廖子亚1, 郭晓萌1, 王海胜1, 闫艳春1, 李俊2

 , 赵百锁1,2
, 赵百锁1,2

1.中国农业科学院研究生院, 北京 100081;
2.农业部微生物产品质量安全风险评估实验室, 北京 100081
收稿日期:2017-11-29;修回日期:2018-02-02;网络出版日期:2018-05-25
基金项目:国家自然科学基金(31370158,31570110);中国农业科学院研究生院基本科研业务费(610042017001)
*通信作者:李俊, Tel:+86-10-82106208, E-mail:lijun01@caas.cn
赵百锁, Tel:+86-10-82106784, E-mail:bszhao@163.com
摘要:[目的]确定厌氧盐碱细菌Alkalitalea saponilacus产木聚糖酶所需的碳源,优化木聚糖粗酶的提取条件并分析酶学性质。[方法]应用GC技术分析A.saponilacus发酵木聚糖的主要产物;利用二硝基水杨酸法(DNS)测定木聚糖酶活力以获得最优的碳源、提取粗酶的最佳条件及其酶学特性。[结果]A.saponilacus以不同来源木聚糖为底物时,发酵产生的主要产物丙酸含量都在80%以上。若以0.4%(W/V)蔗糖+0.1%(W/V)桦木木聚糖为复合碳源时,木聚糖酶活力是以桦木木聚糖或者蔗糖为单一碳源时的3.2倍。木聚糖酶的酶活力在盐度2%-6%、pH 7.0和55达到最佳且在该条件下的酶活力为590 IU/mg。此外,该酶活力在0.2% Tween 20存在时增加,而在5 mmol/L Mg2+和0.2% Triton X-100存在时无显著影响,但在Cu2+、Fe3+和Ni2+等金属离子存在时则被显著抑制。[结论]A.saponilacus发酵主产物丙酸以及生物合成的木聚糖酶在工业生产中具有广泛的应用前景。
关键词: 盐碱细菌 Alkalitalea saponilacus 丙酸 木聚糖酶
Carbon source optimization and characterization of a xylanase from Alkalitalea saponilacus SC/BZ-SP2T
Ziya Liao1, Xiaomeng Guo1, Haisheng Wang1, Yanchun Yan1, Jun Li2

 , Baisuo Zhao1,2
, Baisuo Zhao1,2

1.Graduate School, Chinese Academy of Agricultural Sciences, Beijing 100081, China;
2.Laboratory of Quality & Safety Risk Assessment for Microbial Products(Beijing), Ministry of Agriculture, Beijing 100081, China
Received 29 November 2017; Revised 2 February 2018; Published online 25 May 2018
*Corresponding author: Jun Li, Tel:+86-10-82106208, E-mail:lijun01@caas.cn
Baisuo Zhao, Tel:+86-10-82106784, E-mail:bszhao@163.com
Supported by the National Natural Science Foundation of China (31370158, 31570110) and by the Graduate School of Chinese Academy of Agricultural Sciences (610042017001)
Abstract: [Objective]We optimized carbon source for xylanase production by anaerobically halophilic alkaliphilic bacterium Alkalitalea saponilacus and characterized the enzyme.[Methods]Xylanase activity was determined by 2-nitro salicylic acid (DNS) method. The conditions for the extraction of crude xylanase were optimized and the enzyme was characterized.[Results]Xylanase activity by fermentation using 0.4% (W/V) sucrose+0.1% (W/V) birch xylan as carbon source was 3.2 folds higher than that with single substrate of birch xylan or sucrose. The maximal xylanase activity reached 590 IU/mg under the conditions of salinity between 2% and 6%, pH 7.0 and 55℃. Enzyme activity was significantly inhibited with Cu2+, Fe3+and Ni2+.[Conclusion]Xylanase produced by A. saponilacus can have potential for industrial production.
Key words: halo-alkaliphile Alkalitalea saponilacus propionic acid xylanase
木聚糖是半纤维素中主要的碳水化合物,属于较复杂的多聚五碳糖。该化合物主要由β-D-1,4-木糖苷键连接形成主链骨架及多种取代基(如乙酰基和阿拉伯呋喃糖基等)结合形成[1]。此外,有些木聚糖还含有少量的阿拉伯糖、甘露糖、半乳糖、半乳糖醛酸、葡萄糖和葡萄糖醛酸等[2]。因此,自然界中木聚糖的完全降解需要多种水解酶的协同作用[3],而木聚糖酶在系列水解反应中最为关键。
近年来,科学家从极端环境中分离获得了一些嗜极细菌能生物合成木聚糖酶。其中主要有木聚糖兼性芽孢杆菌(Amphibacillus xylanus),发酵兼性芽孢杆菌(Amphibacillus fermentum),热带兼性芽孢杆菌(Amphibacillus tropicus),伊氏碱扰菌(Alkaliflexus imshenetskii),短小芽孢杆菌(Bacillus pumilus)和碱性梭菌(Clostridium alkalicellum)等[4-5]。其中一部分来源于极端条件下的微生物生成的木聚糖酶具有一些特殊的性质。然而,目前能生物合成木聚糖酶的嗜极细菌均为好氧微生物,而厌氧条件下能生成木聚糖酶的嗜极细菌尚未见报道。
本课题组从美国华盛顿州Soap Lake盐碱湖中首次分离得到一株能降解木聚糖的专性厌氧嗜极细菌Alkalitalea saponilacus[4]。该菌株能在Na+浓度为0.35–1.38 mol/L (相当于2%–8% NaCl)和pH为7.5–10.5条件下进行生长繁殖。其适宜生长条件:Na+浓度为0.44–0.69 mol/L (相当于2.6%–4.0% NaCl)和pH为9.7。研究表明,A. saponilacus能在基本培养基中以来源于燕麦、桦木或榉木的木聚糖作为唯一碳源进行生长繁殖。这暗示着A. saponilacus不仅能合成木聚糖酶且能分泌此酶到细胞外。因此,本文首先以木聚糖为底物来分析其主要发酵产物,进一步证明木聚糖可以被降解及厌氧发酵;其次确定A. saponilacus产木聚糖酶所需的最佳碳源组合,为工业化节约发酵成本提供依据;最后优化木聚糖粗酶的提取条件并分析酶学特性,为该木聚糖酶的潜在应用提供了理论支持。
