张燕1, 张阳1, 张博1, 吴小刚1

 , 张力群2
, 张力群2 1.广西农业环境与农产品安全重点实验室培育基地, 植物科学国家级实验教学示范中心, 广西大学农学院, 广西 南宁 530004;
2.中国农业大学植物病理系, 农业部植物病理学重点开放实验室, 北京 100193
收稿日期:2017-07-16;修回日期:2017-10-19;网络出版日期:2017-10-23
基金项目:广西大学科研基金(XGZ160171);广西自然科学基金(2016GXNSFCA380024)
*通信作者:吴小刚, Tel/Fax:+86-771-3234576, E-mail:wuxiaogang@foxmail.com
摘要:[目的]包括碳源代谢等不同环境因子可调控生防菌株生防相关因子表达,进而影响其防病效果。荧光假单胞菌2P24可防治多种植物病原真菌、细菌引起的土传病害,抗生素2,4-二乙酰基间苯三酚(2,4-diacetylphoroglucinol,2,4-DAPG)是其主要生防因子之一。本文利用平板对峙法及遗传学方法研究不同碳源对菌株2P24产生2,4-DAPG的影响及相关的调控途径。[方法]利用平板对峙法检测了菌株2P24在添加葡萄糖、果糖和蔗糖等碳源的土豆浸液培养基中对棉花立枯丝核菌(Rhizoctonia solani)的拮抗能力及菌株2P24中影响2,4-DAPG产生的相关基因的表达。另外,利用Tn5转座子对含有2,4-DAPG合成基因phlA报告质粒p970Gm-phlAp的野生型菌株2P24进行随机突变,在果糖土豆浸液培养基中筛选提高phlA基因表达的突变菌株。[结果]平板对峙实验表明,菌株2P24以葡萄糖为碳源时其抑菌活性最强,蔗糖次之,而以果糖等为碳源时菌株2P24无抑菌活性;转录融合实验进一步表明葡萄糖可促进phlA基因的表达,果糖则不影响phlA基因的表达。在果糖土豆浸液培养基中,转座子随机突变实验获得了5株可明显提高phlA基因表达的突变菌株。Tn5插入位点和序列分析显示其中一个突变体是Tn5破坏了cheB基因。转录检测表明与野生菌株相比,cheB突变体中phlA基因的表达和2,4-DAPG的前体物质间苯三酚(phloroglucinol,PG)产量都显著提高。游动性实验发现突变cheB基因可显著降低该菌株的游动性。[结论]上述结果表明菌株2P24中不同碳源在转录水平上可影响phlA基因的表达,进而影响2,4-DAPG产生。遗传学结果也显示,cheB基因参与调控2,4-DAPG生物合成过程。
关键词: 荧光假单胞菌 碳源代谢 2, 4-二乙酰基间苯三酚 游动性
Effect of carbon sources on production of 2, 4-diacetylphoroglucinol in Pseudomonas fluorescens 2P24
Yan Zhang1, Yang Zhang1, Bo Zhang1, Xiaogang Wu1

 , Liqun Zhang2
, Liqun Zhang2 1.National Demonstration Center for Experimental Plant Science Education, College of Agriculture, Guangxi University, Nanning 530004, Guangxi Zhuang Autonomous Region, China;
2.Key Open Laboratory for Plant Pathology, Ministry of Agriculture, Department of Plant Pathology, China Agricultural University, Beijing 100193, China
Received 16 July 2017; Revised 19 October 2017; Published online 23 October 2017
*Corresponding author: Xiaogang Wu, Tel/Fax: +86-771-3234576; E-mail: wuxiaogang@foxmail.com
Supported by the Scientific Research Foundation of Guangxi University (XGZ160171) and by the Natural Science Foundation of Guangxi (2016GXNSFCA380024)
Abstract: [Objective]Many environmental factors, such as carbon sources, regulate the biosynthesis of antimicrobial compounds and influence the biocontrol capacity of Pseudomonas fluorescens. P. fluorescens 2P24 protects various crop plants against root diseases caused by plant pathogens. Among a range of antimicrobial compounds secreted by 2P24, 2, 4-diacetylphloroglucinol (2, 4-DAPG) is the major determinant of its biocontrol potential. This study investigated the impact of exposing strain 2P24 to selected carbon sources on the production of 2, 4-DAPG.[Methods]Antifungal activity of strain 2P24 was tested on potato infusion agar with different carbon sources against Rhizoctonia solani. The reporter strain 2P24 (p970Gm-phlAp) was subjected to a random mini-Tn5 insertion mutagenesis. The collection of Tn5 insertion mutants was then screened for improved phlA expression on potato infusion agar with fructose.[Results]Strain 2P24 cultured on potato infusion with glucose strongly inhibited the growth of R. solani, whereas no inhibition was observed on potato infusion agar with or without fructose. Five mutants with significantly increased phlA expression were identified and the interrupted locus in one of them was identified as the cheB gene. Genetic analysis showed that the expression of phlA and the production of phloroglucinol (PG) were strongly increased in the cheB mutant as compared with the parental strain.[Conclusion]Carbon resources influenced the expression of phlA and 2, 4-DAPG production and some genetic factors involved in carbon sources to regulate the production of 2, 4-DAPG.
