刘彩月1,2, 程明芳1, 江红梅1, 赵福宽2
 , 范丙全1
, 范丙全1

1. 中国农业科学院农业资源与农业区划研究所, 北京 100081;
2. 北京农学院生物科学与工程学院, 农业部都市农业(北方)重点实验室, 北京 102206
收稿日期:2019-03-31;修回日期:2019-06-06;网络出版日期:2019-11-12
基金项目:农业部“948”重点项目(2011-G25,2016-X21);国家高技术研究发展计划(863计划)(2013AA102801-7,2013AA102802-4)
*通信作者:赵福宽, Tel/Fax:+86-10-82106212;
范丙全, E-mail:fanbingquan@caas.cn.
摘要:[目的] 筛选高效拮抗向日葵菌核菌的细菌菌株,为开发防治菌核菌病害、提高向日葵产量的生物菌剂提供菌种资源。[方法] 以羧甲基纤维素钠(CMC)、小麦秸秆纤维素为唯一碳源的无机盐培养基,分离高效降解纤维素的细菌菌株;采用纤维素降解菌与菌核菌的平板对峙方法,进一步筛选拮抗菌核菌的菌株;利用16SrDNA序列鉴定菌株、PDYA平板对峙实验检验上述所选拮抗菌株的抑菌谱;采用离体向日葵新鲜叶片、草炭土基质盆栽实验,观察拮抗菌菌株抑制菌核菌生长的能力;温室盆栽和田间试验条件下,研究其防治向日葵菌核菌病害、促进生长和提高产量的效果。[结果] 筛选了一株高效抑制菌核菌的细菌YC16,经过16SrDNA序列分析,鉴定为解淀粉芽孢杆菌。YC16菌株能够抑制8种病原真菌生长,包括齐整小核菌、腐皮镰孢菌、尖孢镰刀菌、稻梨孢、辣椒疫霉、镰刀菌、尖镰孢黄瓜专化型和向日葵菌核菌;抑制菌核菌感染叶片,抑制率达到了80.42%;抑制盆栽基质中菌核菌的菌丝生长,基质表面菌丝密度比对照减少了50%以上。盆栽接种YC16的向日葵生物量比对照提高54.9%,田间向日葵接种YC16菌剂对菌核菌引发的盘腐病防治效果达39%–100%,产量提高24.4%–30.2%。[结论] YC16生物菌剂施用于土壤,能够有效防治向日葵的茎腐病和盘腐病,展现了防治向日葵菌核病和提高产量的双重效果,是一株具有良好应用前景的高效菌种资源。
关键词:向日葵菌核菌解淀粉芽孢杆菌防治效果增产作用
Isolation of a high effective antagonistic bacterial strain YC16 against Sclerotinia sclerotiorum diseases in sunflower
Caiyue Liu1,2, Mingfang Cheng1, Hongmei Jiang1, Fukuan Zhao2
 , Bingquan Fan1
, Bingquan Fan1

1. Institute of Agricultural Resources and Regional Planning, Chinese Academy of Agricultural Sciences, Beijing 100081, China;
2. Key Laboratory of Urban Agriculture(North China) of Ministry of Agriculture, College of Biological Science and Engineering, Beijing University of Agriculture, Beijing 102206, China
Received: 31 March 2019; Revised: 6 June 2019; Published online: 12 November 2019
*Corresponding author: Zhao Fukuan, Tel/Fax:+86-10-82106212;
Fan Bingquan, E-mail:fanbingquan@caas.cn.
Foundation item: Supported by the 948 Agricultural Project (2011-G25, 2016-X21) and by the National High Technology Research and Development of China (2013AA102801-7, 2013AA102802-4)
Abstract: [Objective] To isolate antagonistic bacteria against Sclerotinia sclerotiorum diseases in sunflower. [Methods] Cellulose-degrading bacteria were isolated by CMC and wheat straw cellulose as sole carbon and energy source in mineral culture medium, then the ability of cellulose-degrading bacteria to suppress Sclerotinia sclerotiorum mycelium development was recorded under various conditions. The antagonistic spectrum of the isolate YC16 against pathogenic fungi was tested on PDYA petri-dishes, the suppressive ability of YC16 against Sclerotinia sclerotiorum was also observed using fresh detached leaves of sunflower and peat pot experiment. The effect of YC16 inoculation on plant growth promotion and Sclerotinia rot diseases prevention of sunflower was studied in pot and field experiment. [Results] YC16 was isolated and identified as Bacillus amyloliquefaciens. YC16 could suppress eight pathogenic fungi, including Sclerotium rolfsii, Fusarium solani, Fusarium oxysporum, Pyricularia oryzae, Phytophthora capsici, Fusarium sp., Fusarium oxysporum f. sp. cucumerinum Owen and Sclerotinia sclerotiorum. YC16 could inhibit S. sclerotiorum from infecting sunflower leaves by 80.42% and reduce the density of S. sclerotiorum mycelia by more than 50% on the surface of the Peat media compared with the control under pot condition. YC16 inoculation increased obviously the fresh biomass weight of sunflower by 54.9%. Under conventional chemical fertilizer application, YC16 inoculation increased sunflower yield by 24.4% to 30.2%, S. sclerotiorum diseases of sunflower was reduced by 39% to 100% through 3 year's field studies. [Conclusion] The isolate YC16 showed the potential for controlling sunflower Sclerotinia rot diseases and increasing sunflower yields as an efficient microbial resource for development of biocontrol agent.
