王雨霏1,2, 陈相书1, 张凌琳1,2


1. 四川大学口腔疾病研究国家重点实验室, 国家口腔疾病临床医学研究中心, 四川 成都 610041;
2. 四川大学华西口腔医院牙体牙髓病科, 四川 成都 610041
收稿日期:2018-09-23;修回日期:2019-01-02;网络出版日期:2019-01-09
基金项目:四川大学大学生创新创业训练计划项目(2018101581,2018101547);国家自然科学基金(81771062)
*通信作者:张凌琳, Tel:+86-28-85503470, E-mail:zhll_sc@163.com.
摘要:细菌对传统抗生素的耐药程度十分严重,寻找克服耐药性的新型抗菌药物已成为当务之急。抗菌肽(antimicrobial peptides,AMPs)是当下较有前景的抗菌药物之一。虽然通常认为,AMPs优先攻击细胞膜的特点使其不会引起广泛的耐药性,但其对特定靶标的识别能力仍为基因突变和细菌耐药性的产生提供了可能。此外,一些细菌还显示出了抵御宿主AMPs的杀伤作用并与宿主细胞共存的能力,相应的细菌防御机制也使其对治疗性AMPs产生抗性,这种交叉抗性近年来也备受关注。这些耐药现象的发现均对AMPs的开发提出了新挑战。本综述就细菌对AMPs耐药的分子机制进行了研究进展的总结,并且对治疗性AMPs与宿主防御肽交叉抗性的相关机制研究进行了归纳,以期寻求新的对抗耐药性的策略。
关键词:交叉耐药性宿主防御肽细胞膜荚膜磷壁酸脂多糖
Advances in studying bacterial resistance to antimicrobial peptides
Yufei Wang1,2, Xiangshu Chen1, Linglin Zhang1,2


1. State Key Laboratory of Oral Diseases & National Clinical Research Centre for Oral Disease, Sichuan University, Chengdu 610041, Sichuan Province, China;
2. Department of Cariology and Endodontics, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, Sichuan Province, China
Received: 23 September 2018; Revised: 2 January 2019; Published online: 9 January 2019
*Corresponding author: Linglin Zhang, Tel:+86-28-85503470, E-mail:zhll_sc@163.com.
Foundation item: Supported by the National Innovation and Entrepreneurship Training Project of Sichuan University (2018101581, 2018101547) and by the National Natural Science Foundation of China (81771062)
Abstract: Bacteria have developed severe resistance to traditional antibiotics, demanding novel antimicrobial agents to overcome resistance. Antimicrobial peptides are promising novel antimicrobial agents. Owing to preferential attack on the cell membrane, antimicrobial peptides will not lead to widespread resistance, although their recognition of specific targets might cause genetic mutations and resistance of the targets. Additionally, some bacteria can overcome endogenous antimicrobial peptides and survive with the host cells. The corresponding defense mechanisms of bacteria also bring cross-resistance to therapeutic antimicrobial peptides. The resistance mentioned above poses a challenge to the development of antimicrobial peptides. In this review, we summarize the research progress in studying the mechanisms of antimicrobial peptides resistance, to provide the reference for developing strategies to fight bacterial resistance.
Keywords: cross-resistancehost defense peptidecell membranecapsuleteichoic acidlipopolysaccharide
细菌对传统抗生素的耐药程度十分严重,可能令抗感染治疗重新成为棘手问题。据美国疾病控制中心(American Centers for Disease Control)的最新报告估计,耐药微生物在2013年导致了美国200多万人患病和23000人死亡[1]。因此,寻找克服耐药性的新型抗菌药物已成为当务之急。抗菌肽(antimicrobial peptides,AMPs)是当下较有前景的抗菌药物之一。AMPs是两亲性多肽,通常带正电荷,从细菌到人类的各种各样的生物均可产生,按来源可分为内源性AMPs和人工设计合成AMPs。人的体内源性AMPs,如α-防御素(α-defensins,HNP)、β-防御素(β-defensins,HBD)、LL-37等,具有广谱的抗菌能力和系列免疫调节功能,在机体内环境稳态的维持中发挥重要作用[2]。人工设计合成的AMPs如万古霉素、多粘菌素、OP-145、GH12等,则被期望发挥治疗性的功效,处于实验室研究至临床应用各阶段[3-5]。AMPs的作用范围很广,通常对细菌、真菌和原生动物都有活性。它们通过不同的作用机制来发挥抗菌作用,包括破坏细胞膜的完整性,干扰核酸功能,影响蛋白质合成,调节相关酶活性[3, 6-7]等。