Key words: Pseudomonas fluorescens carbon catabolic 2, 4-diacetylphoroglucinol swarming motility
土壤中尤其是植物根围栖息着许多具有生防潜力的微生物资源,这些微生物通过调节根部微生态环境及竞争生态位点等方式,抑制土壤中病原菌对植物造成的危害[1]。另外,由于化学农药引起的抗药性以及可持续农业发展的需求,生物杀菌剂研发与生产存在着无可置疑的市场潜力。然而,大多数生防菌株由于种植季节、田间位点、作物差异等因素,导致防治效果不稳定,阻碍了其商业发展[1]。生防菌株田间防病效果不稳定性的原因,很大程度上是由于自然环境中物理或化学等环境因素导致的。因此解析环境因素影响生防效果发挥的分子机制是提高生防菌株生防效果及可靠性的关键。
荧光假单胞菌(Pseudomonas fluorescens)是一类广泛用于研究生防菌株作用机理的模式菌株[2]。前期研究表明,荧光假单胞菌的主要生防机制之一是产生抑菌次生代谢产物,如2, 4-二乙酰基间苯三酚(2, 4-diacetylphloroglucinol,2, 4-DAPG)、藤黄绿脓菌素(pyoluteorin)、硝吡咯菌素(pyrrolnitrin)和吩嗪-1-羧酸(phenzaine-1-carboxylate)等。这些次生代谢产物不仅直接抑制病原菌的生长,还可改善生防细菌在作物根围的生态适应性,从而进一步影响生防细菌的长期防病效果[3]。荧光假单胞菌中2, 4-DAPG的合成主要以乙酰辅酶A为底物,在聚酮合成酶PhlD的催化下合成前体物质间苯三酚(phloroglucinol,PG),之后PhlA、PhlB和PhlC三个蛋白共同作用将乙酰基团转移到PG上,最终合成2, 4-DAPG[4]。前期遗传学研究表明,许多遗传因子在转录水平及转录后水平参与调控2, 4-DAPG的生物合成过程。如P. fluorescens F113中,特异性调控因子PhlF通过结合2, 4-DAPG合成基因簇phlACBD启动子区域的特定位点pho结合位点,从而抑制phlACBD的转录,影响2, 4-DAPG产量[4]。除特异性调控因子外,目前也发现多个遗传因子在转录后水平调控2, 4-DAPG的产生,如双组分调控系统GacS/GacA (two-component system GacS/GacA)通过激活非编码RNA (non-coding small RNA) RsmX、RsmY和RsmZ的表达,阻遏其相对应的RNA结合蛋白(RNA binding protein) RsmA和RsmE功能,从而精细调控2, 4-DAPG的产生[5]。
生防荧光假单胞菌2P24分离自小麦全蚀病自然衰退土壤,可防治多种土传病害[6]。前期已发现多个新颖的遗传因子调控该菌株主要生防性状2, 4-DAPG的产生,然而非遗传因子及其调控网络对2, 4-DAPG产生的影响目前还不清楚。本文检测了不同碳源条件下菌株2P24中2, 4-DAPG合成基因phlA表达及PG产量的变化,并通过Tn5随机突变的方法筛选果糖培养基中影响2, 4-DAPG产生的调控因子,从而进一步明确环境因子-遗传因子相互作用对2, 4-DAPG产生的影响。
1 材料和方法 1.1 菌株、质粒、引物及培养条件 本实验中用到的菌株、质粒和引物列于表 1中。菌株2P24及其突变菌株、质粒重组所需的Escherichia coli培养条件参照文献[7]。土豆浸液培养基中各种碳源终浓度为0.1 mol/L。抗生素使用浓度分别为:氨苄青霉素(Ap) 50 μg/mL,卡那霉素(Km) 50 μg/mL,四环素(Tet) 20 μg/mL,庆大霉素(Gm) 50 μg/mL。用于重组质粒筛选的5-溴-4-氯-3-吲哚-β-D-半乳糖苷(X-Gal)终浓度为40 μg/mL。
表 1. 本实验所用菌株、质粒及引物 Table 1. Bacterial strains, plasmids and primers used in this study
| Name | Relevant characteristics or sequences (5′→3′) | Reference or source |
| Strains Pseudomonas fluorescens | ||
| 2P24 | Wild type | Laboratory |
| 2P24ΔgacA | Derivative of 2P24, gacA gene in-frame deletion | [8] |
| 2P24Δvfr | Derivative of 2P24, vfr gene in-frame deletion | This work |
| 2P24ΔphlA | Derivative of 2P24, phlA gene in-frame deletion | Laboratory |
| 2P24Δcra | Derivative of 2P24, cra gene in-frame deletion | This work |
| 2P24ΔcheB | Derivative of 2P24, cheB gene in-frame deletion | This work |
| 2P24(p970Gm-phlA) | 2P24 containing the phlA-lacZ transcriptional fusion | Laboratory |
| Escherichia coli | ||
| DH5α | supE 44 ΔlacU 169 (80lacZ ΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | Laboratory |
| S17-1(λ-π) | pro thi hsdR Tpr Smr: chromosome::RP4-2 Rc::Mu-Km::Tn7 | Laboratory |
| Agrobacterium tumefaciens NTL4(pZLR4) | A. tumefaciens NT1 derivative carrying a traG-lacZ reporter fusion, AHL biosensor | [9] |
| Rhizoctonia solani | Plant pathogen for cotton | Laboratory |