1 材料和方法 1.1 主要试剂和仪器 酒石酸钾钠、无水硫酸钠和桦木木聚糖购买于Sigma公司;3, 5–二硝基水杨酸和牛血清白蛋白BSA(V)标准品购买于Solarbio公司;苯酚购买于北京百灵威科技有限公司。DK-8D电热恒温三孔水箱(上海跃进医疗器械有限公司);HACH DR 2800分光光度计(HACH公司);INFINITE 200 PRO多功能酶标仪(TECAN公司)。
1.2 菌株和液体培养基 Alkalitalea saponilacus菌株来源于本实验室。A. saponilacus菌株培养基成分及其配制参考文献[4]。
1.3 A. saponilacus发酵木聚糖终产物的分析 以燕麦木聚糖为底物,在固体培养基上将A. saponilacus活化,挑取单菌落到液体培养基中,转接3次后以1%接种量分别接种于以燕麦木聚糖(1%,W/V)、桦木木聚糖(1%,W/V)或者榉木木聚糖(1%,W/V)为底物的液体培养基中。在35 ℃和100 r/min条件下培养,培养5 d。每24 h收集样品,用GC技术测定混合酸中各组分的含量[6]。
1.4 A. saponilacus产酶所需碳源的筛选 以1%接种量将A. saponilacus分别接种于以蔗糖(0.5%,W/V)、桦木木聚糖(0.5%,W/V)、麦芽糖(0.5%,W/V)、葡萄糖(0.5%,W/V)或者纤维二糖(0.5%,W/V)为底物的液体培养基中。在35 ℃和100 r/min条件下培养36 h。利用DNS法(如下所述)测定所产木聚糖酶的总酶活,以确定该菌株产木聚糖酶的所需碳源。
1.5 A. saponilacus产酶所需最佳碳源的确定 以1%接种量将A. saponilacus分别接种于以蔗糖(0.5%,W/V)、桦木木聚糖(0.5%,W/V)或者蔗糖(0.4%,W/V)+桦木木聚糖(0.1%,W/V)为底物的液体培养基中。在35 ℃和100 r/min条件下培养36 h。利用DNS法(如下所述)测定发酵液所含木聚糖酶的总酶活,以确定该菌株产木聚糖酶的所需最佳碳源组合。
1.6 硫酸铵盐析条件确定及A. saponilacus产木聚糖粗酶的制备 为获取硫酸铵沉淀木聚糖粗酶所需的饱和度,将培养36 h菌液在功率200 W、工作时间:间歇时间=3:3 (s/s)条件下超声破碎10 min,然后在13572×g和4 ℃条件下离心20 min,收集上清液。分别取20 mL的上清液,冰浴下缓慢加入饱和度为20%、30%、40%、50%、60%、70%、80%、90%和100%的(NH4)2SO4,4 ℃静置过夜。最后取上清液在13572×g和4 ℃条件下离心20 min,以获得含木聚糖酶的蛋白沉淀。用磷酸氢二钠-磷酸二氢钠缓冲液(pH 7.0)重悬蛋白沉淀以获得木聚糖粗酶液。每个处理重复3次。
1.7 木聚糖酶酶活力的测定 根据Amore A等[7]所用测定木聚糖酶酶活力的方法,经过适当改进用二硝基水杨酸(DNS)测定木聚糖酶酶活力。具体操作如下:
木糖标准曲线的制作:用蒸馏水制备1%木糖标准液,再用蒸馏水将1%木糖标准液分别稀释成100、200、300、400、500 μg/mL木糖标准液。分别取不同浓度的木糖标准液200 μL于1.5 mL离心管中,再加入200 μL DNS溶液混匀,然后在100 ℃条件下反应5 min,最后迅速取出后放入冰浴冷却。制作空白对照如下:取200 μL蒸馏水代替200 μL木糖标准液重复以上反应。调空白对照为零,测定540 nm波长下的吸光值,以OD540为纵坐标,以相应的木糖标准液为横坐标,制作木糖标准曲线。
木聚糖酶酶活力的测定:用蒸馏水制备0.5%桦木木聚糖为底物,在1.5 mL离心管中加入0.5%木聚糖底物150 μL,再加入粗酶液50 μL混匀,在55 ℃水浴中反应10 min,最后迅速放入冰浴中冷却。然后加入200 μL DNS溶液混匀,在100 ℃条件下反应5 min,再迅速放入冰浴中冷却,测定OD540。每个样品重复3次,取平均值计算酶活力。空白对照:将待测粗酶液100 ℃加热10 min失活后,取50 μL重复以上反应。木糖生成量:待测粗酶液OD540–空白OD540。根据木糖标准曲线方程计算出反应产生的木糖含量,从而换算到木聚糖酶酶活力。
木聚糖酶酶活力单位定义:每分钟水解木聚糖生成1 μmol木糖同系物所需木聚糖酶的酶量(其中,酶反应条件为55 ℃、pH 7.0和2%盐度)。即:木聚糖酶酶活力单位(IU/mL)=N×C×0.4×1000/M× T×V,其中N:粗酶液的稀释倍数,C:由木糖标准曲线得到的木糖含量(μg/mL),0.4:反应的总体积(mL),M:木糖的分子量,T:反应时间(min),V:加入的粗酶液的体积。
1.7.1 蛋白浓度的测定: 根据Solarbio公司试剂盒说明测定(No. PC0020)。
1.7.2 温度对木聚糖酶酶活力的影响: pH 7.0条件下,在30–90 ℃ (温度间隔为5 ℃)水浴中对粗酶液测定其木聚糖酶酶活力,以最高值为参照组,相对酶活力=(不同温度下的木聚糖酶酶活力/参照组木聚糖酶酶活力)×100%,以确定该菌株木聚糖酶最适反应温度。每个处理重复3次。
1.7.3 盐度对木聚糖酶酶活力的影响: 配制不同NaCl浓度的0.5%木聚糖底物,将等量的木聚糖酶与木聚糖底物混匀,使NaCl终浓度达到2%、4%、6%、8%、10%、12%、14%、16%、18%、20%或22%。测定不同盐度下木聚糖酶的酶活力,以最高值为参照组,相对酶活力定义如上。每个处理重复3次。
1.7.4 pH对木聚糖酶酶活力的影响: 配制50 mmol/L柠檬酸-柠檬酸钠缓冲液(pH 4–6),磷酸氢二钠-磷酸二氢钠缓冲液(pH 6.0–8.0),甘氨酸-氢氧化钠缓冲液(pH 8–10),并用配好的缓冲液制备0.5%的木聚糖底物。将等量的木聚糖酶加入各缓冲液中,使酶在不同pH条件下进行反应,测定木聚糖酶酶活力。每个处理重复3次。
1.7.5 金属离子及表面活性剂对木聚糖酶酶活力的影响: 在最适条件下,分别在稀释后的粗酶液中加入5 mmol/L CuCl2、FeCl3、CaCl2、AlCl3、CoCl2、ZnCl2、MnCl2、NiCl2、MgCl2、0.2% (V/V) Triton X-100或吐温20 (Tween 20)。测定加入金属离子或表面活性剂后的木聚糖酶酶活力,以未添加金属离子及表面活性剂的粗酶液的酶活力为100%,相对酶活力定义如上,每个处理重复3次。
2 结果和分析 2.1 A. saponilacus以木聚糖为底物产酸分析 A. saponilacus能够在厌氧条件下转化来源于榉木、桦木和燕麦的木聚糖,生成乙酸、丙酸和丁酸混合物,其中丙酸约占80%以上(图 1)。在发酵过程中,第1天燕麦木聚糖发酵产生了较多的丙酸,这可能是燕麦木聚糖的成分及其结构有利于A. saponilacus利用;第4天桦木木聚糖和燕麦木聚糖被转化生成的丙酸含量几乎相同,且两者产酸浓度均比榉木木聚糖高,这说明A. saponilacus更青睐以桦木和燕麦木聚糖作为发酵底物。此外,以不同木聚糖为发酵底物时,第4天产酸量都达到最大,第5天则都有所降低。究其原因可能是木聚糖被消耗殆尽,A. saponilacus可以利用混合酸为自身的生长繁殖提供营养。
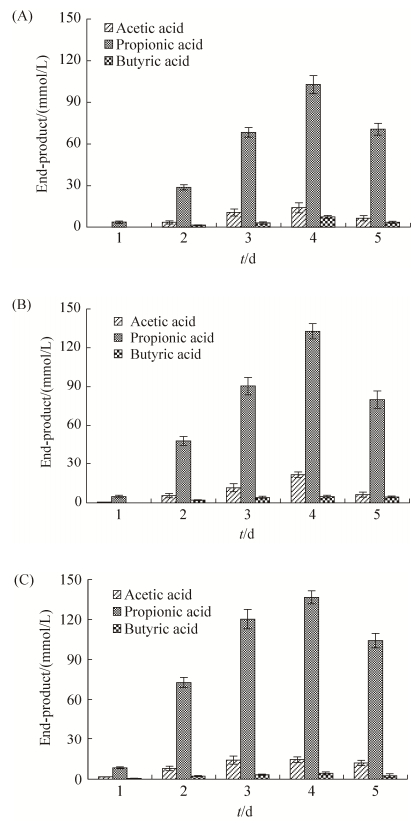 |