虽然人们通常认为,AMPs优先攻击细胞膜的特点使其不会引起广泛的耐药性,但其对特定靶标的识别能力仍为基因突变和细菌耐药性的产生提供了可能。随着研究的推进,细菌对AMPs耐药机制的认识正被逐步加深。细菌对AMPs具有固有(intrinsic)耐药和获得性(acquired)耐药两大类机制[8]:固有耐药机制是细菌与生俱来的一些耐药特性,该特性可使细菌具有广泛的抵御抗菌药物的能力;获得性耐药机制则是指原本对抗菌药物敏感的细菌通过获取耐药基因或自发产生耐药突变的方式来产生耐药性。这些耐药机制间并没有严格界线,单个菌种可同时具有以上两种耐药机制。近年来,细菌赖以产生耐药性的截留作用及细胞表面改性机制被肯定,该分子机制在后续研究中被不断更新并被加以更全面的探讨。然而又有近期研究发现,寄生在宿主体内的细菌和一些入侵的细菌显然有能力战胜宿主的AMPs并与宿主细胞共存,相应的细菌防御机制也让细菌对治疗性AMPs产生了抵抗能力。这种交叉抗性的出现使其成为了AMPs耐药方面新的研究热点。因此,更好地了解这些耐药机制对于寻求新的对抗耐药性的策略是至关重要的,本文即就近年来细菌对AMPs耐药分子机制的更新及交叉抗性相关机制的进展作一综述。
1 AMPs耐药的分子机制 针对AMPs的多肽本质及杀菌机制,与AMPs相互作用的细菌会在转录和翻译层面利用多种分子机制抵御AMPs的攻击(表 1)。这些细菌可利用其分泌的多种蛋白酶以及细胞外结构对AMPs进行抵御,同时还能适应性地对细胞表面结构进行改性,从而阻碍AMPs对细菌的静电吸附与打孔。
表 1. 抗菌肽耐药的分子机制 Table 1. Molecular mechanisms of bacteria resistance to antimicrobial peptides
| Mechanisms | AMPs | Bacteria | Structure/protein | References | |
| Extracellular mechanisms | Entrapment by surface proteins | HNP 1-3 | S. aureus | Staphylokinase | [9] |
| LL-37 | S. pyogenes | Streptococcal inhibitor of complement | [10] | ||
| Entrapment by surface polysaccharides | GH12 | S. mutans | Extracellular matrix | [3] | |
| Proteolytic processing of AMPs | HBD-1 | P. mirabilis | Metalloprotease | [11] | |
| LL-37 | |||||
| LL-37 | S. aureus | Aureolysin proteases | [12] | ||
| LL-37 | S. pyogenes | Cysteine protease | [13] | ||
| LL-37 | enterohemorrhagic E. coli | Outer membrane protease T | [14] | ||
| LL-37 | group A hemolytic Streptococcus | Plasminogen-activating streptokinase | [15] | ||
| Modifications of cell surface and membrane | Inhibition of capsular polysaccharides | HBD | K. pneumoniae | Capsule | [16] |
| HBD-3 | S. mitis | Capsule | [17] | ||
| LL-37 | |||||
| HNP-1 | F. nucleatum | Capsule and biofilms | [18] | ||
| Aminoacylation of teichoic acids | LL-37 | group B Streptococcus | Teichoic acids | [19] | |
| Polymyxin B | |||||
| Magainin 2 | |||||
| HBD | S. aureus | Teichoic acids | [20] | ||
| Lysostaphinn | S. aureus | Teichoic acids | [21] | ||
| LL-37 | S. aureus | Teichoic acids | [4] | ||
| PBP 10 | |||||
| Melittin | |||||
| OP-145 | |||||
| LL-37 | group B Streptococcus | ||||
| Magainin 2 | |||||
| Polymyxin B | |||||
| Modification of LPS | Polymyxin B | P. gingivali | LPS | [22] | |
| Polymyxin B | V. cholera | Lipid A | [23] | ||
| Polymyxin B | V. cholera | LPS | [24] | ||
| Polymyxin B | S. typhimurium | LPS | [5] | ||
| Polymyxin | E. coli | LPS | [25-26] | ||
| Aminoacylation of phosphatidylglycerol | Daptomycin | S. aureus | Phosphatidylglycerol | [27] | |
表选项
1.1 AMPs耐药的细胞外机制