| Plasmids | ||
| pUT-Km | Mini-Tn5 delivery plasmid with Kmr, Apr | [10] |
| p970Gm-phlAp | phlA-lacZ transcriptional fusion, Gmr | [11] |
| p970Km-rsmXp | rsmX-lacZ transcriptional fusion, Kmr | [11] |
| p970Gm-rsmYp | rsmY-lacZ transcriptional fusion, Gmr | [11] |
| p970Km-rsmZp | rsmZ-lacZ transcriptional fusion, Kmr | [11] |
| pRK415 | Broad-host-range cloning vector, Tetr | [12] |
| p2P24Km | Cloning vector, Kmr | Laboratory |
| p2P24△cra | p2P24Km carrying a 1.6 kb Hind Ⅲ-EcoR Ⅰ insert with a deletion in cra, Kmr | This work |
| p2P24△vfr | p2P24Km carrying a 2 kb BamH Ⅰ-EcoR Ⅰ insert with a deletion in vfr, Kmr | This work |
| p2P24△cheB | p2P24Km carrying a 1.9 kb Hind Ⅲ-EcoR Ⅰ insert with a deletion in cheB, Kmr | This work |
| p6013-phlA | phlA′-′lacZ translational fusion, Tetr | Laboratory |
| p415-cheB | pRK415 with a 2 kb Pst Ⅰ-Xba Ⅰ fragment containing cheB gene of 2P24, Tetr | This work |
| Primers | Sequence (5′→3′) | Restriction site |
| Cra-EcoRI1 | ATGAATTCCAGCACCACCGCTTCCAGCGGC | EcoR Ⅰ |
| Cra-840-2 | ACGCATCAGCAGCGCCACCGTCGAACAATTGCTCGACTTCCTGCCGCTGC | |
| Cra-3 | GCAGCGGCAGGAAGTCGAGCAATTGTTCGACGGTGGCGCTGCTGATGCGT | |
| Cra-780-HindⅢ4 | ATGAAGCTTGCAGCAGGCGCAGGTGCTCGTC | Hind Ⅲ |
| vfr-EcoRI-F1 | ACGAATTCGATGATTTCATAATCGTGGGTG | |
| vfr-R1 | ACCAGGTTGCGCTCTTCCAGGTCCTAGTTTGTCGAGATTCTTGATCTTGA | |
| Vfr-F2 | TCAAGATCAAGAATCTCGACAAACTAGGACCTGGAAGAGCGCAACCTGGT | |
| vfr-BamHI-R2 | ACGGATCCAATGATCGTGCGCTGGTCATGC | BamH Ⅰ |
| cheB1EcoRI | ATGAATTCCGACGACGGCAAAGGCATGGAC | EcoR Ⅰ |
| 1KBcheB2 | CCATGCCGGTGAGTACCACCGCCAGCTGAATGCTCGTGTCCGCCGAAAGA | |
| 900bpcheB3 | TCTTTCGGCGGACACGAGCATTCAGCTGGCGGTGGTACTCACCGGCATGG | |
| cheB4HindⅢ | AGTAAGCTTCGCGGCCCAGCGGGGATAAATC | Hind Ⅲ |
| CheBPstI | ATGCTGCAGTCCGGCGCCACCATCACCGGCGACG | PstⅠ |
| CheBXbaI | GATCTAGAACGCCATGATGATGCCGATC | XbaⅠ |
| The underline in the sequence represents the restriction site. | ||
表选项
1.2 P. fluorescens 2P24菌株及其突变菌株对病原菌的拮抗作用 将棉花立枯丝核菌Rhizoctonia solani菌饼接入含有不同碳源的土豆浸液培养基平板中央,在距离菌饼边缘2.5 cm处接种5 μL待测菌液,培养24 h后,测量抑菌带距离。每个处理重复3次。
1.3 DNA操作和序列分析 质粒DNA提取、限制性酶切、琼脂糖凝胶电泳、感受态制备、连接、转化等操作参考文献[13]。质粒电击转化到P. fluorescens菌株中的方法参照文献[7]。DNA序列测序由生工生物工程(上海)股份有限公司完成。核苷酸序列以及推测的蛋白序列分析由在线BLAST搜索引擎完成(https://blast.ncbi.nlm.nih.gov/Blast.cgi)。
1.4 菌株2P24中间苯三酚定量检测 将菌株2P24及其衍生菌株接种于相应培养基中28 ℃摇培48 h。取150 μL菌液,12000 r/min离心1 min,将上清液转移至新的离心管中。依次加入50 μL浓HCL和200 μL肉桂醛盐酸试剂,混匀室温反应5 min后,分光光度计测量OD550吸光值。具体过程参照文献[14]。本实验重复3次。
1.5 转录、转录后报告融合结构活性检测 将非编码RNA编码基因rsmX、rsmY和rsmZ转录报告质粒p970Km-rsmXp[11]、p970Gm-rsmYp[11]和p970Km-rsmZp[11]及phlA基因转录报告质粒p970Gm-phlAp[11]和phlA基因转录后报告质粒p6013-phlA (实验室保存)转入相应菌株中,用以检测相关基因转录水平或转录后水平的表达情况。β-半乳糖苷酶活性的检测方法参照文献[15]。本实验重复3次。