Keywords: sunflowerSclerotinia sclerotiorumBacillus amyloliquefaciensbiocontrol effectyield increase
向日葵菌核病包括茎腐病和盘腐病,是当前威胁向日葵生产的主要病害。发病轻时减产10%–30%,严重时高达60%,甚至绝收[1-2]。长期以来,向日葵菌核病以化学防治为主,鉴于化学农药污染环境,生物防治正在成为向日葵菌核病的主要防治措施[3-6]。当前细菌防治菌核病的研究方兴未艾,集中于芽孢杆菌。其中,以枯草芽孢杆菌[7-8]、地衣芽孢杆菌[9-10]、蜡样芽孢杆菌[11]、短小芽孢杆菌[12]、巨大芽孢杆菌[13]、蜂房类芽孢杆菌[14]和解淀粉芽孢杆菌[15-16]为主。
目前,受菌核菌病严重危害的主要作物是油菜、大豆和向日葵,其他作物危害较小。芽孢杆菌类细菌多具有抑制菌核菌的菌核形成[9]、菌丝生长[17-18]、子囊孢子萌发[17]以及破坏菌丝结构[19]的能力,在作物菌核菌病害防治中扮演重要角色。
枯草芽孢杆菌Em7对苗期油菜菌核病防治效果达97.5%,降低田间油菜发病率50%–70%[17-19];枯草芽孢杆菌菌株NJ-18降低油菜茎腐病77.1%[20]、大田油菜接种NJ-18处理菌核病防治率57.4%[21];枯草芽孢杆菌菌株YS45对田间油菜茎腐病防治效果为50%[22]、菌株EDR2防治油菜苗期菌核病效果达80%以上[23]。解淀粉芽胞杆菌GM-1对盆栽油菜菌核病防治效果达68%[24]。接种解淀粉芽胞杆菌BS6,大田油菜茎腐病发病率下降为5.00%–29.55%[25]。芽孢杆菌A5F菌株能够抑制大豆菌核菌引起的茎腐病[26]。蜡样芽孢杆菌alf-87A菌株降低豌豆荚果基腐病39%–55%[27];蜡样芽孢杆菌SB24菌株减少了子囊孢子形成率90%以上,发病程度降低了45%–90%[28]。解淀粉芽孢杆菌PGPBacCA1对大豆菌核菌生长抑制率达76.5%[16]。利用芽孢杆菌防治向日葵菌核病的研究报道较少。枯草芽孢杆菌ZH-2菌剂防治向日葵菌核菌效率达72.4%[3];盆栽条件下,枯草芽孢杆菌S.16菌液对向日葵菌核病的防效达73.33%[29];解淀粉芽孢杆菌B14菌株与其他2株芽孢菌组合的3个菌群,能够将向日葵菌核菌发病率下降至25%–46%[5]。非芽孢细菌也有一些报道,此处不予赘述。
纤维素降解菌能够依靠自身产生的纤维素酶参与纤维素的降解,而且在防治作物菌核菌病害中发挥重要作用。报道显示,粘帚霉对菌核菌具有很强的抑制能力[30],枯草芽孢杆菌Z19抑制油菜菌核菌[31],哈兹木霉菌和康宁木霉菌抑制鹰嘴豆菌核菌[32],戴尔福特菌NF83-1抑制油菜菌核菌[33],甲基营养型芽孢杆菌wswshg-10抑制大豆菌核菌[34],体现了产纤维素酶微生物防治菌核菌病害的作用效果。但是,纤维素降解菌防治向日葵菌核菌病害的研究未见报道。
目前,关于解淀粉芽孢杆菌防治菌核菌病害的研究,无论是油菜、大豆、黄瓜、芥末菜、莴苣、麝香石竹还是向日葵,主要集中于菌株筛选、抑菌能力、防治效果,乃至抑菌机理研究[35]。显然,同时拥有防治菌核菌病害和提高向日葵产量能力的解淀粉芽孢杆菌菌株,鲜有报道。
本文旨在筛选既能高效防治向日葵菌核病、又能提高产量的芽孢杆菌菌株。通过对筛选到的拮抗菌株的抑菌能力、促生效果、提高产量的作用研究,明确其应用潜力,为研制高效防治向日葵菌核菌病害的生物菌剂提供菌种资源。
1 材料和方法 1.1 土壤来源 (1) 筛选菌株土壤样品采自吉林省长白山森林土、吉林省公主岭市农田土壤、黑龙江漠河县森林和麦田土壤,密封于塑料袋,带回实验室置于4 ℃保存;(2)盆栽供试土壤取自内蒙古自治区乌拉特前旗大佘太镇。
1.2 培养基 (1) 纤维素降解菌分离培养基:无机盐基础培养基,分别加入羧甲基纤维素钠(CMC)、1%球磨200目小麦秸秆纤维素粉末,pH 7.0[36]。(2)细菌保藏采用牛肉膏蛋白胨培养基(g/L):牛肉膏3,蛋白胨5,NaCl 5,水1000 mL,pH 7.0。(3) PDA培养基(g/L)用于筛选拮抗菌;(4) PDY培养基:PDA培养基中不加琼脂,加入酵母粉2 g/L。
1.3 供试菌种来源 地衣芽孢杆菌ACCC11106、侧孢芽孢杆菌ACCC11079、刺孢吸水链霉菌北京变种ACCC40033由中国农业科学院农业微生物菌种保藏管理中心提供。哈茨木霉ATCC20847(T22)、球状茎点霉ATCC MYA-2373购于美国ATCC菌种保藏中心;地衣芽孢杆菌DSM8785、丙酮丁醇梭菌DSM1732购于德国DSMZ菌种保藏中心。菌株Ymy3、JK22为苍白杆菌,CC1D、Y1为粪产碱菌,JQ-1、Y5、F2、C8为芽孢杆菌属,QU5、QQ6为黑曲霉,均为本课题组筛选保存菌株。向日葵菌核菌NQ-1为课题组采自内蒙古自治区乌拉特前旗向日葵菌核。
1.4 拮抗菌的筛选
1.4.1 系列稀释-平板法: 称取1.0 g新鲜土壤样品置于含有99 mL无菌水的200 mL三角瓶中,制备土壤悬液,系列稀释到10–4、10–5和10–6,土壤悬液分别涂布于含CMC、纤维素粉分别为唯一碳源的两种无机盐固体平板上,30 ℃培养2–3 d,待长出菌落后,挑取产生透明圈的单菌落,接种到含CMC的无机盐斜面上,用于后续拮抗菌的能力研究。
1.4.2 平板对峙筛选: 以向日葵菌核菌NQ-1作为病原菌,将菌核菌菌丝体接种于PDA平板中央,分离的纤维素降解菌接种于距病原菌两侧各2.5 cm的位置,28 ℃培养5 d观察并记录结果。挑取抑菌带宽>5.0 mm的菌落,进行平板对峙复筛,每株菌重复3次,抑菌效果稳定的拮抗菌株用于后续实验。
1.5 拮抗菌菌株分子鉴定 细菌16S rDNA扩增测序通用引物:正向引物16S rDNA:5′-AGAGTTTGATCCTGGCTCAGAA CGAACGCT-3′,反向引物16S rDNA:5′-TACGG CTACCTTGTTACGACTTCACCCC-3′。前后引物各1 μL,2×Taq PCR Master Mix (购自北京天根生化科技有限公司) 12.5 μL,ddH2O 8.5 μL,模板DNA 2 μL。反应条件:94 ℃ 4 min;94 ℃ 1 min,53 ℃ 40 s,72 ℃ 90 s,30个循环;72 ℃ 10 min。扩增产物进行1%琼脂糖凝胶电泳检测,序列测定由生工生物工程(上海)股份有限公司完成。利用BLAST软件与GenBank的序列进行同源性比较,使用MEGA 6.0的Neighbor-Joining软件构建系统发育树。
1.6 菌株YC16抑制病原菌种类与能力
1.6.1 菌株YC16抑菌谱: 以菌核菌NQ-1、齐整小核菌ACCC 37946、腐皮镰孢菌ACCC36241、尖孢镰刀菌ACCC 31357、稻梨孢ACCC 37631、辣椒疫霉ACCC 36278、镰刀菌ACCC 36242、尖镰孢黄瓜专化型ACCC 37438作为靶标菌,分别接种于PDA平板中央,菌株YC16接种于距病原菌两侧2.5 cm处,28 ℃培养5 d,每处理重复3次。
1.6.2 向日葵离体叶片实验: YC16菌株于LB液体培养基中摇床培养48 h,用无菌水把菌液稀释为1×109 CFU/mL。向日葵新鲜叶片表面灭菌,移液枪吸取1.0 mL YC16菌液涂布于叶片中央,菌核菌NQ-1接种方法见参考文献[37]。每个处理重复8次即处理8个叶片。
1.6.3 YC16抑制菌核菌的毒性效果试验: (1) 试验设计:①对照(CK),灭菌草炭,添加2.5 mL PDY培养基;② NQ-120:病原菌20 g/盆;③ NQ-140:病原菌40 g/盆;④ NQ-160:病原菌60 g/盆;⑤拮抗菌菌剂5 g/盆;⑥病原菌20 g/盆,YC16菌剂5 g/盆,混合均匀;⑦病原菌40 g/盆,YC16菌剂5 g/盆,混合均匀;⑧病原菌60 g/盆,YC16菌剂5 g/盆,混合均匀。2月26日布置实验,3月5日播种向日葵,每盆播种3粒向日葵种子S998,4月28日收获。(2)病原菌接种剂制备:从NQ-1菌丝体长满PDA平板上切取3方块(1 cm2)的菌丝体转入盛有50 g/瓶灭菌的麦麸-豆粕-稻壳粉组成的固体培养基的三角瓶中,搅拌均匀,共计10瓶,置于室温(19 ℃)培养10 d。(3) YC16菌剂制备:将YC16单菌落接种于PDY液体培养基中,摇床28 ℃、170 r/min培养3 d,灭菌草炭吸附,菌液与草炭用量比为1:1 (V/W)。(4)盆栽记录:病原菌接入草炭基质后的繁殖情况、死苗数量。