1.1.1 表面蛋白和多糖的截留作用: 有些病原体可以产生AMPs结合蛋白,这些蛋白可通过与AMPs结合来阻止AMPs与细胞的接触。这种蛋白的典型代表就是金黄色葡萄球菌(Staphylococcus aureus)产生的葡萄球菌激酶(staphylokinase)。这种酶可以与HNP-1-3形成复合体,使后者的抗菌活性下降80%以上[9]。链球菌补体抑制剂(streptococcal inhibitor of complement, SIC)可直接与AMPs结合,使其失活[28]。
由大量胞外多糖和DNA组成的胞外基质通常会紧紧包裹细菌,以阻断AMP和细菌胞膜表面的直接接触。这些胞外基质会对阳离子AMPs产生静电排斥或拦截作用,以阻止它们和细菌接触,因此往往需要使用更高的药物浓度才能对生物膜产生一定的抑制作用。Wang等[3]发现变异链球菌在形成生物膜后对合成AMPs GH12的抵抗能力显著上升,其最低杀菌浓度由浮游菌形式下的8 mg/L激增至256 mg/L,即其抵抗能力因生物膜的存在提高了数百倍。
1.1.2 蛋白酶对AMPs的水解作用: 一些具有广泛底物特异性的蛋白酶仍可有效地裂解和灭活AMPs。研究较多的这类蛋白酶包括在该类研究的最早期发现的由S. aureus释放的金属蛋白酶(aureolysin)[12],由奇异变形杆菌(Proteus mirabilis)释放的金属蛋白酶ZapA[11],以及由S. pyogenes释放的广谱的半胱氨酸蛋白酶SpeB[13]。最近的研究发现由A组溶血性链球菌(group A Streptococcus,GAS)分泌的原质活化链激酶(plasminogen-activating streptokinase)可导致细胞表面质粒活性积累,从而使LL-37降解[15]。另外,肠出血性大肠埃希菌(enterohemorrhagic Escherichia coli)高表达的外膜蛋白酶OmpT亦可高效降解LL-37[14]。
1.2 AMPs耐药的细胞表面机制
1.2.1 荚膜多糖阻挡作用的利用: 有些细菌可表达由高分子量多糖组成的荚膜,阻碍AMPs和细菌表面的接触。肺炎克雷伯菌(Klebsiella pneumoniae)的荚膜阻止了TLR2和TLR4受体的联系和下游核因子-κB通路(nuclear factor-κB,NF-κb)、促分裂素原活化蛋白激酶通路(mitogen-activated protein kinases,MAPK)的激活,从而抑制HBD的表达[16]。近几年来,基于该机制的耐药情形被发现于更多的细菌中,如轻型链球菌(Streptococcus mitis)也可利用荚膜,对HBD-3和LL-37产生耐受[17]。相似地,还有新近报道称具核梭杆菌(Fusobacterium nucleatum)亦可通过降低膜通透性、加速增殖和菌膜形成来产生对人体内HNP-1的耐药性[18]。
1.2.2 磷壁酸的氨酰化: 带正电荷的AMPs可以被细胞膜或细胞壁上的负电荷吸引,但是这个过程反过来又降低了细菌的表面电荷,增加了表面密度,限制了多肽的吸附作用。在革兰阳性菌中,若将带正电荷的分子转移到细胞壁上的磷壁酸(teichoic acids, TA)上,细菌便会产生对AMPs的抵抗性[21]。这种修饰主要是指D-丙氨酸对TA原有结构的取代。由单一启动子编码的4个蛋白对D-丙氨酰化过程是必要的。由dltA编码的D-丙氨酰载体蛋白连接酶(D-alanyl carrier protein ligase,DCL)首先使用ATP活化D-丙氨酸,在dltD编码的DltD蛋白的帮助下,活化复合体被传递到由dltC编码的D-丙氨酸载体蛋白(D-alanine carrier protein,DCP)上。由dltB编码的DltB是一种跨膜蛋白,参与D-alanyl-DCP复合体的跨膜转运,在此处,D-氨基酸被转移到细菌胞膜的磷脂骨架上。
D-丙氨酸带正电的自由氨基可部分影响TA的净负电荷,取代后的D-丙氨酰脂结构调节了TA的功能,显著增加了革兰阳性菌对AMPs的耐药性,如S. aureus即通过磷壁酸的D-丙氨酰化冲破了皮肤屏障的保护作用[20]。此后,Malanovic等[4]也对PBP 10、LL-37、蜂毒肽和OP-145等AMPs被这种机制阻挡的情况进行了详细的综述。而不能进行磷壁酸D-丙氨酰化的Δdlt缺陷株会对阳离子AMPs相当敏感。另一项类似的研究也发现,敲除了dltA的B组溶血性链球菌较野生株脆弱得多[19]。另外,D-丙氨酰化引起的肽聚糖球囊密度的增加也会在空间上阻碍AMPs进入胞膜,从而增强其对AMPs的抗性[19]。
1.2.3 脂多糖的修饰: 相似的中和过程也可发生在革兰阴性菌中。脂多糖(lipopolysaccharide, LPS)位于革兰阴性菌的外膜。LPS的脂质A成分带负电荷,它由2个葡萄糖胺基组成,其游离的磷酸基可以与4个或更多的酰基链相连。增加4-氨基-4脱氧阿拉伯糖(4-amino-4-deoxy-L-arabinose,L-Ara4N)或磷酸乙醇胺(phosphoethanolamine, pEtN)可调节脂多糖的结构,从而中和细菌表面的负电荷,这种调节作用被认为是P. gingivalis产生耐药性的重要机制[22]。而在较近的研究中,又一则基于该种调节作用的细菌耐药情况被报道,研究者发现鼠伤寒沙门菌(Salmonella typhimurium)同样能通过该脂多糖的调节对一系列AMPs产生耐药[5]。LPS的酰化作用也可降低细菌表面的负电荷。Matson等[23]发现,霍乱弧菌(Vibrio cholerae)的msbB基因对脂质A的全酰化是必需的,并且严重影响了细菌对AMPs的抗药性。后续的研究表明V. cholerae的另一组基因,almEFG启动子编码了LPS的糖基化作用,引发了细菌对多粘菌素(polymyxins)的耐药性[24]。更值得关注的是,中国科学家于2016年正式报道了多粘菌素耐药基因mcr-1,mcr-1可编码磷酸乙醇胺转移酶,因其存在于质粒pHNSHP45,是已知的第一个能够在不同菌株间水平转移的多粘菌素抗性基因[25]。Gao等[26]从E. coli多重耐药菌株中分离获得具有多粘菌素抗性的菌株WH12,并成功提取到其携带的mcr-1质粒pWH12,pWH12的二代测序结果分析发现其来源于质粒pHNSHP45,但发生了进化和变异,证明了mcr-1质粒存在多样性。