| 图 1 A. saponilacus以木聚糖为底物发酵产生乙酸、丙酸和丁酸的含量 Figure 1 Acetic acid, propionic acid and butyric acid content from the fermentation of xylan by A. saponilacus. A: the fermentation of beech wood xylan; B: the fermentation of birch wood xylan; C: the fermentation of oat spelt xylan. Data are presented as mean±SD and are representatives of three independent experiments. |
| 图选项 |
2.2 不同碳源对A. saponilacus产木聚糖酶的影响 碳源是影响微生物生长代谢重要的环境因素之一,也对微生物产酶至关重要[8]。将A. saponilacus以桦木木聚糖、蔗糖、麦芽糖、葡萄糖或者纤维二糖为唯一碳源培养36 h后收集菌体,提取木聚糖酶进行DNS反应,最后进行颜色观察(图 2)和酶标仪测定。结果表明,以5种不同碳源为底物时,A. saponilacus单位体积内产木聚糖酶的酶活力依次为桦木木聚糖 > 蔗糖 > 麦芽糖 > 葡萄糖 > 纤维二糖。因此本研究将以桦木木聚糖和蔗糖作为碳源组合来优化A. saponilacus发酵产木聚糖酶。
 |
| 图 2 A. saponilacus在不同碳源下合成木聚糖酶的酶活力 Figure 2 Influence of carbon sources on xylanase activity of A. saponilacus. A1: control of birch wood xylan; A2: test of birch wood xylan; B1: control of maltose; B2: test of maltose; C1: control of sucrose; C2: test of sucrose; D1: control of glucose; D2: test of glucose; E1: control of cellobiose; E2: test of cellobios. Compared with the control, the deeper the color, the higher the xylanase activity. |
| 图选项 |
2.3 不同碳源配比对A. saponilacus产木聚糖酶的影响 A. saponilacus在0.5%的桦木木聚糖或者蔗糖为底物产木聚糖酶酶活力较高,因此研究以桦木木聚糖和蔗糖作为碳源组合对A. saponilacus发酵产木聚糖酶的影响。由于蔗糖来源广泛且廉价易得,若能以其为底物产木聚糖酶可以有效地降低生物合成木聚糖酶的生产成本。图 3所示,分别以0.5%蔗糖、0.5%木聚糖或者0.4%蔗糖+0.1%木聚糖为底物均能产生木聚糖酶,其中以0.4% (W/V)蔗糖+0.1% (W/V)木聚糖为碳源的培养基生物合成木聚糖酶酶活力显著高于其他组合,其酶活力高达(527.3±3.2) IU/mL,为单一碳源的3.2倍,木聚糖酶比活力(295.6±25.9) IU/mg亦远远高于单一碳源蔗糖(200.0±44.3) IU/mg或者木聚糖(166.3±6.1) IU/mg。同时蛋白含量(3.2±0.3) mg/mL也得到了很大的提高,约是单一碳源的4倍,蛋白含量与菌体数量在一定程度上呈正相关关系,这表明两种碳源的组合大大促进了菌体的生长和木聚糖酶的合成,以此提高单位体积木聚糖酶量和其比活力。
 |
| 图 3 A. saponilacus在不同碳源组合下木聚糖酶的酶活力及其蛋白浓度 Figure 3 Influence of carbon sources on xylanase activity and protein concentration of A. saponilacus. Data are presented as mean±SD and are representatives of three independent experiments. |
| 图选项 |
2.4 最适硫酸铵用量在粗酶提取中的确定 硫酸铵可以浓缩、纯化某些蛋白质,且成本低、操作简单、不易使蛋白质变性,因此需要获得硫酸铵最适用量以得到由等体积发酵液提取而来酶活力最高的木聚糖粗酶液。图 4结果表明,硫酸铵饱和度在20%–60%范围内均能沉淀得到木聚糖酶且保持其酶活力。当硫酸铵饱和度为40%时,所收集到的总木聚糖酶酶活力最高。因此,本研究中的后续实验均用40%饱和度的硫酸铵来获取木聚糖酶沉淀,进而再次制备木聚糖酶粗酶液。
 |
| 图 4 硫酸铵盐析A. saponilacus木聚糖粗酶的优化 Figure 4 Opitmization of ammonium sulfate precipitation of crude xylanase from A. saponilacus. Data are presented as mean±SD and are representatives of three independent experiments. |
| 图选项 |
2.5 温度对木聚糖酶酶活力的影响 A. saponilacus生长温度范围为8–40 ℃且适宜生长温度为35–37 ℃,因此推测温度可能会对木聚糖酶酶活力影响较大。结果表明,A. saponilacus产生的木聚糖酶在30–90 ℃条件下均有活性(图 5)。在30–90 ℃范围内,酶活力先升高后降低且最适温度为55 ℃。在30–60 ℃条件下,其相对酶活力维持40%以上;在45–55 ℃条件下,其相对酶活力达到了96%以上;在60 ℃时,该酶活力急剧下降且至65 ℃后下降趋势开始变缓;在80 ℃时,其相对酶活力约为24%;在90 ℃时,其相对酶活力约为6%,表明该木聚糖酶在高温时有较强的耐受力。
 |
| 图 5 温度对A. saponilacus木聚糖酶酶活力的影响 Figure 5 Influence of temperature on xylanase activity of A. saponilacus. Data are presented as mean±SD and are representatives of three independent experiments. |
| 图选项 |
2.6 NaCl浓度对木聚糖酶酶活力的影响 A. saponilacus是一株能够在0.35–1.38 mol/L (相当于2%–8% NaCl)的盐条件下进行生长繁殖的中度嗜盐细菌。这暗示着该菌分泌胞外的木聚糖酶的酶活力可能会对盐有一定的依赖。图 6结果表明,在NaCl浓度为2%–6%时,木聚糖酶酶活力达到了峰值,该值比对照(无NaCl)提高了约1.5倍,甚至在盐度高达12%的条件下酶活力也比无盐时高。这充分说明在2%–12% NaCl范围内,盐会对该木聚糖酶的酶促反应有一定的促进作用。当NaCl浓度达到14%,该酶的酶活力才开始受到抑制而减弱;在NaCl浓度高达20%时,相对酶活力仍然还有约30%。此外,A. saponilacus生长的最适盐浓度为0.44–0.69 mol/L (相当于2.6%–4.0% NaCl)[4],因此,来源于A. saponilacus的木聚糖酶的酶活力适宜反应所需盐度与该菌生长适宜盐度相吻合。
 |
| 图 6 盐度对A. saponilacus木聚糖酶酶活力的影响 Figure 6 Influence of different salinities on xylanase activity of A. saponilacus. Data are presented as mean±SD and are representatives of three independent experiments. |
| 图选项 |
