高清清, 邵启文, 叶正琴, 夏乐, 高崧

 , 焦新安, 刘秀梵
, 焦新安, 刘秀梵 扬州大学兽医学院, 江苏省动物重要疫病与人兽共患病防控协同创新中心, 农业部禽用生物制剂创制重点实验室, 江苏 扬州 225009
收稿日期:2017-05-16;修回日期:2017-05-31;网络出版日期:2017-07-21
基金项目:国家自然科学基金(31272559,30972196,30771604,30471281,31672553,31602059);农业部公益性行业专项(201303044);中国博士后科学基金(2015M580477);江苏省博士后基金(1501076C);江苏省自然科学基金(BK20140485,BK20151308);江苏省高校自然科学基金(14KJB230001);江苏高校优势学科建设工程资助项目(PAPD);扬州大学科技创新培育基金(2014CXJ051,2015CXJ057)
*通信作者:高崧, Tel:+86-514-87972117;E-mail:gsong@yzu.edu.cn
摘要:[目的]探究双组份系统RstA/RstB中效应基因rstA对尿道致病性大肠杆菌毒力的影响。[方法]利用λ Red重组系统构建UPEC U17 rstA缺失株U17ΔrstA,并构建相应的回复株,通过体内外试验评价rstA基因缺失对UPEC毒力的影响。[结果]生长曲线的测定结果显示,在LB普通培养基中,缺失株生长速度与野生株相似,但在LB贫铁培养基中,缺失株生长速度较野生株相比明显下降;体外环境应激试验结果显示,缺失株在强酸、强碱、高渗透压、尿素、氧化应激等环境压力下的生存能力与野生株相似;菌株生物被膜检测结果显示,缺失株的生物被膜形成能力与野生株相当;荧光定量PCR检测结果显示,rstA基因在贫铁环境下的表达水平较正常条件下显著上调,暗示贫铁环境可能是rstA发挥效应的刺激信号。6周龄BALB/c小鼠尿道感染试验结果显示,rstA缺失株在尿液、膀胱、肾脏中的带菌量显著低于野生株,而回复株毒力恢复至野生株水平,表明rstA缺失能显著降低UPEC U17的毒力。[结论]rstA与尿道致病性大肠杆菌的致病性相关,为潜在的毒力因子。
关键词: 尿道致病性大肠杆菌 双组份系统 rstA 缺失株 毒力
Deletion of the rstA gene of uropathogenic Escherichia coli strain U17 led to attenuation of virulence in mouse
Qingqing Gao, Qiwen Shao, Zhengqin Ye, Le Xia, Song Gao

 , Xin'an Jiao, Xiufan Liu
, Xin'an Jiao, Xiufan Liu Key Laboratory of Avian Bioproducts Development, Ministry of Agriculture, Jiangsu Co-innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, College of Veterinary Medicine, Yangzhou University, Yangzhou 225009, Jiangsu Province, China
Received 16 May 2017; Revised 31 May 2017; Published online 21 July 2017
*Corresponding author: Song Gao, Tel:+86-514-87972117;E-mail:gsong@yzu.edu.cn
Supported by the National Natural Science Foundation of China (31272559, 30972196, 30771604, 30471281, 31672553, 31602059), by the Special Fund for Agroscientific Research in the Public Interest (201303044), by the General Financial Grant from the China Postdoctoral Science Foundation (2015M580477), by the Jiangsu Postdoctoral Science Foundation (1501076C), by the Natural Science Foundation of Jiangsu Province (BK20140485, BK20151308), by the Natural Science Foundation of the Higher Education Institutions of Jiangsu Province (14KJB230001), by the Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) and by the Science and Technology Innovation Fund of Yangzhou University (2014CXJ051, 2015CXJ057)
Abstract: [Objective]To study the role of response regulator rstA of TCS RstA/RstB in pathogenesis of uropathogenic Escherichia coli (UPEC).[Methods]By using λ Red recombination system, we generated the rstA knockout mutant U17ΔrstA and the complementation strain ReU17ΔrstA. We then compared and analyzed the characterization of the mutant strain and the wild type strain in vivo and in vitro.[Results]The growth curves in LB showed that the deletion of rstA did not affect growth kinetics of mutant, whereas in LB-Fe medium, the growth rate of U17ΔrstA was lower than that of the wild-type strain U17. Under the selected environmental stress conditions in vitro, the bacterial survival experimental results showed that the mutant was not sensitive to acid, alkali, high osmotic pressure, urea and oxidants. Strain biofilm assay showed that the biofilm formation ability of the mutant was similar to that of the wild-type strain. qRT-PCR results showed that the rstA gene was significantly upregulated in LB-Fe medium, indicating that the iron-deficiency environment may be the stimulus signal evoking the RstA regulator. The mouse model of ascending urinary tract infection demonstrated that the deletion of rstA led to attenuation of virulence, because the mutant showed significantly decreased colonization compared with the wild type strain in urine, bladder and kidney, whereas the complementation strain restored the virulence to resemble that of wild-type strain.[Conclusion]The rstA gene was a potential virulence factor and associated with the pathogenesis of UPEC.