1.2.4 磷脂酰甘油的氨酰化: 细菌细胞膜的主要成分,磷脂酰甘油(phosphatidylglycerol, PG)也可以被赖氨酸或精氨酸修饰。赖氨酰磷脂酰甘油(lysyl-PG)依靠MprF蛋白(multiple peptide resistance factor,MprF)合成。MprF在革兰阴性菌和革兰阳性菌中都高度保守,是一个97 kDa的跨膜蛋白,并包含两个功能域。C末端结构域负责在生物膜的内侧,以PG和赖氨酰-tRNA为底物,合成赖氨酰磷脂酰甘油,而N末端,即翻转酶功能域,则负责将赖氨酰磷脂酰甘油转移至外膜[29]。增加的lysyl-PG和MprF的获得性点突变被认为与达托霉素(daptomycin,DAP)的体内外抗性密切相关[27]。多种细菌都可通过PG的氨酰化作用发挥对AMPs的抵抗作用[30],包括产气荚膜梭菌(Clostridium perfringens)、粪肠球菌(Enterococcus faecalis)、铜绿假单胞菌(P. aeruginosa)、结核分枝杆菌(Mycobacterium tuberculosis)、炭疽杆菌(Bacillus anthracis)、枯草芽胞杆菌(Bacillus subtilis)和屎肠球菌(Enterococcus faecium)。
综上,如图 1所总结的,特定的细菌可在AMPs进入细胞前,利用细胞外的蛋白及多糖对其进行截留,或分泌蛋白酶降解胞外基质中的AMPs;当AMPs逐步接近细菌时,特定细菌又可利用荚膜阻隔其与细菌内膜的接触;而当AMPs成功到达其作用的主要目标——细胞膜时,细菌又可利用多种细胞表面改性来减少AMPs与细菌胞膜的静电吸附。
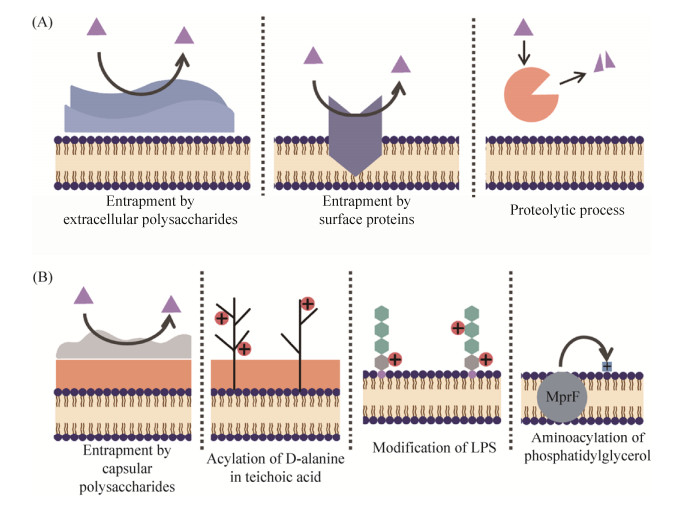 |
| 图 1 细菌对抗菌肽的耐药分子机制 Figure 1 Molecular mechanisms of bacterial resistance to antimicrobial peptides. A: Extracellular mechanisms; B: Modifications of cell surface and membrane |
| 图选项 |
2 与宿主防御肽的交叉抗性相关的AMPs耐药机制 新近研究发现,反复将细菌暴露于AMPs可在体内外选择出耐药株,而耐药株产生获得性耐药性的成本很低[10],并且细菌可产生对治疗性AMPs和宿主防御性AMPs的交叉抗性。
在Habets和Brockhurst[31]将递增浓度的培西加南(Pexiganan)处理后的S. aureus连续传代后,可诱导耐药菌株出现,而耐培西加南的S. aureus对人内源性AMP HNP-1也表现出了交叉抗性。Dobson等[10, 32]使用对合成AMPs Iseganan、培西加南耐药的菌株感染黄粉虫,结果表明,这些在体外对治疗性AMPs逐步产生耐药作用的细菌在黄粉虫体内的存活率也显著上升。对DAP耐药的耐甲氧西林的金黄色葡萄球菌(methicillin- resistant Staphylococcus aureus,MRSA)与HNP-1[33]和LL-37[34]之间也存在交叉耐性。最近亦有研究发现了类似的情况,即MRSA还与宿主防御素血小板抗菌蛋白(platelet microbicidal protein, tPMPs)存在交叉耐药[35]。
另一方面,暴露于内源性AMPs这一手段可以在应用DAP之前筛选出对DAP有交叉耐药性的菌株[36]。Lofton等[37]发现使用含有递增浓度LL-37的培养基进行传代后,S. Typhimurium LT2对合成AMP CNY100HL也逐步产生了耐药性。编码了WaaY激酶的waaY基因的突变参与了这些多肽的交叉耐药,这种激酶介导了LPS内核的二庚糖磷酸化。并且,新近的研究对其进行了更深入的探讨,称这些突变对细菌在宿主中的适应性和存活性影响很小[38]。
因此,如表 2总结的,内源性AMPs和治疗性AMPs之间的交叉耐药是一种双向的关系。使用外源性的治疗性AMPs后,细菌可能对人体的宿主防御肽产生耐受,同时,细菌对内源性AMPs的交叉抗性很可能引起细菌对治疗性AMPs的耐药性。
表 2. 治疗性AMPs和宿主防御肽的交叉抗性 Table 2. Cross-resistance between therapeutic AMPs and host-defense peptides
| Bacteria resistant to therapeutic AMPs | Corresponding resistance to host defense peptides | References |
| Pexiganan resistant S. aureus | Resistant to HNP-1 in vitro | [31] |
| Iseganan and pexiganan resistant S. aureus | A rise in livability in vivo | [10, 32] |
| Daptomycin resistant MRSA | Resistant to HNP-1 in vitro | [33] |
| Daptomycin resistant MRSA | Resistant to platelet microbicidal protein in vitro | [35] |
| Bacteria resistant to host-defense peptides | Corresponding resistance to therapeutic AMPs | |
| LL-37 resistant S. typhimurium | Resistant to CNY100HL in vitro | [37] |
表选项
3 总结和展望 AMPs是人体重要的抗菌物质,首先被报道的耐药现象多针对内源性AMPs。此后,在使用治疗性AMPs的过程中,如作为外用抗感染药物已有较长应用史的多粘菌素,研究人员也逐渐发现了对应的耐药菌株,继而出现了相关耐药机制,如关于耐药基因mcr-1[25]的报道。在此之外,对于还未临床广泛应用的AMPs,其耐药情况则大多在基础研究中被发现或诱导。目前我们对细菌AMPs耐药性的机制了解相对有限,仅有很少的报道提示了耐药性出现的速度和耐药机制对相关突变株适应性的影响。但就现有研究而言,不难发现,不管革兰阳性菌还是革兰阴性菌,都可通过一种或几种机制产生对AMPs的耐药性,包括截留AMPs、分泌蛋白酶、利用荚膜多糖及氨酰化的磷脂酰甘油阻挡AMPs。但涉及到两类细菌各自特有的结构,其耐AMPs的机制也有差异。革兰阳性菌细胞壁较厚,含有丰富的肽聚糖和磷壁酸,革兰氏阴性细菌的细胞壁中肽聚糖含量低,而脂类物质含量高,脂多糖为其细胞壁的特有结构,只有具有该结构的相应细菌,才能对其进行修饰加工以获得对AMPs的抗性。