1.7 向日葵盆栽接种菌株YC16的促生效果 试验处理:(1)对照(CK):不接菌,使用与其他处理等量草炭加等量液体培养基;(2) YC16菌剂;(3) CC1D菌剂;(4) DSM8785菌剂;(5) Y5菌剂;(6) ACCC11106菌剂;(7) JK22菌剂;(8) F2菌剂;(9) C8菌剂;(10) Y1菌剂。菌剂使用量为2 g/盆土壤,每盆装土壤500 g,菌剂含菌量2×109 CFU/g。每个处理重复6次。试验在温室进行,向日葵品种为LD5009,播种种子2粒/盆。生长30 d收获植株,称量植株鲜重、记录发生茎腐病的株数。
1.8 田间条件下YC16菌剂的抗病与增产作用 3年田间试验都在内蒙古乌拉特前旗南昌新村的不同地块进行,氮磷钾复合肥(15:15:15)用量为600 kg/hm2,菌剂使用量900 kg/hm2,所有对照(CK)都使用等量草炭和等量液体培养基,与其他菌剂保持一致。所有肥料作为底肥一次施入,采用机械使用化肥和播种,同时覆盖地膜。
2014年田间试验土壤为轻度盐碱土,试验处理包括CK、YMA-2373、ATCC20847(T22)、Ymy3和YC16菌剂。向日葵品种为LD5009,小区面积9.9 m2,重复3次。
2015年田间试验土壤为中度盐碱土,试验处理包括CK、JQ-1、ACCC11079、QU5、YC16菌剂。向日葵品种为393,小区面积20.4 m2,重复3次。
2016年田间试验土壤为非盐碱土,试验处理包括CK、Y5、Y1、ACCC40033、QQ6、YC16,向日葵品种为JK601,小区面积为15 m2,重复3次。
生防菌剂制备。将菌株YC16、ACCC40033、YMA-2373、T22、Ymy3、JQ-1、ACCC11079、QU5、Y5、Y1、QQ6分别接种于LB液体培养基进行摇瓶培养,培养24 h。按1%接种量接入5 L发酵罐中,培养基为PDY,培养48 h,以灭菌草炭分别吸附上述菌液,细菌菌剂含菌量2×109 CFU/g,真菌菌剂含孢子量2×108 CFU/g。
2 结果和分析 2.1 拮抗菌株筛选与鉴定 从吉林省公主岭市农田土壤中分离并纯化获得一株高效拮抗向日葵菌核病的菌株YC16。经过提取YC16菌株DNA,利用16S rDNA通用引物进行PCR扩增,获得1600 bp的核苷酸序列,提交到GenBank (登录号为MK713569)。将扩增并验证得到的序列在GenBank上进行同源性比较,发现该序列与Bacillus amyloliquefaciens菌株的序列同源性 > 98%,利用MEGA 6.0的Neighbor-Joining软件进行系统发育树的构建(图 1),遗传距离显示菌株YC16与Bacillus amyloliquefaciens菌株的遗传距离最近。结合形态观察,参照《细菌鉴定手册》相关属,初步确定YC16菌株为解淀粉芽孢杆菌(Bacillus amyloliquefaciens)。
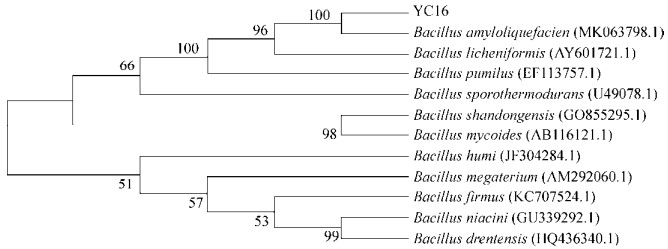 |
| 图 1 基于菌株YC16的16S rDNA序列同源性构建的系统发育树 Figure 1 Phylogenetic tree of YC16 and reference Bacillus species. Evolutionary distances showed in Figure 1 were calculated by MEGA 6.0; Bootstrap=1000. Bar, 0.05 substitution per nucleotide. Numbers in parentheses represent the sequences accession number in GenBank. The number at each branch points is the percentage supported by bootstrap. |
| 图选项 |
2.2 菌株YC16抑菌谱及其拮抗离体叶片感病能力
2.2.1 菌株YC16对不同病原菌的抑菌能力: 固体培养基平板上,菌株YC16的发酵菌液对齐整小核菌、腐皮镰孢菌、尖孢镰刀菌、稻梨孢、辣椒疫霉、镰刀菌、尖镰孢黄瓜专化型和向日葵菌核菌都有明显的抑制能力,表明菌株YC16具有广泛的抑菌谱(图 2)。
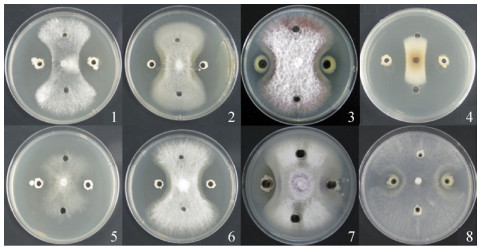 |
| 图 2 菌株YC16接种72 h对不同病原真菌的抑菌能力 Figure 2 Antagonistic ability of isolate YC16 against pathogenic fungus after 72 h. |
| 图选项 |
固体平板对峙法实验结果显示,菌株YC16对8种常见病原菌均有明显的抑制活性,抑菌率均在50%以上。其中,对腐皮镰孢菌、齐整小核菌、镰刀菌、向日葵菌核菌的抑菌效果较好,抑菌率均在60% (表 1)。
表 1. 菌株YC16对不同病原真菌的抑菌能力 Table 1. Antagonistic ability of isolate YC16 to pathogenic fungus
| No. | Pathogenic fungus | Inhibitary rate/% |
| 1 | Sclerotium rolfsii | 68.95±0.38a |
| 2 | Fusarium solani | 69.55±1.68a |
| 3 | Fusarium oxysporum | 54.18±1.79e |
| 4 | Pyricularia oryzae | 62.16±1.31c |
| 5 | Phytophthora capsici | 53.06±0.54e |
| 6 | Fusarium sp. | 66.97±0.63b |
| 7 | Fusarium oxysporum f. sp. cucumerinum Owen | 56.46±0.74d |
| 8 | Sclerotinia sclerotiorum | 62.14±0.86c |
| The different small letters in the same column indicate significant differences among treatments at P<0.05 level. | ||
表选项
2.2.2 菌株YC16拮抗菌核菌感染叶片的效果: 向日葵离体叶片实验结果显示,YC16菌液能够抑制菌核菌侵染叶片,表明菌液YC16对菌核菌侵染离体叶片具有防治效果(图 3),接种不足24 h时,CK处理的叶片已出现菌斑,24 h时菌斑侵染面积为12.23%,48 h已达到38.17%。涂抹YC16菌液的叶片于72 h时才出现菌斑,而此时CK叶片的侵染菌斑已达到64.87%,菌液YC16的抑菌率达到80.42%,具有良好的抑菌效果(表 2)。
 |
| 图 3 YC16接种96 h对抑菌菌核菌感染叶片的效果 Figure 3 Antagonistic effect of YC16 on S.sclerotiorum in detached leaves after 96 h. |
| 图选项 |
表 2. 菌株YC16拮抗菌核菌感病离体叶片的能力 Table 2. The inhibition ability of isolate YC16 against S. sclerotiorum in detached leaves
| Percent of disease area/% | Time point/h | ||
| 24 | 48 | 72 | |
| CK | 12.23 b | 38.17 b | 64.87 b |
| YC16 | 0 a | 0 a | 12.7 a |
| The different small letters in the same column indicate significant differences among treatments at P<0.05 level. | |||