1.6 突变菌株的筛选及Tn5的定位 以含有phlA-lacZ转录报告质粒(p970Gm-phlAp)的2P24菌株为出发菌株,利用Tn5转座子对该菌株进行随机突变,果糖土豆浸液培养基(含X-Gal)中筛选菌落颜色明显变蓝(表明phlA基因表达增强)的突变菌株。利用鸟枪法克隆Tn5及其两侧侧翼序列基因并测序,以确定Tn5插入位置。
1.7 cra、vfr及cheB基因内缺失突变菌株的构建 cra、vfr及cheB基因内缺失突变菌株通过双交换同源重组的方法得到,具体方法如下:分别利用引物Cra-EcoRI1/Cra-840-2和Cra-3/Cra-780- HindⅢ4、vfr-EcoRI-F1/vfr-R1和vfr-F2/vfr-BamHI- R2及cheB1EcoRI/1KBcheB2和900bpcheB3/ cheB4HindⅢ,通过PCR的方法分别得到cra、vfr及cheB基因上下两段侧翼序列,将得到的片段酶切后克隆到自杀载体p2P24Km中,获得自杀载体p2P24△cra、p2P24△vfr和p2P24△cheB。将获得的载体分别电击转入菌株2P24中,二次同源交换后即获得cra、vfr及cheB基因内缺失突变菌株。为互补2P24△cheB突变菌株,利用引物CheBPstI和CheBXbaI (表 1)以2P24基因组为模板PCR扩增得到完整的cheB基因,克隆到穿梭载体pRK415中得到互补载体p415-cheB。
1.8 游动性、生物膜的检测和群体感应系统信号分子(quorum sensing, QS)检测 菌株2P24及其突变菌株过夜培养后,将5 μL培养液滴到琼脂浓度0.3%的LB平板上,28 ℃过夜培养后,测量细菌游动性。生物膜的检测方法参照文献[7]。为检测QS系统信号分子的产量,菌株2P24及cheB突变体28 ℃培养12 h后,取3 μL菌液滴于QS系统报告菌A. tumefaciens NTL4 (pZLR4)检测平板中,28 ℃过夜培养后即可观察结果。本实验重复3次,每个处理3个重复。
2 结果和分析 2.1 不同碳源对菌株2P24中2, 4-DAPG产生的影响 前期研究表明,环境因子可影响抗生素2, 4-DAPG的产生[2]。菌株2P24中2, 4-DAPG产量的高低与其对病原菌拮抗能力强弱呈正相关[6]。本研究测定了不同碳源条件下菌株2P24对棉花立枯丝核菌(Rhizoctonia solani)抑制能力的影响。实验结果表明在不添加任何碳源的土豆浸汁培养基中菌株2P24不能抑制棉花立枯丝核菌的生长;添加0.1 mol/L葡萄糖则可明显抑制棉花立枯丝核菌的生长,添加0.1 mol/L蔗糖其抑菌效果弱于葡萄糖,而添加果糖、甘露醇、葡萄糖酸钠和丁二酸钠等碳源则完全不能抑制棉花立枯丝核菌的生长(表 2)。而对照菌株phlA基因突变菌株在上述碳源条件下对R. solani病原菌都没有拮抗能力(表 2)。有意思的是,在0.1 mol/L葡萄糖土豆浸汁培养基中额外添加0.1 mol/L果糖可削弱菌株2P24对R. solani的抑制作用(表 2)。这些结果表明菌株2P24中葡萄糖可促进2, 4-DAPG的产生,而果糖、甘露醇、葡萄糖酸钠和丁二酸钠等碳源不影响2, 4-DAPG的产生。
表 2. 不同碳源影响菌株2P24对棉花立枯丝核菌的抑制作用 Table 2. Inhibition of R. solani by 2P24 on potato infusion agar added different carbon sources
| Carbon source | Inhibitory zone*/cm | |
| 2P24 | 2P24ΔphlA | |
| Glucose | 0.61±0.05a | 0 |
| Fructose | 0 d | 0 |
| Mannitol | 0 d | 0 |
| Sucrose | 0.32±0.05c | 0 |
| Sodium D-gluconate | 0 d | 0 |
| Sodium succinate | 0 d | 0 |
| Glucose+Frucose | 0.43±0.04b | 0 |
| Potato infusion | 0 d | 0 |
| *Data represent the averages of 3 replicates per treatment. Means followed by different letters differ significantly at P=0.05, according to Tukey′s honestly significant difference post-hoc teat. | ||
表选项
为研究碳源对2, 4-DAPG产生调控的分子机制,本研究检测了葡萄糖、果糖和蔗糖对2, 4-DAPG合成基因簇phlACBD表达的影响。检测菌株生长量发现,在土豆浸汁培养基中添加或不添加葡萄糖、果糖或蔗糖并不影响菌株2P24的生长(数据未列)。而转录报告实验发现,与对照菌株相比,葡萄糖可明显诱导phlACBD基因簇的表达,蔗糖对phlACBD基因簇的诱导作用弱于葡萄糖,而果糖则不影响phlACBD基因簇的表达(图 1-A)。同样,转录后水平检测也表明与对照相比,葡萄糖转录后促进phlA表达,而果糖则不影响该基因表达(图 1-B)。这些结果表明葡萄糖通过激活2, 4-DAPG合成基因簇的表达,从而促进2, 4-DAPG的产生。
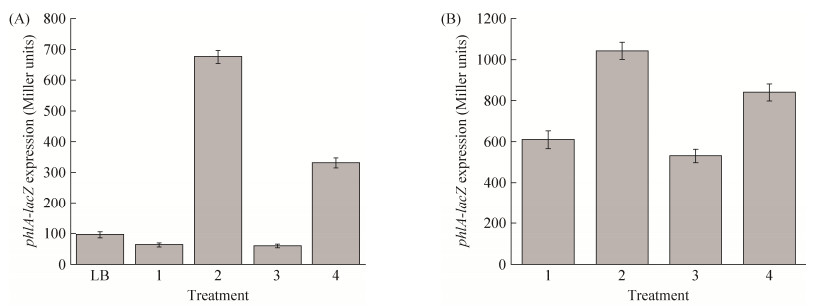 |
| 图 1 不同碳源条件下菌株2P24中2, 4-DAPG合成基因簇的表达 Figure 1 The effect of carbon sources on the expression of 2, 4-DAPG biosynthesis genes in P. fluorescens 2P24. Strain 2P24 was cultured in the LB broth, the potato fusion broth (1), the potato fusion broth with 0.1 mol/L glucose (2), the potato fusion broth with 0.1 mol/L fructose (3), and the potato fusion broth with 0.1 mol/L surcose (4) at 28 ℃ and the expression of the phlA-lacZ transcriptional fusion (A) or the phlA′-′lacZ translational fusion (B) was checked at 12 h post-inoculation. All experiments are performed in triplicate, and the x±s are indicated. |