2.7 pH对木聚糖酶酶活力的影响 由于A. saponilacus最适生长pH为9.7,希望其所产木聚糖酶嗜碱或者耐碱,所以在3种不同的缓冲液多种不同pH的条件下研究木聚糖酶的最适反应pH。结果如图 7所示,木聚糖酶酶活力在pH 4.0–6.0时逐渐升高,在pH 6.0–8.0时先升高后降低,在pH 8.0–10.0时逐渐降低,在pH 7.0时木聚糖酶酶活力达到了最大值,说明A. saponilacus所产木聚糖酶最适反应pH为7.0,同时,该木聚糖酶在pH 5.5–8.0的条件下仍保持着超过40%的木聚糖酶相对酶活力。pH 9.7时A. saponilacus所产木聚糖酶只有约15.0%的相对酶活力,与pH 4.5时的木聚糖酶酶活力差不多,表明该酶在较宽的酸性pH和碱性pH范围内都有一定的耐受力。在最适反应温度、盐度、pH条件下测定木聚糖酶活力,约为发酵液的2倍(590 IU/mg)。
 |
| 图 7 pH对A. saponilacus木聚糖酶酶活力的影响 Figure 7 Influence of pH on xylanase activity of A. saponilacus. Data are presented as mean±SD and are representatives of three independent experiments. |
| 图选项 |
2.8 金属离子及表面活性剂对木聚糖酶酶活力的影响 加入5 mmol/L不同金属离子测定木聚糖酶酶活力,结果如表 1所示,CuCl2、FeCl3对木聚糖酶抑制作用效果最大,被抑制后的木聚糖酶酶活力仅有空白对照的11.1%和17.6%,NiCl2、AlCl3、MnCl2、CoCl2、ZnCl2、CaCl2亦对菌株所产木聚糖酶酶活力有较大的抑制作用,而MgCl2则对酶活力没有明显的影响。加入Tween 20可以增加约1.16倍的木聚糖酶酶活力,而加入Triton X-100后酶活力却没有观察到明显的变化。加入Tween 20可以增加酶活力可能是因为表面活性剂可以改变作用底物的结构,使酶更容易与底物结合,进而有效地作用于底物或者是通过阻止酶的降解来维持酶的稳定性[9]。
表 1. 金属离子和表面活性剂对A. saponilacus木聚糖酶酶活力的影响 Table 1. Influence of metal compounds and surfactant on xylanase activity of A. saponilacus
| Metal compounds and surfactant | Relative activity/% |
| Control | 100.00±4.19 |
| CuCl2 | 11.05±2.45 |
| FeCl3 | 17.63±2.49 |
| NiCl2 | 34.66±1.57 |
| AlCl3 | 38.43±7.06 |
| MnCl2 | 54.08±2.20 |
| CoCl2 | 55.58±3.80 |
| ZnCl2 | 62.12±6.24 |
| CaCl2 | 68.31±4.31 |
| MgCl2 | 98.88±4.08 |
| Triton X-100 | 98.20±2.74 |
| Tween 20 | 116.61±1.36 |
表选项
3 讨论 据文献报道,通常微生物发酵木聚糖的主要终产物为乙酸[10-12]。而本研究中的海洋滑动菌科(Marinilabiliaceae)中的模式菌种A. saponilacus能以不同来源的木聚糖(榉木、桦木和燕麦木聚糖)为唯一碳源且终产物主要为丙酸。因此,A. saponilacus产丙酸这一重要的生理代谢特征对应用微生物法生产丙酸具有很重要意义。丙酸是生产纤维素塑料、除草剂和香水等工业中关键的中间体。其次,丙酸也是霉菌的强抑制剂,其钙、钠和钾盐可以被用作食品和饲料防腐剂[13]。此外,丙酸还能增强废水中的生物除磷效率[14]。然而,目前丙酸基本都来源于石化工业[13],因此A. saponilacus发酵木聚糖为丙酸可望为现代工业中提供新思路。
本研究首次证明了在厌氧条件下木聚糖酶能被盐碱细菌生物合成。A. saponilacus能利用多种碳源生长且合成木聚糖酶。其以桦木木聚糖为底物时比活力可达295.6 IU/mg,远远高于高地芽胞杆菌(Bacillus altitudinis DHN8)(40.5 IU/mg)[15]、梭杆菌(Clostridium PXYL1)(37.1 IU/mg)[16]、假单胞菌(Pseudomonas sp. CL3)(40.0 IU/mg)[17]等产木聚糖酶的细菌以及毁丝霉(Myceliophthora sp. IMI 387099)(124.8 IU/mg)[18]、嗜热毛壳菌(Chaetomium thermophile NIBGE)(95.3 IU/mg)[19]、胶囊青霉(Penicillium capsulatum)(42.37 IU/mg)[20]等真菌。其中A. saponilacus的木聚糖酶粗酶液的酶活力高于来源于胶囊青霉(Penicillium capsulatum) (42.37 IU/mg)[20]和木霉(Trichoderma harzianum T4)(248.66 IU/mg)[21]的木聚糖酶粗酶液(表 2),却低于来源于胶囊青霉(761 IU/mg)和木霉(3578 IU/mg)经过精细纯化的木聚糖酶,所以为了获得更高的木聚糖酶活力,还需对来源于A. saponilacus菌株的木聚糖酶进行精细纯化。
表 2. 不同微生物产生的木聚糖酶及其酶活力比较 Table 2. Comparison of the xylanase production by various microorganisms
| Microorganisms | Substrate | Cultivation conditions | Xylanase activity | References |
| Bacteria | ||||
| A. saponilacus | Birchwood and sucrose | pH 9.7, 35 ℃, 36 h | 527.3 IU/mL (295.6 IU/mg) | This study |
| Bacillus altitudinis DHN8 | Oat spelt xylan | pH 7.0, 37 ℃, 48 h | 40.5 IU/mg | [15] |
| Bacillus coagulans | Wheat straw | pH 7.0, 45 ℃, 48 h | 165.0 IU/mL | [22] |
| Bacillus sp. QH14 | Birchwood | pH 9.0, 37 ℃, 48 h | 260.3 IU/mg | [5] |
| Bacillus sp. SPS-0 | Wheat bran | pH 8.2, 60 ℃, 24 h | 31.8 IU/mg | [23] |
| Cellulomonas flavigena | Bagasses | 37 ℃ | 12.8 IU/mg | [24] |
| Clostridium PXYL1 | Birchwood | 20 ℃ | 37.0 IU/mg | [16] |
| Pseudomonas sp. CL3 | Carboxymethyl cellulose | 37 ℃, 48 h | 40.0 U/mL | [17] |
| Pseudomonas sp. WLUN024 | Xylan (Sigma) | pH 8.5, 37 ℃, 48 h | 190.2 IU/mL | [25] |
| Fungi | ||||
| Acrophialophora nainiana | Birchwood | 40 ℃, 72 h | 45.1 IU/mg | [26] |