表选项
2.2.3 YC16菌剂对菌核菌不同接种量和苗期向日葵生长的影响: 菌核菌NQ-1接入盆装草炭土基质后,繁殖能力表现差异。48 h时,加入60 g病原菌NQ-1的菌丝已然长满基质表层,同时接种菌核菌NQ-1菌剂60 g/盆和YC16菌剂5 g/盆的处理基质表层刚开始出现白色菌丝;72 h时,加入菌核菌NQ-1菌剂40 g/盆的菌丝已经长满草炭基质表层,同时接种菌核菌NQ-1菌剂40 g/盆和YC16菌剂5 g/盆的处理表层刚开始出现白色菌丝;96 h时,加入菌核菌NQ-1菌剂20 g/盆的菌丝长满基质表层,同时接种菌核菌NQ-1菌剂20 g/盆与YC16菌剂5 g/盆处理的草炭基质表层刚开始出现白色菌丝,并且基质表面菌丝密度比对照减少了50%以上。表明YC16菌剂能够抑制菌核菌NQ-1的生长。
向日葵种子播种于基质后,幼苗的生长情况不同但无死苗。生长至15 d可以看出,接种YC16菌剂处理的向日葵株高和叶片面积均大于只接种病原菌NQ-1的处理,表现出良好的生长趋势,并且同时接种40 g/盆菌核菌NQ-1与5 g/盆YC16菌剂的处理植株长势最好。表明YC16菌剂在抑制菌核菌的菌丝生长的同时,能够促进向日葵生长。
2.3 盆栽土壤接种YC16菌剂对向日葵幼苗的促生效果 土壤盆栽条件下,向日葵分别接种的10种生物菌剂,都能够提高向日葵的生物量(表 3)。Y1菌剂处理的生物量最高,达到了8.61 g/株,比对照提高54.9%;YC16菌剂处理的生物量次高,为8.58 g/株,略低于Y1菌剂0.03 g/株,比对照提高54.3%;JK22、F2、C8三种菌剂的生物量也比较高,分别为8.04 g/株、8.13 g/株和8.22 g/株,比对照分别增加44.6%、46.2%和47.8%;CC1D、DSM8785、Y5、ACCC11106四种菌剂的生物量比较低,分别比对照增加36.0%、38.8%、37.4%和43.7%。向日葵生长30 d收获,没有一株发生菌核菌引起的茎腐病。
表 3. 土壤盆栽条件下不同细菌菌剂对向日葵生物量的影响 Table 3. Effect of 9 bacterial inoculant on sunflower biomass in soil pot experiment
| Inoculant | Stem rot incidence/% | Biomass Wt/(g/plant) | Biomass increase/(g/plant) | Increase rate/% |
| CK | 0 | 5.56c | – | – |
| CC1D | 0 | 7.56b | 2.00 | 36.0 |
| DSM8785 | 0 | 7.72b | 2.16 | 38.8 |
| Y5 | 0 | 7.64b | 2.08 | 37.4 |
| ACCC11106 | 0 | 7.99ab | 2.43 | 43.7 |
| JK22 | 0 | 8.04ab | 2.48 | 44.6 |
| F2 | 0 | 8.13ab | 2.57 | 46.2 |
| C8 | 0 | 8.22ab | 2.66 | 47.8 |
| YC16 | 0 | 8.58a | 3.02 | 54.3 |
| Y1 | 0 | 8.61a | 3.05 | 54.9 |
| The different small letters in the same column indicate significant differences among treatments at P<0.05 level. | ||||
表选项
综上表明,10种不同菌剂中,YC16菌剂促进向日葵生长的效果显著,并且显著高于对照菌株DSM8785和ACCC11106,与Y1菌剂的生物量之间差异甚微。
2.4 田间向日葵接种YC16菌剂的抗病增产效果 2014年向日葵收获时,生物菌剂试验区内所有植株全部为黄绿色,没有一株病死。结果显示,YC16菌剂产量最高,为3838.6 kg/hm2,比对照增产28.8%;苍白杆菌Ymy3菌剂次之,产量为3333.5 kg/hm2,增产11.9%;T22菌剂的产量为3303.2 kg/hm2,增产10.8%;YMA-2373菌剂的产量为3131.5 kg/hm2,仅增产5.1% (表 4)。2014年内蒙古乌拉特前旗降水充沛,向日葵茎腐病、盘腐病、秆锈病严重,大面积绝收。试验区周围大田的向日葵除了地边少数存活之外,全部死亡于锈病和菌核菌病害。说明YC16菌剂在菌核菌病害严重的年份,能够显著防治菌核菌病害,同时提高向日葵产量。
表 4. YC16生物菌剂防治向日葵菌核菌病和提高产量的作用 Table 4. Effect of isolate YC16 on S. sclerotiorum disease incidence and sunflower yields
| Inoculant | S. sclerotiorum rot incidence/% | Puccinia helianthi Schw. rust incidence/% | Yield/(kg/hm2) | Yield increase/(kg/hm2) | Increase rate/% |
| CK | 0 | 0 | 2979.9c | – | – |
| YMA-2373 | 0 | 0 | 3131.5c | 151.6 | 5.1 |
| T22 | 0 | 0 | 3303.2b | 323.3 | 10.8 |
| Ymy3 | 0 | 0 | 3333.5b | 353.6 | 11.9 |
| YC16 | 0 | 0 | 3838.6a | 858.7 | 28.8 |
| The different small letters in the same column indicate significant differences among treatments at P<0.05 level. | |||||
表选项
2015年试验结果显示,YC16菌剂接种向日葵的产量最高,达到了3946.3 kg/hm2,比对照增产792.5 kg/hm2,增产率达24.4%;黑曲霉QU5菌剂的产量达3774.7 kg/hm2,增产620.9 kg/hm2,增产率为19.1%;ACCC11079菌剂的产量达3578.6 kg/hm2,增产13.1%;芽孢杆菌JQ-1菌剂的产量为3456.1 kg/hm2,仅增产9.3% (表 5)。上述结果说明,不发生严重菌核菌病害的年份,YC16菌株表现了显著提高产量的作用。
表 5. YC16生物菌剂防治向日葵菌核菌病和提高产量的作用 Table 5. Effect of isolate YC16 on S. sclerotiorum disease incidence and sunflower yields
| Inoculant | S. sclerotiorum rot incidence/% | Puccinia helianthi Schw. rust incidence/% | Yield/(kg/hm2) | Yield increase/(kg/hm2) | Increase rate/% |
| CK | 0 | 0 | 3153.8c | – | – |
| JQ-1 | 0 | 0 | 3456.1b | 302.3 | 9.3 |
| ACCC11079 | 0 | 0 | 3578.6b | 424.8 | 13.1 |
| QU5 | 0 | 0 | 3774.7a | 620.9 | 19.1 |
| YC16 | 0 | 0 | 3946.3a | 792.5 | 24.4 |
| The different small letters in the same column indicate significant differences among treatments at P<0.05 level. | |||||
表选项