Key words: Uropathogenic Escherichia coli two-component regulatory system rstA mutant virulence
肠道外致病性大肠杆菌(Extraintestinal pathogenic E. coli,ExPEC)是一类能够引起人和动物肠道外感染的病原菌,主要包括:尿道致病性大肠杆菌(Uropathogenic E. coli,UPEC)、新生儿脑膜炎大肠杆菌(Neonatal meningitis-associated E. coli,NMEC)和禽病原性大肠杆菌(Avian pathogenic E. coli,APEC)[1]。在ExPEC中,UPEC是引起人和动物(犬、猫)尿道感染的最常见的病原体。
双组份系统(Two-component regulatory systems,TCSs)广泛分布于多种细菌中,能够调控与营养摄取、压力应激、耐药性以及其它信号转导途径相关基因的表达[2-4],尤其在致病性方面的作用,正逐渐成为研究的热点。TCSs通常由位于跨膜区的具有感应作用的组氨酸激酶感受元件(Sensor)及其相应的效应元件(Response regulator)两部分组成。感受元件能够识别特定环境信号的改变,并将信号传递给效应元件使其活化,活化的效应元件,可直接结合到靶基因启动子上,从而调控下游基因的转录表达[5]。RstA/RstB系统是一种典型的双组份系统,由跨膜感受蛋白RstB及其相应的效应蛋白RstA组成。研究表明RstA蛋白可被延伸入细胞质中的RstB末端磷酸化,从而激活RstA的DNA结合结构域,调控相关基因的表达[6]。国外研究发现,在大肠杆菌中,RstA/RstB涉及细菌对酪洛芬、普立地诺和醋竹桃霉素的抗性作用[7];RstA的多重拷贝能够抑制编码ATP酶的yjeE基因缺失株的致死性表型[8],表明其能代偿ATP酶合成途径;Ogasawara等还指出大肠杆菌中的RstA可以使asr (酸应激RNA)表达上调[9];另有研究发现在沙门菌中,RstA在细菌应对饥饿环境与各种应激过程中发挥着关键作用[10-11];此外,RstA对于维持胞内铁离子浓度的平衡也起着重要作用[12],因铁离子是细菌生存所必须的元素,因此也有****认为该系统可能参与病原菌的致病过程,但缺少直接证据证明该推论。
本研究为了探讨TCS RstA/RstB中效应基因rstA与UPEC致病性的关系,利用λ Red重组系统,构建了rstA缺失株UPEC U17ΔrstA,旨在进一步了解TCS在UPEC致病过程中的作用及深入研究UPEC的致病机理。
1 材料和方法 1.1 菌株和质粒 UPEC U17菌株为本实验室由临床尿道感染病例中分离的人源强毒株[13],血清型未定。质粒pACYC184由本校朱国强教授惠赠。本研究所用的质粒和菌株见表 1。
表 1. 本研究中使用菌株和质粒 Table 1. Bacterial strains and plasmids used in this study
| Strain or plasmid | Characteristics | Source or reference |
| Strains | ||
| U17 | Wild-type strain of UPEC serotype nontypable | Human |
| U17ΔrstA | U17ΔrstA | This study |
| DH5α | endA1 hsdR17(rk–mk+)supE44 thi-1 recA1 gyrA (NalR) RelA1Δ(lacIZYA-argF) U169deoR (ф80d lac Δ(lacZ) M15) | Invitrogen |
| Plasmids | ||
| pACYC184 | low-copy expression vector, Catr Tetr | gifted |
| pACYC184-rstA | rstA fragment cloned into Hind Ⅲ-Xba Ⅰ site of pACYC184 vector | This study |
| pKD46 | Amp; expresses λ Red recombinase | [14] |
| pKD3 | cat gene, template plasmid | [14] |
表选项
1.2 运用λ Red重组系统构建基因缺失株U17ΔrstA