| 图选项 |
2.2 菌株2P24中与碳源代谢相关遗传因子对2, 4-DAPG产生的影响 前期研究表明,多个遗传因子参与碳源代谢[16]。其中Cra蛋白(catabolite repressor/activator)通过中央代谢途径影响碳源通量,从而调控多个基因表达[17]。Pseudomonas属中碳源代谢抑制蛋白(carbon catabolite repression) Vfr蛋白通过结合胞内cAMP调控细菌一些重要的生物性状,包括多种次生代谢物的产生[18]。本文构建了cra和vfr突变菌株并检测生防相关性状。平板拮抗及转录后水平实验表明,缺失cra和vfr基因不影响果糖及葡萄糖培养基中菌株2P24对棉花立枯丝核菌的抑制作用(数据未列)及phlA基因转录后水平的表达(图 2-A)。
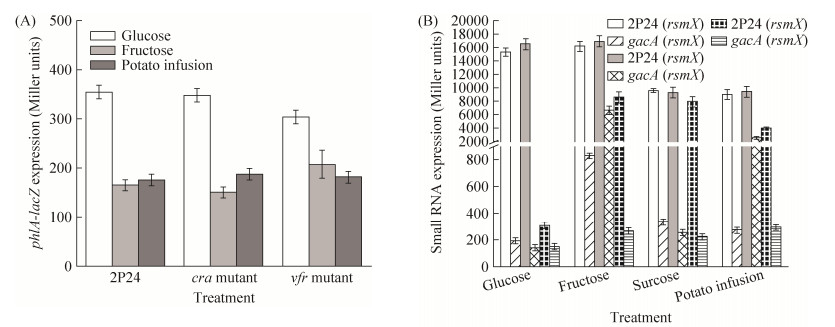 |
| 图 2 菌株2P24中cra和vfr基因对phlA表达的影响(A)及不同碳源对非编码RNA编码基因的影响(B) Figure 2 The effect of cra and vfr genes on phlA gene expression (A) and different carbon resources on non-coding RNAs expression (B) in P. fluorescens 2P24. Strain 2P24 and its derivatives were cultured in indicated broth at 28 ℃ and the expression of the phlA′-′lacZ translational fusion or rsmX/Y/Z-lacZ transcriptional fusion were checked at 12 h post-inoculation. All experiments are performed in triplicate, and the x±s are indicated. |
| 图选项 |
除此之外,E. coli菌株中CsrA-CsrB调控系统调控糖原异生过程,且P. fluorescens CHA0中三羧酸循环中间产物通过CsrA-CsrB类调控系统RsmA/RsmE影响生防相关因子表达[19]。本研究也检测了不同碳源条件下菌株2P24中非编码RNA编码基因rsmX、rsmY和rsmZ的表达情况。结果表明与野生型菌株相比,不同碳源条件下缺失gacA基因rsmX、rsmY和rsmZ基因的表达都显著降低,但碳源本身并不影响这些基因的表达(图 2-B)。以上结果表明,不同碳源条件下菌株2P24中抗生素2, 4-DAPG产量的变化并不是通过Vfr、Cra和非编码RNA调控途径参与调控的。
2.3 果糖条件下影响2, 4-DAPG产生的突变体筛选 不同菌株对碳源利用不同,对下游性状如次生代谢产物的影响也不尽相同(图 2)。为进一步研究菌株2P24中碳源代谢对抗生素2, 4-DAPG调控途径,本研究利用Tn5转座子对含有2, 4-DAPG合成基因phlA转录报告质粒p970Gm-phlAp的野生型菌株进行随机突变,在果糖条件下筛选影响phlA基因表达的调控因子。在大约10000株突变体中筛选得到5株可明显提高phlA基因表达(即菌落颜色明显变蓝)的突变体。
转录水平检测发现,与野生菌株相比,在果糖培养液中这些突变体中phlA基因表达显著提高(图 3-A)。进一步检测PG时发现,这些突变体中PG产量也显著高于菌株2P24 (图 3-B)。平板拮抗实验进一步表明果糖土豆浸液培养基中这些突变体可抑制棉花立枯丝核菌的生长。另外在葡萄糖土豆浸液培养基中,与野生菌株相比,这些突变体对棉花立枯丝核菌的拮抗能力也明显发生变化,如突变体M2其拮抗能力较野生型明显增强,而突变体M12的拮抗能力较野生型明显降低(表 3)。这些结果表明菌株2P24中可能存在多个遗传因子在不同碳源条件下影响2, 4-DAPG的产生。
 |
| 图 3 各突变体中phlA基因表达的变化(A)及PG产量测定(B) Figure 3 The expression of phlA gene (A) and production of PG (B) were measured in strain 2P24 and its mutants. Expression of the phlA-lacZ transcriptional fusion in strain 2P24 and its mutants was measured the potato fusion broth with 0.1 mol/L fructose at 28 ℃ after12 h post-inoculation. All experiments are performed in triplicate, and the x±s are indicated. |
| 图选项 |
表 3. 不同碳源影响菌株2P24及其突变菌株对棉花立枯丝核菌的抑制作用 Table 3. Inhibition of R. solani by 2P24 and its derivatives on different carbon sources medium
| Strains | Inhibitory zone*/cm | |
| Fructose | Glucose | |
| M1 | 0.13±0.03d | 0.50±0.05b |
| M2 | 0.70±0.06b | 0.70±0.05a |
| M4 | 0.98±0.07a | 0.30±0.02d |
| M11 | 0.10±0.02d | 0.30±0.03d |
| M12 | 0.37±0.03c | 0.27±0.02d |
| 2P24 | 0e | 0.40±0.05c |