| Aspergillus nidulans KK-99 | Wheat bran | pH 10.0, 37 ℃, 144 h | 40.0 IU/mL | [27] |
| Chaetomium thermophile NIBGE | Wheat straw | pH 5.0, 45 ℃, 120 h | 95.3 IU/mg | [19] |
| Myceliophthora sp. IMI 387099 | Corn cob | pH 6.0, 42 ℃, 144 h | 124.8 IU/mg | [18] |
| Penicillium canescens | Casein peptone and bean cake | pH 7.0, 30 ℃, 168 h | 18.8 IU/ mg | [28] |
| Penicillium capsulatum | Birchwood | 37 ℃, 120 h | 42.37 IU/mg; 761.00 IU/ mg (after purification) | [20] |
| Thermomyces lanuginosus | Cob | pH 6.5, 50 ℃, 144 h | 3576.0 IU/mL | [29] |
| Trichoderma reesei Rut C-30 | Lactose | pH 6.0, 28 ℃, 120 h | 94.7 IU/mL | [30] |
| Trichoderma harzianum T4 | Oat spelt xylan or wheat bran | pH 7.0, 28 ℃, 168 h | 248.66 IU/mg; 3578.26 IU/mg (after purification) | [21] |
表选项
A. saponilacus所合成木聚糖酶具有其独特的酶学性质。首先,该木聚糖酶有较高的耐盐性,能在2%–12%较宽的盐度范围下保持高效酶活力。而目前报道的多数细菌产生的木聚糖酶的酶活力适宜盐度范围较窄[31-32]或者在盐条件下会受到抑制[19]而不利于其在实践中的广泛应用。通过比较A. saponilacus和一些嗜盐细菌所合成木聚糖酶的耐盐特征(表 3),发现A. saponilacus所合成木聚糖酶的酶最适反应盐度范围相对较宽且该酶在适宜盐度时酶活力比无NaCl提升幅度也较大。此外,该酶的相对酶活力在盐度高达20%时还仍有约30%。因此,A. saponilacus生物合成的耐盐木聚糖酶对处理高盐及盐浓度波动较大条件下的农业废弃物有明显的优势。其次,A. saponilacus生物合成的木聚糖酶与其他细菌的木聚糖酶相比较(表 4),在较宽的温度和pH范围内也有相对高的酶活力。例如来源于A. saponilacus木聚糖酶最适反应温度较高(55 ℃)且耐高温(如在80 ℃时仍保持着20%以上的相对酶活力)。再次,A. saponilacus合成的木聚糖酶在去垢剂Triton X-100存在的情况下酶活力没有受到任何影响,且能在Tween 20存在下提高了酶活力,这表现出了该木聚糖酶的潜在工业应用价值。除此之外,A. saponilacus发酵生产木聚糖酶兼具细菌发酵周期短和真菌发酵酶活力高的优势,亦显示出广泛的工业应用前景(表 2)。
表 3. 来源于不同嗜盐细菌的木聚糖酶酶学性质比较 Table 3. Comparison of the xylanase characteristic from various halophiles
| Strain | Optimal salinity/% | Multiple of increase of xylanase activity in optimal salinity | References |
| A. saponilacus | 2.0–6.0 | 1.5 | This study |
| Thermoanaerobacterium saccharolyticum NTOU1 | 12.5 | 1.6 | [33] |
| Halophilic Bacterium-OKH | 15.0 | 5.0 | [34] |
| Strain CL8 DSM 12619 | 5.8 | 1.1 | [35] |
| Gracilibacillus sp. TSCPVG | 3.5 | 1.3 | [36] |
| Bacillus pumilus GESF-1 | 2.5 | 1.6 | [31] |
表选项
表 4. 不同细菌所产木聚糖酶酶学性质比较 Table 4. Comparison of the xylanase characteristic from various bacterium
| Strain | Medium pH | Optimal pH | The pH range at which the relative activity is more 40% | Optimal temperature/℃ | Temperature range at which the relative activity is more 40% | References |
| A. saponilacus | 9.7 | 7.0 | 5.5–8.0 | 55 | 30–60 | |
| Bacillus altitudinis DHN8 | 7.0 | 7.0 | 5.0–10.0 | 50 | 40–70 | [15] |
| Bacillus sp. PKD-9 | 6.0 | 8.0 | 5.0–9.0 | 55 | 45–60 | [37] |
| Bacillus pumilus GESF-1 | 7.0 | 8.0 | 6.0–9.0 | 40 | 30–60 | [31] |
| Chromohalobacter sp. TPSV 101 | 9.0 | 9.0 | 4.0–10.0 | 65 | 30–80 | [38] |
| Hypocrea orientalis EU7-22 | – | 4.5 | 4.0–5.0 | 55 | 30–60 | [39] |
| Paenibacillus sp. AR247 | 7.0 | 6.0 | 4.0–8.0 | 60 | 40–70 | [40] |
| Pseudomonas sp. WLUN024 | 8.5 | 7.2–8.0 | 7.0–9.0 | 50 | 25–70 | [25] |
| Pseudomonas sp. CL3 | – | 6.0 | 5.0–7.0 | 55 | 37–65 | [17] |
表选项
但是,A. saponilacus最适生长pH为9.7,而其合成的木聚糖酶最适反应pH为7.0;同时,该菌生长pH范围为7.5–10.5,而其木聚糖酶反应pH范围为4.0–10.0。这与我们在本研究开始时的假说存在很大差异,即预期获得能耐碱性pH环境的木聚糖酶。表 4所示,尽管菌株生长繁殖所需的pH不一定就是木聚糖酶的最适反应pH,但是差异基本都小于1个pH单位。因此,A. saponilacus所产的木聚糖酶的分子机制还需从基因、转录和蛋白水平进行深入探讨,将来可以应用定点突变技术使得该酶成为真正的耐碱酶。此外,本实验室A. saponilacus菌株正在进行全基因组完成图测序工作,期望不久的将来能够揭示其生物合成木聚糖酶的神秘面纱。因此,本研究启示嗜碱菌生物合成的木聚糖酶不一定具有嗜碱或耐碱性,若获得真正的嗜极酶还需进行大量的筛选。
综上所述,A. saponilacus产丙酸及其生物合成的木聚糖酶有一些特殊的特征。若能在全基因完成图基础上对其进行更深入的研究,那么产丙酸和木聚糖酶的特殊合成途径、功能及其调控等会更加清晰,可为研究该嗜极菌的潜在应用前景提供理论指导。
References
