecular Biology Reviews, 2000, 64(3): 515-547. |
| [12] | Van Dijl JM, De Jong A, Vehmaanper? J, Venema G, Bron S. Signal peptidase Ⅰ of Bacillus subtilis: patterns of conserved amino acids in prokaryotic and eukaryotic type Ⅰ signal peptidases. The EMBO Journal, 1992, 11(8): 2819-2828. DOI:10.1002/j.1460-2075.1992.tb05349.x |
| [13] | Dijl JM, Jong A, Smith H, Bron S, Venema G. Signal peptidase Ⅰ overproduction results in increased efficiencies of export and maturation of hybrid secretory proteins in Escherichia coli. Molecular and General Genetics MGG, 1991, 227(1): 40-48. DOI:10.1007/BF00260704 |
| [14] | Bolhuis A, Sorokin A, Azevedo V, Ehrlich SD, Braun PG, De Jong A, Venema G, Bron S, Maarten van Dijl J. Bacillus subtilis can modulate its capacity and specificity for protein secretion through temporally controlled expression of the sipS gene for signal peptidase Ⅰ. Molecular Microbiology, 1996, 22(4): 605-618. DOI:10.1046/j.1365-2958.1996.d01-4676.x |
| [15] | Bron S, Bolhuis A, Tjalsma H, Holsappel S, Venema G, van Dijl JM. Protein secretion and possible roles for multiple signal peptidases for precursor processing in Bacilli. Journal of Biotechnology, 1998, 64(1): 3-13. DOI:10.1016/S0168-1656(98)00099-6 |
| [16] | Miller GL. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical Chemistry, 1959, 31(3): 426-428. DOI:10.1021/ac60147a030 |
| [17] | Li ZW, Li YR, Gu ZH, Ding ZY, Zhang L, Xu S, Shi GY. Chinese Journal of Biotechnology, 2019, 35(3): 458-471. (in Chinese) 李宗文, 李由然, 顾正华, 丁重阳, 张梁, 徐沙, 石贵阳. 地衣芽胞杆菌FLP/FRT基因编辑系统的构建及验证. 生物工程学报, 2019, 35(3): 458-471. |
| [18] | Zhong C, You C, Wei P, Zhang YHP. Simple Cloning by Prolonged Overlap Extension-PCR with Application to the Preparation of Large-Size Random Gene Mutagenesis Library in Escherichia coli. Synthetic DNA, 2017, 1472: 49-61. |
| [19] | Vinayavekhin N, Mahipant G, Vangnai AS, Sangvanich P. Untargeted metabolomics analysis revealed changes in the composition of glycerolipids and phospholipids in Bacillus subtilis under 1-butanol stress. Applied Microbiology and Biotechnology, 2015, 99(14): 5971-5983. DOI:10.1007/s00253-015-6692-0 |
| [20] | Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research, 2001, 29(9): e45. DOI:10.1093/nar/29.9.e45 |
| [21] | Martoglio B, Dobberstein B. Signal sequences: more than just greasy peptides. Trends in Cell Biology, 1998, 8(10): 410-415. DOI:10.1016/S0962-8924(98)01360-9 |
| [22] | Von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. European Journal of Biochemistry, 1983, 133(1): 17-21. DOI:10.1111/j.1432-1033.1983.tb07424.x |
| [23] | Zalucki YM, Jennings MP. Signal peptidase Ⅰ processed secretory signal sequences: Selection for and against specific amino acids at the second position of mature protein. Biochemical and Biophysical Research Communications, 2017, 483(3): 972-977. DOI:10.1016/j.bbrc.2017.01.044 |
| [24] | Duffaud G, Inouye M. Signal peptidases recognize a structural feature at the cleavage site of secretory proteins. Journal of Biological Chemistry, 1988, 263(21): 10224-10228. DOI:10.1016/S0021-9258(19)81502-8 |
| [25] | Chatterjee S, Suciu D, Dalbey RE, Kahn PC, Inouye M. Determination of Km andkcat for Signal peptidase Ⅰ Using a Full Length Secretory Precursor, pro-OmpA-nuclease A. Journal of Molecular Biology, 1995, 245(4): 311-314. DOI:10.1006/jmbi.1994.0025 |
| [26] | Choo KH, Tong JC, Ranganathan S. Modeling Escherichia coli signal peptidase complex with bound substrate: determinants in the mature peptide influencing signal peptide cleavage. BMC Bioinformatics, 2008, 9(1): 1-7. DOI:10.1186/1471-2105-9-1 |
| [27] | Whiteley M, Diggle SP, Greenberg EP. Progress in and promise of bacterial quorum sensing research. Nature, 2017, 551(7680): 313-320. |
| [28] | Tian RZ, Liu YF, Li JH, Liu L, Du GC. Progress in the regulatory tools of gene expression for model microorganisms. Synthetic Biology Journal, 2020, 1(4): 454-469. (in Chinese) 田荣臻, 刘延峰, 李江华, 刘龙, 堵国成. 典型模式微生物基因表达精细调控工具的研究进展. 合成生物学, 2020, 1(4): 454-469. |