2016年试验结果显示,生物菌剂作为底肥使用,可以防治茎腐病发生,盘腐病减轻,没有发生杆锈病,向日葵产量显著提高(表 6)。不接种菌剂的CK处理,盘腐病发病率为54%;接种YC16菌剂和Y1菌剂处理的盘腐病发病率仅为15%,比对照降低了39%;QQ6和40033菌剂处理的盘腐病发病率分别为34%和27%,比对照分别降低发病率20%和27%;Y5菌剂处理的盘腐病发病率为22%,比对照降低32%。
表 6. YC16生物菌剂防治向日葵菌核菌病和提高产量的作用 Table 6. Effect of isolate YC16 on S. sclerotiorum disease incidence and sunflower yields
| Inoculant | S. sclerotiorum rot incidence/% | Puccinia helianthi Schw. rust incidence/% | Yield/(kg/hm2) | Yield increase/(kg/hm2) | Increase rate/% |
| CK | 54a | 0 | 3366.3c | – | – |
| Y5 | 22cd | 0 | 3901.7b | 535.4 | 15.9 |
| Y1 | 15d | 0 | 3943.7b | 577.4 | 17.2 |
| 40033 | 27c | 0 | 4052.0ab | 685.7 | 20.4 |
| QQ6 | 34b | 0 | 4167.1a | 800.8 | 23.8 |
| YC16 | 15d | 0 | 4382.5a | 1016.2 | 30.2 |
| The different small letters in the same column indicate significant differences among treatments at P<0.05 level. | |||||
表选项
不接种菌剂的CK处理,向日葵产量最低,仅为3366.3 kg/hm2;接种YC16处理的产量为4382.5 kg/hm2,比对照增产30.2%;QQ6菌剂产量为4167.1 kg/hm2,增产23.8%;40033菌剂的产量为4052.0 kg/hm2,增产20.4%;Y5和Y1菌剂的产量分别为3901.7 kg/hm2和3943.7 kg/hm2,比对照分别增产15.9%和17.2%。以上结果说明,YC16生物菌剂既能够降低盘腐病发生程度,又能够提高向日葵产量。
3 讨论 解淀粉芽孢杆菌是一种广受青睐的芽孢类细菌。以往研究显示,所筛选的解淀粉芽孢杆菌中,拮抗油菜菌核菌3株[9, 25, 38]、拮抗黄瓜菌核菌2株[39-40]、防治大豆菌核菌茎腐病1株[41]、拮抗芥末菜菌核菌1株[15]、拮抗莴苣菌核菌腐烂病1株[42]、防治麝香石竹菌核菌茎腐病1株[43]、防治向日葵菌核菌1株[5]。菌株YC16是目前世界范围内的第一株既能抑制向日葵菌核菌病害、又能提高产量的解淀粉芽孢杆菌。
目前,国际上解淀粉芽孢杆菌抑制向日葵菌核菌病害的研究只有1篇报道。田间条件下,解淀粉芽孢杆菌B14能够抑制9种土著菌核菌的生长,抑病指数为60%–100%,向日葵发病率降低25%–46%[5]。
3年田间试验表明,YC16菌株能够提高向日葵产量24.4%–30.2%,菌核菌病害防治效果达39%–100%,年际间产量变化受降水量影响较大(表 4–6),截至目前,仅有一株解淀粉芽孢杆菌B14菌株进行了田间接种研究,能够降低向日葵菌核菌发病率,却没有显示提高产量的效果[5]。除此之外,解淀粉芽孢杆菌在田间其他作物上表现抑菌促生作用,解淀粉芽孢杆菌菌剂对黄瓜菌核菌抑制率为69.6%[39],菌株B3对油菜菌核病防治效果达55.8%,同时促进油菜生长[38]。
解淀粉芽孢杆菌YC16是一株拥有防治向日葵菌核病和提高产量的优良细菌菌株。今后,拟对其拮抗、促生和增产的机理,以及土壤定殖、菌剂类型、使用方式、保活材料和发酵工艺进行研究。为更好地发挥YC16菌株防治向日葵茎腐病、盘腐病和提高产量的效果提供科学依据。
References
| [1] | Dan JB, Kong DY, Liu SP, Gao FX, Yang S, Zhang J. Forecast for the occurrence of sunflower Sclerotinia sclerotiorum in Hetao irrigation district. Chinese Journal of Agrometeorology, 2012, 33(1): 142-147. (in Chinese) 淡建兵, 孔德胤, 刘双平, 高飞翔, 杨松, 张静. 河套灌区向日葵菌核病发生程度预测预报. 中国农业气象, 2012, 33(1): 142-147. DOI:10.3969/j.issn.1000-6362.2012.01.023 |
| [2] | Zhao WQ. The mechanism of disease occurrence and integrated control technologies against sunflower Sclerotinia sclerotiorum. Agriculture & Technology, 2017, 37(16): 58-59. (in Chinese) 赵伟权. 向日葵菌核病制病机理和综合防治技术. 农业与技术, 2017, 37(16): 58-59. |
| [3] | Zhang SM, Jiang TR, Wang YX, Zhao XY, Zhang XC, Li J. Initial report on prevention and control effect of sunflower Sclerotinia sclerotiorum by Bacillus subtilis. Modernizing Agriculture, 2008(11): 1-2. (in Chinese) 张淑梅, 姜天瑞, 王玉霞, 赵晓宇, 张先成, 李晶. 枯草芽孢杆菌防治向日葵菌核病效果初报. 现代化农业, 2008(11): 1-2. DOI:10.3969/j.issn.1001-0254.2008.11.007 |
| [4] | Zhang YM, Li XJ, Wang Y, Zhao J, Zhou HY. Bacterial strain S-16 suppressing sclerotial formation of Sclerotinia sclerotiorum. Chinese Journal of Biological Control, 2014, 30(1): 121-127. (in Chinese) 张一名, 李小娟, 王颖, 赵君, 周洪友. 抑制向日葵核盘菌菌核形成的生防菌S-16的鉴定及其生防作用研究. 中国生物防治学报, 2014, 30(1): 121-127. |
| [5] | Sabaté DC, Brandan CP, Petroselli G, Erra-Balsells R, Audisio MC. Biocontrol of Sclerotinia sclerotiorum (Lib.) de Bary on common bean by native lipopeptide-producer Bacillus strains. Microbiological Research, 2018, 211: 21-30. DOI:10.1016/j.micres.2018.04.003 |
| [6] | Smolińska U, Kowalska B. Biological control of the soil-borne fungal pathogen Sclerotinia sclerotiorum-a review. Journal of Plant Pathology, 2018, 100(1): 1-12. |
| [7] | Kamal MM, Lindbeck KD, Savocchia S, Ash GJ. Biological control of sclerotinia stem rot of canola using antagonistic bacteria. Plant Pathology, 2015, 64(6): 1375-1384. DOI:10.1111/ppa.12369 |