1.2.1 引物的设计: 根据质粒pKD3序列,设计引物AF/AR扩增氯霉素基因,并在引物的5′端分别加50 bp的rstA上下游序列,作为同源重组的同源臂(下划线部分)。引物BF和BR为rstA开放阅读框上下游序列,结合CF和CR进行rstA缺失株的鉴定。引物HF和HR扩增rstA ORF及其启动子在内的序列,用以构建回复株。引物QF和QR用于荧光定量PCR检测rstA基因的表达水平。引物序列见表 2。
表 2. 本试验中使用引物 Table 2. Primers used in this study
| Primer | Sequence (5′→3' ) | Target gene |
| AF | ATGAACACTATCGTATTTGTGGAAGATGATGCGGAAGTCGGTTCACTGATTTGTGTAGGCTGGAGCTGCT | pKD3 |
| AR | TTATTCCCATGCATGAGGCGCAAAAAGATAGCCTTTGTTACGCACAGTTTATGGGAATTAGCCATGGTCC | pKD3 |
| BF | ACACTATCGTATTTGTGGA | Upstream region of rstA |
| BR | TTCCCATGCATGAGGCGCA | Downstream region of rstA |
| CF | TTGTGTAGGCTGGAGCTGCT | pKD3 |
| CR | ATGGGAATTAGCCATGGTCC | pKD3 |
| HF | CGCTCTAGAGGAATAATCGGCCACATACT | rstA for complementation |
| HR | TCAAAGCTTAGCCCGACCAGCAGAGACAT | rstA for complementation |
| QF | CAGGTTACCGTAGAGCCG | rstA for qRT-PCR |
| QR | TTCCAGTGCCAGGATGTG | rstA for qRT-PCR |
| 50 bp up-and downstream region of rstA are underlined. | ||
表选项
1.2.2 重组片段的扩增: 以pKD3为模板,使用引物AF/AR扩增并回收两端带有50 bp rstA同源臂的氯霉素基因的PCR产物,用于λ Red同源重组反应的线性打靶DNA。PCR扩增片段约1.1 kb,PCR产物通过0.8%琼脂糖凝胶电泳进行鉴定。经电泳鉴定为预期长度的PCR产物,用Agarose Gel DNA Purification Kit回收纯化DNA片段。
1.2.3 U17ΔrstA缺失株构建: 将PCR扩增的重组片段电转化到含有pKD46的感受态细胞U17中,通过λ Red重组系统构建基因缺失株U17ΔrstA[14]。
1.3 回复株的构建 利用在线软件(http://www.fruitfly.org/seq_tools/promoter.html)预测rstA基因的启动子。根据预测结果,设计引物HF/R,扩增包括rstA ORF及其启动子在内的序列。将目的片段克隆入回复质粒pACYC184,构建重组质粒pACYC184-PnativerstA。将重组质粒电转入缺失株中,构建回复株。
1.4 缺失株生物学特性的研究
1.4.1 细菌在LB普通和贫铁培养基中生长曲线的测定: 分别将野生株和缺失株的单个菌落接种于5 mL新鲜LB培养基中,37 ℃、220 r/min摇振培养过夜,第2天吸取培养物加至10 mL的LB普通或含有200 μmol/L铁离子螯合剂2, 2′-dipyridyl (DIP)的LB贫铁培养基中[15],调节OD600至0.05,37 ℃、220 r/min摇振培养,连续4 h;每0.5 h测定培养物的OD600值,然后根据测定的值对细菌的生长曲线进行绘制,分析野生株与缺失株在LB普通和贫铁培养基中的生长差异。
1.4.2 体外环境应激实验:: 细菌三区划线于LB平板上,37 ℃静置培养16–18 h,挑取约10个单菌落在LB固体培养基上满板划线,37 ℃静置培养4 h。用1 mL含15%甘油的PBS将LB平板上的细菌刮下,将细菌浓度调至1×108 CFU/mL。
各取100 μL细菌悬液分别与900 μL pH 4.0的酸性LB培养基、pH 10.0的100 mmol/L Tris碱溶液、10 mmol/L H2O2溶液混匀,37 ℃培养箱中静置作用30 min;另各取100 μL细菌悬液分别与3 mol/L尿素溶液、4.8 mol/L NaCl溶液1:1等体积混匀,37 ℃培养箱中静置作用60 min。混合液取出用15%甘油PBS连续倍比稀释,平板计数,18 h后观察结果。