| *Data represent the averages of 3 replicates per treatment. Means within the same column followed by different letters differ significantly at P=0.05, according to Tukey′s honestly significant difference post-hoc teat. | ||
表选项
2.4 cheB基因调控群体感应系统及游动性 上述突变体参与2, 4-DAPG的产生,因此本研究利用鸟枪法将这些突变体中Tn5侧翼序列进行克隆、测序,序列分析表明其中一株突变体M4中Tn5破坏了cheB基因。CheB蛋白属于甲基酯酶,主要影响控制细菌游动性过程中化学受体(chemoreceptor)的去甲基化[20]。游动性实验表明突变cheB基因显著影响菌株2P24的游动性(图 4-A)。遗传学研究进一步表明,突变cheB基因可显著提高phlA基因表达,而其互补菌株phlA基因的表达可恢复得到野生型水平,表明果糖土豆浸液培养基条件下CheB抑制phlA基因的表达(图 4-B)。PG检测实验进一步发现,菌株2P24中突变cheB基因也可显著提高PG的产量(图 4-C)。另外,拮抗实验表明破坏cheB基因可降低该菌株在葡萄糖为碳源条件下对棉花立枯丝核菌的拮抗能力(表 3)。这些结果表明菌株2P24中cheB基因可能参与碳源代谢过程中对抗生素2, 4-DAPG产生的调控过程。
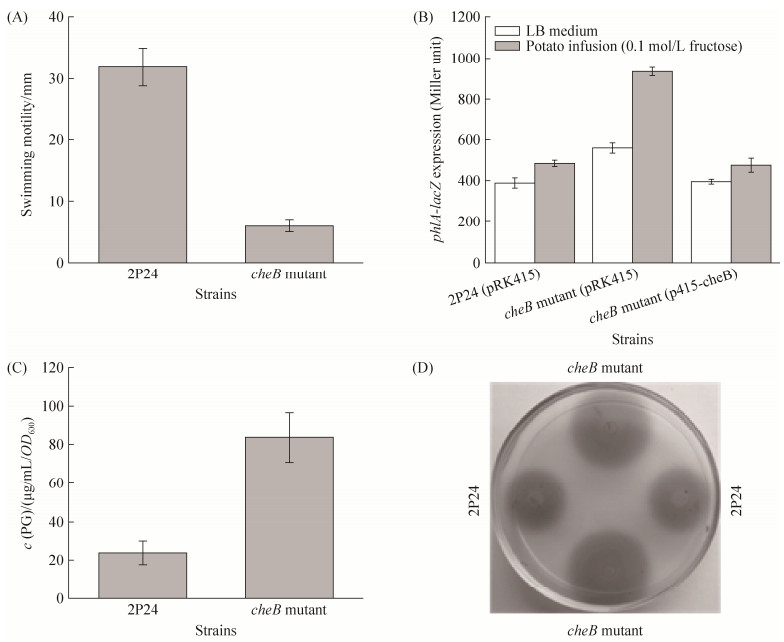 |
| 图 4 菌株2P24中cheB基因调控游动性(A)、phlA基因转录表达(B)及PG产量(C)、群体感应信号分子产生(D) Figure 4 The effect of cheB gene on motility (A), expression of the phlA gene (B), PG production (C) and quorum sensing system (D) in P. fluorescens 2P24. Expression of the phlA-lacZ transcriptional fusion in strain 2P24 and its derivatives was measured the potato fusion broth with 0.1 mol/L fructose or LB broth at 28 ℃ after12 h post-inoculation. For analysis of QS signals, strain 2P24 and the cheB mutant were grown in LB broth at 28 ℃ for 24 h, 3 μL of cultures were spotted on the plates containing QS signals biosensor A. tumefaciens NTL4(pZLR4). After incubation at 28 ℃ for 10 h, the picture was taken. All experiments are performed in triplicate, and the x±s are indicated. |
| 图选项 |
PcoR-PcoI群体感应系统(quorum sensing system,QS)是菌株2P24防治植物土传病害的另一个主要生防因子,该系统参与调控生物膜(biofilm)的形成及菌株2P24在植物根围的定殖能力,从而影响该菌株生防能力[7]。为检测cheB基因是否影响该菌株其他生防相关性状的表达,本研究检测了群体感应系统信号分子的产量,结果表明突变cheB可显著提高群体感应信号分子产生(图 4-D),表明菌株2P24中cheB基因负调控QS系统。这些结果也揭示菌株2P24中CheB蛋白是一个重要的、全局性调控生防相关性状的遗传因子。
3 讨论 生物和非生物因素可调控作物根围有益微生物相关基因表达,从而影响微生物-作物之间相互作用。前期研究表明许多内在的生物遗传因子调控微生物相关基因表达,而非生物因子如碳源影响微生物基因表达的作用机理并不清楚[2]。碳源不仅直接作为能量来源影响微生物生长代谢,而且参与调控根围微生物次生代谢物的产生。除此之外,不同菌株中碳源调控微生物表型的结果截然不同。如生防菌株P. fluorescens F113中,蔗糖、果糖和甘露醇可促进2, 4-DAPG的产量,而葡萄糖则不会促进该次生代谢产物的产生;而菌株CHA0中葡萄糖则可促进生防2, 4-DAPG的积累[21-22]。本研究从分子水平发现菌株2P24中,葡萄糖和蔗糖可促进2, 4-DAPG的产生,主要原因是葡萄糖和蔗糖能激活2, 4-DAPG合成基因簇的表达;而果糖、甘露醇等碳源并不影响2, 4-DAPG合成基因簇的表达(图 1)。检测已知与碳源代谢相关遗传因子的研究发现,Cra蛋白、Vfr蛋白及非编码RNA分子并不参与葡萄糖促进2, 4-DAPG产生的调控网络(图 2),表明菌株2P24中存在其他遗传因子参与调控碳源代谢对生防相关因子的调控。
利用经典遗传学方法,本研究发现多个遗传因子参与碳源影响2, 4-DAPG产生,转录水平检测、PG检测及拮抗实验证实这些突变体影响抗生素2, 4-DAPG产生。另外,研究发现PG产量与phlA转录水平和拮抗实验并不完全一致,表明这些突变体中Tn5破坏的基因可能在不同的合成途径中影响抗生素2, 4-DAPG的产生(图 3,表 3)。对cheB基因突变菌株研究发现,该突变体可显著提高菌株2P24在果糖培养基中对棉花立枯丝核菌的拮抗作用,同时也影响了菌株2P24在葡萄糖土豆浸液培养基中2, 4-DAPG产生的过程(表 3)。除影响2, 4-DAPG产生外,cheB基因负调控群体感应信号分子的合成。革兰氏阴性菌中,群体感应信号分子合成由LuxI类蛋白负责,LuxI类蛋白S-腺苷甲硫氨酸和酰基-酰基转运蛋白(Acyl-ACP)转化为N-乙酰高丝氨酸内酯类信号分子[23]。酰基-酰基转运蛋白是脂肪酸合成的中间产物,而脂肪酸合成与碳源代谢密切相关。这些结果进一步揭示了cheB基因参与碳源代谢过程,进而影响菌株2P24生防相关性状的表达。