| [8] | Dai YL, Pan YM, Fan M, Gan L, Gao ZM. Condition optimization for antibiotic substances produced by Bacillus subtilis strain RSS-1. Chinese Agricultural Science Bulletin, 2018, 34(3): 51-57. (in Chinese) 代玉立, 潘月敏, 樊淼, 甘林, 高智谋. 枯草芽孢杆菌RSS-1菌株产生抗菌物质条件的优化. 中国农学通报, 2018, 34(3): 51-57. |
| [9] | Chen SY, Yang BY, Gao MY, Dai SY. Inhibition of sclerotia formation of Sclerotinia sclerotiorum by Bacillus amyloliquefaciens. Chinese Journal of Applied & Environmental Biology, 2005, 11(3): 373-376. (in Chinese) 陈士云, 杨宝玉, 高梅影, 戴顺英. 一株抑制油菜核盘菌菌核形成的解淀粉芽孢杆菌. 应用与环境生物学报, 2005, 11(3): 373-376. DOI:10.3321/j.issn:1006-687X.2005.03.027 |
| [10] | Nigris S, Baldan E, Tondello A, Zanella F, Vitulo N, Favaro G, Guidolin V, Bordin N, Telatin A, Barizza E, Marcato S, Zottini M, Squartini A, Valle G, Baldan B. Biocontrol traits of Bacillus licheniformis GL174, a culturable endophyte of Vitis vinifera cv. Glera. BMC Microbiology, 2018, 18(1): 133. |
| [11] | Kamal MM, Savocchia S, Lindbeck KD, Ash GJ. Biology and biocontrol of Sclerotinia sclerotiorum (Lib.) de Bary in oilseed Brassicas. Australasian Plant Pathology, 2016, 45(1): 1-14. |
| [12] | Kaushal M, Kumar A, Kaushal R. Bacillus pumilus strain YSPMK11 as plant growth promoter and bicontrol agent against Sclerotinia sclerotiorum. 3 Biotech, 2017, 7(2): 90. DOI:10.1007/s13205-017-0732-7 |
| [13] | Hu XJ, Roberts DP, Xie LH, Maul JE, Yu CB, Li YS, Zhang SJ, Liao X. Bacillus megaterium A6 suppresses Sclerotinia sclerotiorum on oilseed rape in the field and promotes oilseed rape growth. Crop Protection, 2013, 52: 151-158. DOI:10.1016/j.cropro.2013.05.018 |
| [14] | Fatouros G, Gkizi D, Fragkogeorgi GA, Paplomatas EJ, Tjamos SE. Biological control of Pythium, Rhizoctonia and Sclerotinia in lettuce:association of the plant protective activity of the bacterium Paenibacillus alvei K165 with the induction of systemic resistance. Plant Pathology, 2018, 67(2): 418-425. DOI:10.1111/ppa.12747 |
| [15] | Rahman ME, Hossain DM, Suzuki K, Shiiya A, Suzuki K, Dey TK, Nonaka M, Harada N. Suppressive effects of Bacillus spp. on mycelia, apothecia and sclerotia formation of Sclerotinia sclerotiorum and potential as biological control of white mold on mustard. Australasian Plant Pathology, 2016, 45(1): 103-117. |
| [16] | Torres MJ, Brandan CP, Sabaté DC, Petroselli G, Erra-Balsells R, Audisio MC. Biological activity of the lipopeptide-producing Bacillus amyloliquefaciens PGPBacCA1 on common bean Phaseolus vulgaris L. pathogens. Biological Control, 2017, 105: 93-99. DOI:10.1016/j.biocontrol.2016.12.001 |
| [17] | Gao XN, Han QM, Chen YF, Qin HQ, Huang LL, Kang ZS. Biological control of oilseed rape Sclerotinia stem rot by Bacillus subtilis strain Em7. Biocontrol Science and Technology, 2013, 24(1): 39-52. |
| [18] | Min J, Huang LN, Lu JY, Xiang N, Xiao YN. Antagonistic study of Bacillus amyloliquefaciens Ba301 against Sclerotinia sclerotiorum. Guangxi Plant Protection, 2017, 30(3): 21-22. (in Chinese) 闵杰, 黄丽娜, 卢金应, 向妮, 肖炎农. 解淀粉芽孢杆菌Ba301对核盘菌的拮抗研究. 广西植保, 2017, 30(3): 21-22. DOI:10.3969/j.issn.1003-8779.2017.03.006 |
| [19] | Gao XN, Chen JY, Huang LL, Qiao HP, Han QM, Kang ZS. Screening of antagonistic endophytic bacteria and their roles in control of Sclerotinia sclerotiorum in canola. Chinese Journal of Pesticide Science, 2010, 12(2): 161-167. (in Chinese) 高小宁, 陈金艳, 黄丽丽, 乔宏萍, 韩青梅, 康振生. 油菜菌核病内生拮抗细菌的筛选及防病作用研究. 农药学学报, 2010, 12(2): 161-167. DOI:10.3969/j.issn.1008-7303.2010.02.08 |