并且这种耐药性还可能与人固有免疫中的天然防御肽有双向交叉耐药关系。几种AMPs的联用或者AMPs/抗生素联用可以避免细菌对内源性AMPs的耐药或者交叉耐药[10, 39]。DAP和氨苄青霉素联用可有效杀灭从心内膜炎和菌血症患者体内分离出的耐青霉素和耐万古霉素的E. faecium临床株。进一步研究发现,氨苄青霉素可能可以通过重新中和耐药菌表面增加的正电荷,增加DAP对细菌表面的吸附,从而增强DAP的杀菌能力。在同一项研究中,当存在氨苄青霉素时,耐青霉素和耐万古霉素的E. faecium 对LL-37、HNP-1和tPMPs也更敏感[39]。
随着下一代测序技术的发展,针对抗生素耐药性的研究已随着高通量技术的应用而逐步进入更加宏观的耐药组研究层面。尽管针对AMPs耐药性的分子机制的探索仍在不停向前推进,但大多数研究仍停留在单菌及单基因的水平。持续修正我们对AMPs作用机制和细菌耐药性的理解将有利于指导新型治疗性AMPs的设计,从而赋予其更好的活性和安全性。借鉴抗生素耐药研究的经验,明确、主动地进行AMPs耐药性及其机制的鉴定及监测,对于避免耗尽现有的治疗性AMPs资源是非常必要的。最重要的是,在我们研发新的抗感染策略以对抗愈发严重的耐药菌感染的过程中,深刻理解细菌对AMPs的耐药机制将是不可或缺的必由之路。
References
| [1] | United States Department of Health and Human Services Centers for Disease Control and Prevention. Antibiotic resistance threats in the United States. (2017-04-10).[2018-09-24]. https://www.cdc.gov/drugresistance/threat-report-2013/index.html. |
| [2] | Bastos P, Trindade F, Da Costa J, Ferreira R, Vitorino R. Human antimicrobial peptides in bodily fluids: current knowledge and therapeutic perspectives in the postantibiotic era. Medicinal Research Reviews, 2017, 38(1): 101-146. |
| [3] | Wang YF, Fan YY, Zhou ZL, Tu HX, Ren Q, Wang XQ, Ding LJ, Zhou XD, Zhang LL. De novo synthetic short antimicrobial peptides against cariogenic bacteria. Archives of Oral Biology, 2017, 80: 41-50. DOI:10.1016/j.archoralbio.2017.03.017 |
| [4] | Malanovic N, Lohner K. Gram-positive bacterial cell envelopes: The impact on the activity of antimicrobial peptides. Biochimica et Biophysica Acta (BBA) - Biomembranes, 2016, 1858(5): 936-946. DOI:10.1016/j.bbamem.2015.11.004 |
| [5] | Kubicek-Sutherland JZ, Heithoff DM, Ersoy SC, Shimp WR, House JK, Marth JD, Smith JW, Mahan MJ. Host-dependent induction of transient antibiotic resistance: a prelude to treatment failure. Ebiomedicine, 2015, 2(9): 1169-1178. DOI:10.1016/j.ebiom.2015.08.012 |
| [6] | Wang YF, Wang XQ, Jiang WT, Wang K, Luo JY, Li W, Zhou XD, Zhang LL. Antimicrobial peptide GH12 suppresses cariogenic virulence factors of Streptococcus mutans. Journal of Oral Microbiology, 2018, 10(1): 1442089. DOI:10.1080/20002297.2018.1442089 |
| [7] | Kao C, Lin XY, Yi GH, Zhang YL, Rowe-Magnus DA, Bush K. Cathelicidin antimicrobial peptides with reduced activation of toll-like receptor signaling have potent bactericidal activity against colistin-resistant Bacteria. mBio, 2016, 7(5): e01418-16. DOI:10.1128/mBio.01418-16 |
| [8] | Arzanlou M, Chai WC, Venter H. Intrinsic, adaptive and acquired antimicrobial resistance in Gram-negative bacteria. Essays in Biochemistry, 2017, 61(1): 49-59. DOI:10.1042/EBC20160063 |
| [9] | Jin T, Bokarewa M, Foster T, Mitchell J, Higgins J, Tarkowski A. Staphylococcus aureus resists human defensins by production of staphylokinase a novel bacterial evasion mechanism. The Journal of Immunology, 2004, 172(2): 1169-1176. DOI:10.4049/jimmunol.172.2.1169 |