细菌趋化性(Chemotaxis)是细菌感应外界环境变化的运动方式,是细胞对碳源、能源等物质竞争的一种表型。趋化性有多个相关基因负责调控,其中cheB基因编码的甲基酯酶通过调控趋化受体蛋白的甲基化影响鞭毛的运动方式。菌株2P24中突变cheB基因可显著降低菌株的游动性(图 4)。目前并未有CheB蛋白参与调控其他生化过程的报道,因此我们下一步工作将研究CheB是否影响其他蛋白甲基化水平或其他信号传递过程,从而调控菌株2P24生防相关因子的表达。
References
| [1] | Cook RJ. Making greater use of introduced microorganisms for biological control of plant pathogens. Annual Review of Phytopathology, 1993, 31(1): 53-80. DOI:10.1146/annurev.py.31.090193.000413 |
| [2] | Haas D, Défago G. Biological control of soil-borne pathogens by fluorescent pseudomonads. Nature Reviews Microbiology, 2005, 3(4): 307-319. DOI:10.1038/nrmicro1129 |
| [3] | Mazzola M, Cook RJ, Thomashow LS, Weller DM, Pierson Ⅲ LS. Contribution of phenazine antibiotic biosynthesis to the ecological competence of fluorescent pseudomonads in soil habitats. Applied and Environmental Microbiology, 1992, 58(8): 2616-2624. |
| [4] | Abbas A, Morrissey JP, Marquez PC, Sheehan MM, Delany IR, O'Gara F. Characterization of interactions between the transcriptional repressor PhlF and its binding site at the phlA promoter in Pseudomonas fluorescens F113. Journal of Bacteriology, 2002, 184(11): 3008-3016. DOI:10.1128/JB.184.11.3008-3016.2002 |
| [5] | Kay E, Dubuis C, Haas D. Three small RNAs jointly ensure secondary metabolism and biocontrol in Pseudomonas fluorescens CHA0. Proceedings of the National Academy of Sciences of United States of America, 2005, 102(47): 17136-17141. DOI:10.1073/pnas.0505673102 |
| [6] | Wei HL, Wang Y, Zhang LQ, Tang WH. Identification and characterization of biocontrol bacterial strain 2P24 and CPF-10. Acta Phytopathologica Sinica, 2004, 34(1): 80-85. (in Chinese) 魏海雷, 王烨, 张力群, 唐文华. 生防菌株2P24与CPF-10的鉴定及其生防相关性状的初步分析. 植物病理学报, 2004, 34(1): 80-85. |
| [7] | Wei HL, Zhang LQ. Qunrun-sensing system influences root colonization and biological control ability in Pseudomonas fluorescens 2P24. Antonie van Leeuwenhoek, 2006, 89(2): 267-280. DOI:10.1007/s10482-005-9028-8 |
| [8] | Yan XX, Zhang LQ, Yang ZW, Tang WH. The role of regulatory gene gacA in the suppression of soil-borne diseases by Pseudomonas fluorescens 2P24. Acta Phytopathologica Sinica, 2004, 34(3): 272-279. (in Chinese) 闫小雪, 张力群, 杨之为, 唐文华. 调控基因gacA在荧光假单胞菌2P24防治土传病害中的作用. 植物病理学报, 2004, 34(3): 272-279. |
| [9] | Cha C, Gao P, Chen YC, Shaw PD, Farrand SK. Production of acyl-homoserine lactone quorum-sensing signals by gram-negative plant-associated bacteria. Molecular Plant-Microbe Interaction, 1998, 11(11): 1119-1129. DOI:10.1094/MPMI.1998.11.11.1119 |
| [10] | Herrero M, de Lorenzo V, Timmis KN. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. Journal of Bacteriology, 1999, 172(11): 6557-6567. |