| [20] | Yang DJ, Wang B, Wang JX, Chen Y, Zhou MG. Activity and efficacy of Bacillus subtilis strain NJ-18 against rice sheath blight and Sclerotinia stem rot of rape. Biological Control, 2009, 51(1): 61-65. |
| [21] | Hou YP, Zhang SP, Wang JX, Chen CJ, Zhou MG. Biological control of rapeseed sclerotinia stem rot caused by Sclerotinia sclerotiorum with Bacillus subtilis NJ-18 and its colonization dynamics on the plant. Acta Phytopathologica Sinica, 2013, 43(4): 411-417. (in Chinese) 侯毅平, 章四平, 王建新, 陈长军, 周明国. 枯草芽胞杆菌NJ-18对油菜菌核病的防治效果及其定殖动态. 植物病理学报, 2013, 43(4): 411-417. |
| [22] | Zhang Y, Bai C, Ran GH, Zhang ZY, Chen YH, Wu G. Characterization of endophytic bacterial strain YS45 from the citrus xylem and its biocontrol activity against Sclerotinia stem rot of rapeseed. Acta Phytopathologica Sinica, 2009, 39(6): 638-645. (in Chinese) 张翼, 白晨, 冉国华, 张志元, 陈耀辉, 吴刚. 柑橘内生细菌YS45的鉴定、抗菌物质分析及其对油菜菌核病的防治作用. 植物病理学报, 2009, 39(6): 638-645. DOI:10.3321/j.issn:0412-0914.2009.06.011 |
| [23] | Gao XN, Chen YF, Han QM, Qin HQ, Kang ZS, Huang LL. Identification and biocontrol efficacy of endophytic bacterium EDR2 against Sclerotinia sclerotiorum. Journal of Northwest A & F University (Natural Science Edition), 2013, 41(2): 175-181, 188. (in Chinese) 高小宁, 陈亚菲, 韩青梅, 秦虎强, 康振生, 黄丽丽. 内生细菌EDR2的鉴定及其对油菜菌核病的防治. 西北农林科技大学学报(自然科学版), 2013, 41(2): 175-181, 188. |
| [24] | Ge PH, Ma GZ, Fu HR, Bao ZH, Wang SF, Liu ZP. Identification and inhibitory effects of the marine bacterial strain GM-1 antagonistic to Sclerotinia sclerotiorum. Plant Protection, 2013, 39(2): 50-56. (in Chinese) 葛平华, 马桂珍, 付泓润, 暴增海, 王淑芳, 刘兆普. 油菜菌核病菌拮抗海洋细菌GM-1菌株的种类鉴定及抑菌作用研究. 植物保护, 2013, 39(2): 50-56. DOI:10.3969/j.issn.0529-1542.2013.02.010 |
| [25] | Fernando WGD, Nakkeeran S, Zhang Y, Savchuk S. Biological control of Sclerotinia sclerotiorum (Lib.) de Bary by Pseudomonas and Bacillus species on canola petals. Crop Protection, 2007, 26(2): 100-107. DOI:10.1016/j.cropro.2006.04.007 |
| [26] | Kumar A, Saini S, Wray V, Nimtz M, Prakash A, Johri BN. Characterization of an antifungal compound produced by Bacillus sp. strain A5F that inhibits Sclerotinia sclerotiorum. Journal of Basic Microbiology, 2012, 52(6): 670-678. DOI:10.1002/jobm.201100463 |
| [27] | Huang HC, Kokko EG, Yanke LJ, Phillippe RC. Bacterial suppression of basal pod rot and end rot of dry peas caused by Sclerotinia sclerotiorum. Canadian Journal of Microbiology, 1993, 39(2): 227-233. |
| [28] | Zhang JX, Xue AG. Biocontrol of sclerotinia stem rot (Sclerotinia sclerotiorum) of soybean using novel Bacillus subtilis strain SB24 under control conditions. Plant Pathology, 2010, 59(2): 382-391. DOI:10.1111/j.1365-3059.2009.02227.x |
| [29] | Zhang YM, Zhen X, Shi LJ, Zhou HY. Colonization of Bacillus subtilis S-16 and its control effect on Sclerotinia sclerotiorum. Journal of Hebei Agricultural Sciences, 2012, 16(1): 36-38, 75. (in Chinese) 张一名, 甄熙, 石林君, 周洪友. 生防菌S-16的定殖动态以及对向日葵菌核病的生防效果研究. 河北农业科学, 2012, 16(1): 36-38, 75. DOI:10.3969/j.issn.1088-1631.2012.01.012 |
| [30] | Ma GZ, Gao HL, Zhang YH, Li SD, Xie BY. Studies on the cell wall degrading enzymes during the mycoparasitism of Gliocladium spp. isolates with the sclerotia of Sclerotinia sclerotiorum. Journal of Jilin Agricultural University, 2007, 29(6): 628-632. (in Chinese) 马桂珍, 高会兰, 张拥华, 李世东, 谢丙炎. 粘帚霉对核盘菌菌核的寄生作用及其细胞壁降解酶活性分析. 吉林农业大学学报, 2007, 29(6): 628-632. DOI:10.3969/j.issn.1000-5684.2007.06.009 |