| [10] | Dobson AJ, Purves J, Kamysz W, Rolff J. Comparing selection on S. aureus between antimicrobial peptides and common antibiotics. PLoS One, 2013, 8(10): e76521. DOI:10.1371/journal.pone.0076521 |
| [11] | Belas R, Manos J, Suvanasuthi R. Proteus mirabilis ZapA metalloprotease degrades a broad spectrum of substrates, including antimicrobial peptides. Infection and Immunity, 2004, 72(9): 5159-5167. DOI:10.1128/IAI.72.9.5159-5167.2004 |
| [12] | Sieprawska-Lupa M, Mydel P, Krawczyk K, Wójcik K, Puklo M, Lupa B, Suder P, Silberring J, Reed M, Pohl J, Shafer W, McAleese F, Foster T, Travis J, Potempa J. Degradation of human antimicrobial peptide LL-37 by Staphylococcus aureus-derived proteinases. Antimicrobial Agents and Chemotherapy, 2004, 48(12): 4673-4679. DOI:10.1128/AAC.48.12.4673-4679.2004 |
| [13] | Johansson L, Thulin P, Sendi P, Hertzen E, Linder A, Akesson P, Low DE, Agerberth B, Norrbyteglund A. Cathelicidin LL-37 in severe Streptococcus pyogenes soft tissue infections in humans. Infection and Immunity, 2008, 76(8): 3399-3404. DOI:10.1128/IAI.01392-07 |
| [14] | Thomassin JL, Brannon JR, Gibbs BF, Gruenheid S, Le Moual H. OmpT outer membrane proteases of enterohemorrhagic and enteropathogenic Escherichia coli contribute differently to the degradation of human LL-37. Infection and Immunity, 2012, 80(2): 483-492. DOI:10.1128/IAI.05674-11 |
| [15] | Ly D, Taylor JM, Tsatsaronis JA, Monteleone MM, Skora AS, Donald CA, Maddocks T, Nizet V, West NP, Ranson M, Walker MJ, McArthur JD, Sanderson-Smith ML. Plasmin(ogen) acquisition by group a Streptococcus protects against C3b-mediated neutrophil killing. Journal of Innate Immunity, 2014, 6(2): 240-250. DOI:10.1159/000353754 |
| [16] | Moranta D, Regueiro V, March C, Llobet E, Margareto J, Larrate E, Garmendia J, Bengoechea JA. Klebsiella pneumoniae capsule polysaccharide impedes the expression of β-defensins by airway epithelial cells. Infection and Immunity, 2010, 78(3): 1135-1146. DOI:10.1128/IAI.00940-09 |
| [17] | Rukke HV, Engen SA, Schenck K, Petersen FC. Capsule expression in Streptococcus mitis modulates interaction with oral keratinocytes and alters susceptibility to human antimicrobial peptides. Molecular Oral Microbiology, 2016, 31(4): 302-313. DOI:10.1111/omi.12123 |
| [18] | Musrati AA, Fteita D, Paranko J, K?n?nen E, Gürsoy UK. Morphological and functional adaptations of Fusobacterium nucleatum exposed to human neutrophil Peptide-1. Anaerobe, 2016, 39: 31-38. DOI:10.1016/j.anaerobe.2016.02.008 |
| [19] | Saar-Dover R, Bitler A, Nezer R, Shmuel-Galia L, Firon A, Shimoni E, Trieu-Cuot P, Shai Y. D-alanylation of lipoteichoic acids confers resistance to cationic peptides in group B Streptococcus by increasing the cell wall density. PLoS Pathogens, 2012, 8(9): e1002891. DOI:10.1371/journal.ppat.1002891 |