1.4.3 菌株生物被膜检测: 按Srdjan Stepanovi?等描述的微孔板生物被膜检测方法[16]进行,挑取单菌落至液体LB培养基过夜振荡培养,取100 μL母液加到10 mL LB液体培养基中,220 r/min,37 ℃振摇培养2 h,调定菌液浓度至OD600为0.1,96孔培养板中每孔加入100 μL菌液,放入37 ℃培养箱中静置培养24 h,小心弃去培养液,PBS洗3遍,自然风干,每孔加0.1%结晶紫染液室温染色30 min,PBS洗3遍,风干后加入100 μL乙醇脱色,充分溶解结晶紫后,脱色液用酶标仪测定OD550值,重复3次取平均值。
1.5 细菌RNA提取及荧光定量PCR
1.5.1 细菌RNA的提取: 挑取U17菌落至3 mL LB液体培养基中,37 ℃、220 r/min摇振培养过夜,按1:100比例分别加至新鲜LB和LB-Fe (200 μmol/L DIP)液体培养基中,37 ℃摇振培养至OD600为0.4–0.6,提取细菌RNA,具体操作按照TaKaRa公司的RNAiso Plus Total RNA提取试剂说明书进行,并使用gDNA Eraser (TaKaRa)去除基因组DNA污染,测定A260/A280分析RNA的浓度。
1.5.2 cDNA的合成: 利用PrimeScript?RT reagent kit (TaKaRa)进行cDNA的合成。加入组分如下:4 μL 5×PrimeScript? Buffer,1 μL PrimeScript? RT Enzyme Mix I,1 μL RT Primer Mix,10 μL RNA,加RNase Free dH2O补足至20 μL,混匀。进行反转录反应,程序为37 ℃ 10 min,85 ℃ 5 s。
1.5.3 荧光定量PCR: 利用软件设计rstA荧光定量PCR的引物QF/R (表 2)。以管家基因gapA为内参,利用荧光定量PCR检测rstA基因在贫铁环境中的相对表达水平。荧光定量PCR的反应体系参照SYBR Premix Ex Taq (TaKaRa)进行,20 μL反应体系中加入2×SYBR Premix Ex Taq 10 μL,上、下游引物各0.8 μL,50×ROX Reference Dye 0.4 μL,cDNA模板2 μL,加灭菌超纯水至20 μL。混匀后在ABI 7500 Real-time PCR扩增仪中进行,反应条件为:95 ℃ 30 s;95 ℃ 5 s,60 ℃ 31 s,40个循环;95 ℃ 15 s,60 ℃ 60 s,95 ℃ 15 s。
1.6 6周龄BALB/c小鼠尿道感染试验 利用6周龄BALB/c小鼠尿道感染模型[17],分别测定野生株、缺失株和回复株对小鼠的致病性。在小鼠尿道攻毒前24 h,收集尿液并作细菌计数,计数结果在102 CFU/mL以上的予以剔除。在小鼠攻毒之前,轻轻挤压其腹部以尽可能排净膀胱内尿液,用适量2%戊巴比妥钠(40–70 mg/kg体重)腹腔注射麻醉。按照Model“11”Plus Syringe Pump的使用说明,将其连接内径0.28 mm的塑料导管经尿道接种50 μL菌液(含108 CFU),操作要谨慎防止人为原因导致输尿管回流。感染后48 h扑杀,取其膀胱、肾脏做细菌计数,扑杀前取尿液做细菌计数,检测细菌经尿道感染48 h后在小鼠体内的动态分布。
2 结果和分析 2.1 U17ΔrstA缺失株的构建及鉴定 将扩增出的带rstA同源臂的氯霉素抗性基因片段rstA-cat电转化入含有pKD46的野生株U17菌株中,诱导同源重组,挑取在氯霉素抗性平板上生长的阳性克隆株进行PCR鉴定,以BF/R引物扩增出带氯霉素抗性基因片段,对照野生株扩增片段为720 bp,突变株条带大小为1100 bp (图 1),表明缺失株U17ΔrstA构建成功。
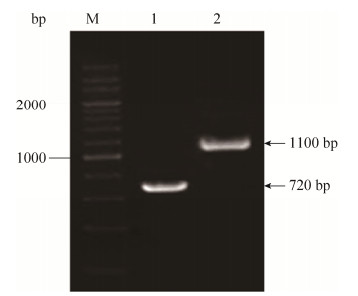 |
| 图 1 U17ΔrstA突变株PCR鉴定 Figure 1 Identification of U17ΔrstA by PCR. M: 200 bp DNA marker (TaKaRa); lane 1: the amplified rstA fragment of U17 by primer BF/BR; lane 2: the amplified rstA-cat fragment of U17ΔrstA by primer BF/BR. |
| 图选项 |
2.2 回复株的构建及鉴定 构建回复质粒pACYC184-PnativerstA后,电转化U17ΔrstA缺失株。PCR鉴定筛选出的拯救回复株,PCR扩增出大小为720 bp左右的rstA野生株条带,说明rstA回复成功并命名为ReU17ΔrstA (图 2)。
 |