| [11] | Wu XG, Liu JC, Zhang W, Zhang LQ. Multiple-level regulation of 2, 4-diacetylphoroglucinol production by the sigma regulator PsrA in Pseudomonas fluorescens 2P24. PLoS One, 2012, 7(11): e50149. DOI:10.1371/journal.pone.0050149 |
| [12] | Keen NT, Tamaki S, Kobayashi D, Trollinger D. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene, 1988, 70(1): 191-197. DOI:10.1016/0378-1119(88)90117-5 |
| [13] | Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning:A Laboratory Manual. 2nd ed. New York: Cold Spring Harbor Laboratory Press, 1989. |
| [14] | Kidarsa TA, Goebel NC, Zabriskie TM, Loper JE. Phloroglucinol mediates cross-talk between the pyoluteorin and 2, 4-diacetylphloroglucinol biosynthetic pathways in Pseudomonas fluorescens Pf-5. Molecular Microbiology, 2011, 81(2): 395-414. DOI:10.1111/mmi.2011.81.issue-2 |
| [15] | Miller JH. Experiments in Molecular Genetics. New York: Cold Spring Harbor Laboratory Press, 1972. |
| [16] | Brückner R, Titgemeyer F. Carbon catabolite repression in bacteria:choice of the carbon source and autoregularoty limitation of sugar utilization. FEMS Microbiology Letters, 2002, 209(2): 141-148. DOI:10.1111/fml.2002.209.issue-2 |
| [17] | Saier Jr MH, Ramseier TM. The catabolite repressor/activator (Cra) protein of enteric bacteria. Journal of Bacteriology, 1996, 178(12): 3411-3417. DOI:10.1128/jb.178.12.3411-3417.1996 |
| [18] | Fox á, Haas D, Reimmann C, Heeb S, Filloux A, Voulhoux R. Emergence of secretion-defective sublines of Pseudomonas aeruginosa PAO1 resulting from spontaneous mutations in the vfr global regulatory gene. Applied and Environmental Microbiology, 2008, 74(6): 1902-1908. DOI:10.1128/AEM.02539-07 |
| [19] | Takeuchi K, Kiefer P, Reimmann C, Keel C, Dubuis C, Rolli J, Vorholt JA, Haas D. Small RNA-dependent expression of secondary metabolism is controlled by Krebs cycle function in Pseudomonas fluorescens. Journal of Biological Chemistry, 2009, 284(50): 34976-34985. DOI:10.1074/jbc.M109.052571 |
| [20] | Parkinson JS. cheA, cheB, and cheC genes of Escherichia coli and their role in chemotaxis. Journal of Bacteriology, 1976, 126(2): 758-770. |
| [21] | Shanahan P, O'Sullivan DJ, Simpson P, Glennon JD, O'Gara F. Isolation of 2, 4-diacetylphloroglucinol from a fluorescent pseudomonad and investigation of physiological parameters influencing its production. Applied and Environmental Microbiology, 1992, 58(1): 353-358. |
| [22] | Duffy BK, Défago G. Environmental factors modulating antibiotic and siderophore biosynthesis by Pseudomonas fluorescens biocontrol strains. Applied and Environmental Microbiology, 1999, 65(6): 2429-2438. |
| [23] | Miller MB, Bassler BL. Quorum sensing in bacteria. Annual Review of Microbiology, 2001, 55(1): 165-199. DOI:10.1146/annurev.micro.55.1.165 |