| [20] | Simanski M, Gl?eser R, K?eten B, Meyer-Hoffert U, Wanner S, Weidenmaier C, Peschel A, Harder J. Staphylococcus aureus subverts cutaneous defense by D-alanylation of teichoic acids. Experimental Dermatology, 2013, 22(4): 294-296. DOI:10.1111/exd.12114 |
| [21] | Brown S, Maria JPS Jr, Walker S. Wall teichoic acids of gram-positive bacteria. Annual Review of Microbiology, 2013, 67: 313-336. DOI:10.1146/annurev-micro-092412-155620 |
| [22] | Jain S, Darveau RP. Contribution of Porphyromonas gingivalis lipopolysaccharide to periodontitis. Periodontology, 2010, 54(1): 53-70. DOI:10.1111/j.1600-0757.2009.00333.x |
| [23] | Matson JS, Yoo HJ, Hakansson K, DiRita VJ. Polymyxin B resistance in El Tor Vibrio cholerae requires lipid acylation catalyzed by MsbB. Journal of Bacteriology, 2010, 192(8): 2044-2052. DOI:10.1128/JB.00023-10 |
| [24] | Henderson JC, Fage CD, Cannon JR, Brodbelt JS, Keatinge-Clay AT, Trent MS. Antimicrobial peptide resistance of Vibrio cholerae results from an LPS modification pathway related to nonribosomal peptide synthetases. ACS Chemical Biology, 2014, 9(10): 2382-2392. DOI:10.1021/cb500438x |
| [25] | Liu YY, Wang Y, Walsh TR, Yi LX, Zhang R, Spencer J, Doi Y, Tian GB, Dong BL, Huang XJH, Yu LF, Gu DX, Ren HW, Chen XJ, Lv LC, He DD, Zhou HW, Liang ZS, Shen JZ. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. The Lancet Infectious Diseases, 2016, 16(2): 161-168. DOI:10.1016/S1473-3099(15)00424-7 |
| [26] | Gao RS, Wang QJ, Li P, Li ZC, Feng YJ. Genome sequence and characteristics of plasmid pWH12, a variant of the mcr-1-harbouring plasmid pHNSHP45, from the multi-drug resistant E. coli. Virulence, 2016, 7(6): 732-735. DOI:10.1080/21505594.2016.1193279 |
| [27] | Rubio A, Conrad M, Haselbeck RJ, Kedar GC, Brown-Driver V, Finn J, Silverman JA. Regulation of mprF by antisense RNA restores daptomycin susceptibility to daptomycin-resistant isolates of Staphylococcus aureus. Antimicrobial Agents and Chemotherapy, 2011, 55(1): 364-367. DOI:10.1128/AAC.00429-10 |
| [28] | Frick IM, ?kesson P, Rasmussen M, Schmidtchen A, Bj?rck L. SIC, a secreted protein of Streptococcus pyogenes that inactivates antibacterial peptides. Journal of Biological Chemistry, 2003, 278(19): 16561-16566. DOI:10.1074/jbc.M301995200 |
| [29] | Ernst CM, Staubitz P, Mishra NN, Yang SJ, Hornig G, Kalbacher H, Bayer AS, Kraus D, Peschel A. The bacterial defensin resistance protein MprF consists of separable domains for lipid lysinylation and antimicrobial peptide repulsion. PLoS Pathogens, 2009, 5(11): e1000660. DOI:10.1371/journal.ppat.1000660 |
| [30] | Nawrocki KL, Crispell EK, McBride SM. Antimicrobial peptide resistance mechanisms of gram-positive Bacteria. Antibiotics, 2014, 3(4): 461-492. DOI:10.3390/antibiotics3040461 |