| [1] | Coughlan MP, Hazlewood GP. Beta-1, 4-D-xylan-degrading enzyme systems:biochemistry, molecular biology and applications. Biotechnology and Applied Biochemistry, 1993, 17(3): 259-289. |
| [2] | Motta FL, Andrade CCP, Santana MHA. A review of xylanase production by the fermentation of xylan: classification, characterization and applications//Chandel AK, Da Silva SS. Sustainable Degradation of Lignocellulosic Biomass-Techniques, Applications and Commercialization. New York: InTech, 2012. |
| [3] | Beg QK, Kapoor M, Mahajan L, Hoondal GS. Microbial xylanases and their industrial applications:a review. Applied Microbiology and Biotechnology, 2001, 56(3/4): 326-338. |
| [4] | Zhao BS, Chen SL. Alkalitalea saponilacus gen. nov., sp. nov., an obligately anaerobic, alkaliphilic, xylanolytic bacterium from a meromictic soda lake. International Journal of Systematic and Evolutionary Microbiology, 2012, 62(Pt 11): 2619-2623. |
| [5] | Shan ZQ, Zhou JG, Zhou YF, Yuan HY, Lü H. Isolation and characterization of an alkaline xylanasefrom a newly isolated Bacillus sp. QH14. Hereditas (Beijing), 2012, 34(3): 356-365. (in Chinese) 单志琼, 周峻岗, 周宇飞, 袁汉英, 吕红. 产碱性木聚糖酶菌株的筛选及酶学性质. 遗传, 2012, 34(3): 356-365. DOI:10.3760/cma.j.issn.1003-9406.2012.03.024 |
| [6] | Zhao BS, Liu J, Frear C, Holtzapple M, Chen SL. Consolidated bioprocessing of microalgal biomass to carboxylates by a mixed culture of cow rumen bacteria using anaerobic sequencing batch reactor (ASBR). Bioresource Technology, 2016, 222: 517-522. DOI:10.1016/j.biortech.2016.09.120 |
| [7] | Amore A, Parameswaran B, Kumar R, Birolo L, Vinciguerra R, Marcolongo L, Ionata E, La Cara F, Pandey A, Faraco V. Application of a new xylanase activity from Bacillus amyloliquefaciens XR44A in brewer's spent grain saccharification. Journal of Chemical Technology and Biotechnology, 2015, 90(3): 573-581. DOI:10.1002/jctb.2015.90.issue-3 |
| [8] | Seyis I, Aksoz N. Xylanase production from Trichoderma harzianum 1073 D3 with alternative carbon and nitrogen sources. Food Technology and Biotechnology, 2005, 43(1): 37-40. |
| [9] | Kapoor M, Nair LM, Kuhad RC. Cost-effective xylanase production from free and immobilized Bacillus pumilus strain MK001 and its application in saccharification of Prosopis juliflora. Biochemical Engineering Journal, 2008, 38(1): 88-97. DOI:10.1016/j.bej.2007.06.009 |
| [10] | Shen DK, Gu S, Bridgwater AV. Study on the pyrolytic behaviour of xylan-based hemicellulose using TG-FTIR and Py-GC-FTIR. Journal of Analytical and Applied Pyrolysis, 2010, 87(2): 199-206. DOI:10.1016/j.jaap.2009.12.001 |
| [11] | Shao W, Wiegel J. Purification and characterization of two thermostable acetyl xylan esterases from Thermoanaerobacterium sp. strain JW/SL-YS485. Applied and Environmental Microbiology, 1995, 61(2): 729-733. |
| [12] | Parajó JC, Domínguez H, Domínguez JM. Production of xylitol from concentrated wood hydrolysates by Debaryomyces hansenii:effect of the initial cell concentration. Biotechnology Letters, 1996, 18(5): 593-598. DOI:10.1007/BF00140209 |
| [13] | Suwannakham S, Yang ST. Enhanced propionic acid fermentation by Propionibacterium acidipropionici mutant obtained by adaptation in a fibrous-bed bioreactor. Biotechnology and Bioengineering, 2005, 91(3): 325-337. DOI:10.1002/(ISSN)1097-0290 |