| [31] | Yang CD, Li ZD, Chen XR, Xu CL, Xue L. Identification, pathogen inhibiting and nitrogen fixation of endophytic bacterium Z19 of Polygonum viviparum. Microbiology China, 2014, 41(2): 267-273. (in Chinese) 杨成德, 李振东, 陈秀蓉, 徐长林, 薛莉. 高寒草地珠芽蓼内生拮抗固氮菌Z19的鉴定及其固氮功能. 微生物学通报, 2014, 41(2): 267-273. |
| [32] | Saxena A, Raghuwanshi R, Singh HB. Trichoderma species mediated differential tolerance against biotic stress of phytopathogens in Cicer arietinum L.. Journal of Basic Microbiology, 2015, 55(2): 195-206. DOI:10.1002/jobm.201400317 |
| [33] | Liang XJ, Guo DH, Liu YZ, Tang YQ, Qiao JQ, Du Y. Identification and evaluation of Delftia tsuruhatensis strain NF83-1. Journal of Plant Protection, 2016, 43(2): 248-254. (in Chinese) 梁雪杰, 郭殿豪, 刘邮洲, 唐永清, 乔俊卿, 杜艳. 一株戴尔福特菌NF83-1的鉴定及评价. 植物保护学报, 2016, 43(2): 248-254. |
| [34] | Wang YX, Jiang W, Liu YS, Meng LQ, Li J, Cao X, Hu JH, Chen JY, Zhang SM. Study on isolation and characterization of a psychrotrophic antagonistic bacterium from cold area. Journal of Northeast Agricultural University, 2016, 47(8): 31-38. (in Chinese) 王玉霞, 姜威, 刘宇帅, 孟利强, 李晶, 曹旭, 胡基华, 陈静宇, 张淑梅. 寒地耐冷生防菌株筛选鉴定及特性研究. 东北农业大学学报, 2016, 47(8): 31-38. DOI:10.3969/j.issn.1005-9369.2016.08.004 |
| [35] | Fira D, Dimki? I, Beri? T, Lozo J, Stankovi? S. Biological control of plant pathogens by Bacillus species. Journal of Biotechnology, 2018, 285: 44-55. DOI:10.1016/j.jbiotec.2018.07.044 |
| [36] | Yin ZW, Fan BQ, Ren P. Isolation and identification of a cellulose degrading fungus Y5 and its capability of degradating wheat straw. Chinese Journal of Environmental Science, 2011, 32(1): 247-252. (in Chinese) 殷中伟, 范丙全, 任萍. 纤维素降解真菌Y5的筛选及其对小麦秸秆降解效果. 环境科学, 2011, 32(1): 247-252. |
| [37] | Hu BC, Rimmer SR. Preliminary study of artificial inoculation for resistance (tolerance) to Sclerotinia sclerotiorum in rapeseed using detached leaves. Journal of Anhui Agricultural Sciences, 1989(3): 56-58. (in Chinese) 胡宝成, Rimmer SR. 油菜菌核病离体叶片接种法研究初报. 安徽农业科学, 1989(3): 56-58. |
| [38] | Wu HJ, Wang S, Zhan J, Ma LL, Gao XW. Biocontrol effect of lipopeptide compands produced by Bacillus spp. against rape Sclerotinia disease. Journal of Northeast Agricultural University, 2012, 43(7): 84-88. (in Chinese) 伍辉军, 王帅, 湛江, 马玲莉, 高学文. 芽胞杆菌产生的脂肽类化合物在防治油菜菌核病中的作用. 东北农业大学学报, 2012, 43(7): 84-88. |
| [39] | Rostami S, Maleki M, Shahriari D. The use of bacillus amyloliquefaciens to control of sclerotinia stem rot (Sclerotinia sclerotiorum) of cucumber. International Journal of Farming and Allied Sciences, 2013, 2(22): 965-970. |
| [40] | Kim BY, Lee SY, Ahn JH, Song J, Kim WG, Weon HY. Complete genome sequence of Bacillus amyloliquefaciens subsp. plantarum CC178, a phyllosphere bacterium antagonistic to plant pathogenic fungi. Genome Announcements, 2015, 3(1): e01368-14. |
| [41] | Alvarez F, Castro M, Príncipe A, Borioli G, Fischer S, Mori G, Jofré E. The plant-associated Bacillus amyloliquefaciens strains MEP218 and ARP23 capable of producing the cyclic lipopeptides iturin or surfactin and fengycin are effective in biocontrol of sclerotinia stem rot disease. Journal of Applied Microbiology, 2012, 112(1): 159-174. DOI:10.1111/j.1365-2672.2011.05182.x |
| [42] | Lee SY, Weon HY, Kim WG, Kim JJ, Han JH. Selection of Bacillus amyloliquefaciens M27 for biocontrol on lettuce sclerotinia rot. The Korean Journal of Mycology, 2015, 43(3): 180-184. |
| [43] | Selvaraj V, Sevugapper N. Synergistic action of anti-microbial peptide (AMP) genes in Bacillus amyloliquefaciens for the anagement of stem rot of carnation. Journal of Mycology and Plant Pathology, 2015, 45(4): 330-335. |