| [31] | Habets MGJL, Brockhurst MA. Therapeutic antimicrobial peptides may compromise natural immunity. Biology Letters, 2012, 8(3): 416-418. DOI:10.1098/rsbl.2011.1203 |
| [32] | Dobson AJ, Purves J, Rolff J. Increased survival of experimentally evolved antimicrobial peptide-resistant Staphylococcus aureus in an animal host. Evolutionary Applications, 2014, 7(8): 905-912. DOI:10.1111/eva.12184 |
| [33] | Bayer AS, Mishra NN, Sakoulas G, Nonejuie P, Nast CC, Pogliano J, Chen KT, Ellison SN, Yeaman MR, Yang SJ. Heterogeneity of mprF sequences in methicillin-resistant Staphylococcus aureus clinical isolates: role in cross-resistance between daptomycin and host defense antimicrobial peptides. Antimicrobial Agents and Chemotherapy, 2014, 58(12): 7462-7467. DOI:10.1128/AAC.03422-14 |
| [34] | Mishra NN, Bayer AS, Weidenmaier C, Grau T, Wanner S, Stefani S, Cafiso V, Bertuccio T, Yeaman MR, Nast CC, Yang SJ. Phenotypic and genotypic characterization of daptomycin-resistant methicillin-resistant Staphylococcus aureus strains: relative roles of mprF and dlt operons. PLoS One, 2014, 9(9): e107426. DOI:10.1371/journal.pone.0107426 |
| [35] | Bayer AS, Mishra NN, Chen L, Kreiswirth BN, Rubio A, Yang SJ. Frequency and distribution of single-nucleotide polymorphisms within mprF in methicillin-resistant Staphylococcus aureus clinical isolates and their role in cross-resistance to daptomycin and host defense antimicrobial peptides. Antimicrobial Agents and Chemotherapy, 2015, 59(8): 4930-4937. DOI:10.1128/AAC.00970-15 |
| [36] | Mishra NN, Yang SJ, Chen L, Muller C, Saleh-Mghir A, Kuhn S, Peschel A, Yeaman MR, Nast CC, Kreiswirth BN, Cremieux AC, Bayer AS. Emergence of daptomycin resistance in daptomycin-naive rabbits with methicillin-resistant Staphylococcus aureus prosthetic joint infection is associated with resistance to host defense cationic peptides and mprF polymorphisms. PLoS One, 2013, 8(8): e71151. DOI:10.1371/journal.pone.0071151 |
| [37] | Lofton H, Pranting M, Thulin E, Andersson DI. Mechanisms and fitness costs of resistance to antimicrobial peptides LL-37, CNY100HL and wheat germ histones. PLoS One, 2013, 8(7): e68875. DOI:10.1371/journal.pone.0068875 |
| [38] | Lofton H, Anwar N, Rhen M, Andersson DI. Fitness of Salmonella mutants resistant to antimicrobial peptides. Journal of Antimicrobial Chemotherapy, 2015, 70(2): 432-440. DOI:10.1093/jac/dku423 |
| [39] | Sakoulas G, Bayer AS, Pogliano J, Tsuji BT, Yang SJ, Mishra NN, Nizet V, Yeaman MR, Moise PA. Ampicillin enhances daptomycin- and cationic host defense peptide-mediated killing of ampicillin- and vancomycin-resistant Enterococcus faecium. Antimicrobial Agents and Chemotherapy, 2012, 56(2): 838-844. DOI:10.1128/AAC.05551-11 |