| [14] | Chen YG, Randall AA, McCue T. The efficiency of enhanced biological phosphorus removal from real wastewater affected by different ratios of acetic to propionic acid. Water Research, 2004, 38(1): 27-36. DOI:10.1016/j.watres.2003.08.025 |
| [15] | Adhyaru DN, Bhatt NS, Modi HA. Enhanced production of cellulase-free, thermo-alkali-solvent-stable xylanase from Bacillus altitudinis DHN8, its characterization and application in sorghum straw saccharification. Biocatalysis and Agricultural Biotechnology, 2014, 3(2): 182-190. DOI:10.1016/j.bcab.2013.10.003 |
| [16] | Akila G, Chandra TS. A novel cold-tolerant Clostridium strain PXYL1 isolated from a psychrophilic cattle manure digester that secretes thermolabile xylanase and cellulase. FEMS Microbiology Letters, 2003, 219(1): 63-67. DOI:10.1016/S0378-1097(02)01196-5 |
| [17] | Cheng CL, Chang JS. Hydrolysis of lignocellulosic feedstock by novel cellulases originating from Pseudomonas sp. CL3 for fermentative hydrogen production. Bioresource Technology, 2011, 102(18): 8628-8634. DOI:10.1016/j.biortech.2011.03.053 |
| [18] | Chadha BS, Ajay BK, Mellon F, Bhat MK. Two endoxylanases active and stable at alkaline pH from the newly isolated thermophilic fungus, Myceliophthora sp. IMI 387099. Journal of Biotechnology, 2004, 109(3): 227-237. DOI:10.1016/j.jbiotec.2003.12.010 |
| [19] | Latif F, Asgher M, Saleem R, Akrem A, Legge RL. Purification and characterization of a xylanase produced by Chaetomium thermophile NIBGE. World Journal of Microbiology and Biotechnology, 2006, 22(1): 45-50. DOI:10.1007/s11274-005-5745-4 |
| [20] | Ryan SE, Nolan K, Thompson R, Gubitz GM, Savage AV, Tuohy MG. Purification and characterization of a new low molecular weight endoxylanase from Penicillium capsulatum. Enzyme and Microbial Technology, 2003, 33(6): 775-785. DOI:10.1016/S0141-0229(03)00176-5 |
| [21] | Franco PF, Ferreira HM, Filho EXF. Production and characterization of hemicellulase activities from Trichoderma harzianum strain T4. Biotechnology and Applied Biochemistry, 2004, 40(3): 255-259. DOI:10.1042/BA20030161 |
| [22] | Choudhury B, Sunita C, Singh SN, Ghosh P. Production of xylanase of Bacillus coagulans and its bleaching potential. World Journal of Microbiology and Biotechnology, 2006, 22(3): 283-288. DOI:10.1007/s11274-005-9033-0 |
| [23] | Bataillon M, Cardinali APN, Castillon N, Duchiron F. Purification and characterization of a moderately thermostable xylanase from Bacillus sp. strain SPS-0. Enzyme and Microbial Technology, 2000, 26(2/4): 187-192. |
| [24] | Sánchez-Herrera LM, Ramos-Valdivia AC, De La Torre M, Salgado LM, Ponce-Noyola T. Differential expression of cellulases and xylanases by Cellulomonas flavigena grown on different carbon sources. Applied Microbiology and Biotechnology, 2007, 77(3): 589-595. DOI:10.1007/s00253-007-1190-7 |
| [25] | Xu ZH, Bai YL, Xu X, Shi JS, Tao WY. Production of alkali-tolerant cellulase-free xylanase by Pseudomonas sp. WLUN024 with wheat bran as the main substrate. World Journal of Microbiology and Biotechnology, 2005, 21(4): 575-581. DOI:10.1007/s11274-004-3491-7 |
| [26] | Salles BC, Cunha RB, Fontes W, Sousa MV, Filho EXF. Purification and characterization of a new xylanase from Acrophialophora nainiana. Journal of Biotechnology, 2000, 81(2/3): 199-204. |
| [27] | Taneja K, Gupta S, Kuhad RC. Properties and application of a partially purified alkaline xylanase from an alkalophilic fungus Aspergillus nidulans KK-99. Bioresource Technology, 2002, 85(1): 39-42. DOI:10.1016/S0960-8524(02)00064-0 |
| [28] | Antoine AA, Jacqueline D, Thonart P. Xylanase production by Penicillium canescens on soya oil cake in solid-state fermentation. Applied Biochemistry and Biotechnology, 2010, 160(1): 50-62. DOI:10.1007/s12010-008-8496-5 |
| [29] | Singh S, Reddy P, Haarhoff J, Biely P, Janse B, Pillay B, Pillay D, Prior BA. Relatedness of Thermomyces lanuginosus strains producing a thermostable xylanase. Journal of Biotechnology, 2000, 81(2/3): 119-128. |
| [30] | Xiong HR, Von Weymarn N, Leisola M, Turunen O. Influence of pH on the production of xylanases by Trichoderma reesei Rut C-30. Process Biochemistry, 2004, 39(6): 731-736. DOI:10.1016/S0032-9592(03)00178-X |
| [31] | Menon G, Mody K, Keshri J, Jha B. Isolation, purification, and characterization of haloalkaline xylanase from a marine Bacillus pumilus strain, GESF-1. Biotechnology and Bioprocess Engineering, 2010, 15(6): 998-1005. DOI:10.1007/s12257-010-0116-x |
| [32] | Wang CY, Chan H, Lin HT, Shyu YT. Production, purification and characterisation of a novel halostable xylanase from Bacillus sp. NTU-06. Annals of Applied Biology, 2010, 156(2): 187-197. DOI:10.1111/aab.2010.156.issue-2 |
| [33] | Hung KS, Liu SM, Tzou WS, Lin FP, Pan CL, Fang TY, Sun KH, Tang SJ. Characterization of a novel GH10 thermostable, halophilic xylanase from the marine bacterium Thermoanaerobacterium saccharolyticum NTOU1. Process Biochemistry, 2011, 46(6): 1257-1263. DOI:10.1016/j.procbio.2011.02.009 |
| [34] | Sanghvi G, Jivrajani M, Patel N, Jivrajani H, Bhaskara GB, Patel S. Purification and characterization of haloalkaline, organic solvent stable xylanase from newly isolated halophilic bacterium-OKH. International Scholarly Research Notices, 2014, 2014: 198251. |
| [35] | Wejse PL, Ingvorsen K, Mortensen KK. Purification and characterisation of two extremely halotolerant xylanases from a novel halophilic bacterium. Extremophiles, 2003, 7(5): 423-431. DOI:10.1007/s00792-003-0342-7 |
| [36] | Chakdar H, Kumar M, Pandiyan K, Singh A, Nanjappan K, Kashyap PL, Srivastava AK. Bacterial xylanases:biology to biotechnology. 3 Biotech, 2016, 6(2): 150. DOI:10.1007/s13205-016-0457-z |
| [37] | Panwar D, Srivastava PK, Kapoor M. Production, extraction and characterization of alkaline xylanase from Bacillus sp. PKD-9 with potential for poultry feed. Biocatalysis and Agricultural Biotechnology, 2014, 3(2): 118-125. DOI:10.1016/j.bcab.2013.09.006 |
| [38] | Prakash S, Veeranagouda Y, Kyoung L, Sreeramulu K. Xylanase production using inexpensive agricultural wastes and its partial characterization from a halophilic Chromohalobacter sp. TPSV 101. World Journal of Microbiology and Biotechnology, 2009, 25(2): 197-204. DOI:10.1007/s11274-008-9880-6 |
| [39] | Li HL, Wu HL, Jiang FJ, Wu JL, Xue Y, Gan LH, Liu J, Long MN. Heterologous expression and characterization of an acidic GH11 family xylanase from Hypocrea orientalis. Applied Biochemistry and Biotechnology, 2018, 184(1): 228-238. DOI:10.1007/s12010-017-2532-2 |
| [40] | Di Marco E, Soraire PM, Romero CM, Villegas LB, Martínez MA. Raw sugarcane bagasse as carbon source for xylanase production by Paenibacillus species:a potential degrader of agricultural wastes. Environmental Science and Pollution Research, 2017, 24(23): 19057-19067. DOI:10.1007/s11356-017-9494-3 |