| 图 2 回复株ReU17ΔrstAPCR鉴定 Figure 2 Identification of complementation strain ReU17ΔrstA by PCR. M: 200 bp DNA Marker (TaKaRa); lane 1: the amplified rstA fragment of U17; lane 2: the amplified rstA fragment of ReU17ΔrstA; lane 3: the amplified rstA fragment of U17ΔrstA. |
| 图选项 |
2.3 缺失株的生物学特性
2.3.1 U17及缺失株在LB普通培养基中的生长曲线: 根据U17及缺失株在LB中分别培养0.5、1.0、1.5、2.0、2.5、3.0、3.5、4.0 h时的OD600测结果,绘制的生长曲线可以看出rstA基因缺失株的生长速度较野生株相比无明显差异(图 3)。
 |
| 图 3 野生株和缺失株在LB中的生长曲线 Figure 3 Growth curves of wild type strain and isogenic mutant in LB at 37 ℃, and their optical density checked at different times. |
| 图选项 |
2.3.2 U17及缺失株在LB贫铁培养基中的生长曲线: 将野生株和突变株分别接种于10 mL含有200 μmol/L铁离子螯合剂DIP的LB贫铁培养基中,从生长曲线可以看出,自3 h后,缺失株的生长速度较野生株相比明显下降(**P < 0.01) (图 4)。
 |
| 图 4 野生株和缺失株在LB-Fe培养基中的生长曲线 Figure 4 Growth curves of wild type strain and isogenic mutant in LB-Fe medium (200 μmol/L iron chelator DIP) at 37 ℃, and their optical density checked at different times. |
| 图选项 |
2.4 体外环境应激实验 比较缺失株和野生株在不同的环境压力条件下的存活情况,结果显示缺失株在pH 4.0的酸性LB培养基、100 mmol/L Tris碱、3 mol/L尿素、4.8 mol/L NaCl高渗透压溶液以及10 mmol/L H2O2中的存活率与野生株相似(P > 0.05)(图 5),表明rstA的缺失对于细菌在这几种环境条件下的生存没有影响。
 |
| 图 5 野生株和突变株体外环境应激试验 Figure 5 Bacterial resistance to environmental stress. Each strain was tested for different environmental stress including acid, alkali, high urea, high osmolarity and oxidants challenge. Results were expressed as survival relative to wild-type strain. |
| 图选项 |
2.5 菌株生物被膜检测 生物被膜检测结果显示,野生株U17 OD550值为0.326±0.022,而U17ΔrstA的OD550值为0.342±0.018,与野生株相比差异不显著(P > 0.05),表明rstA基因缺失对UPEC生物被膜形成能力没有影响。
2.6 荧光定量PCR结果 qRT-PCR结果显示,U17在LB贫铁环境下,rstA基因的表达量较在LB正常培养条件下显著上调2.38倍(**P < 0.01) (图 6),暗示贫铁环境可能是rstA发挥效应的刺激信号。
 |
| 图 6 U17在LB-Fe环境下的rstA相对表达水平 Figure 6 Quantitative RT-PCR analysis of rstA genes' transcription levels in wild type strains U17 under LB-Fe environment. Transcript levels were measured in cDNA preparations from each strain and normalized to the gapA level, and results are shown as fold changes relative to wild-type level. Asterisks indicate statistically significant differences (**P < 0.01). |
| 图选项 |
2.7 6周龄BALB/c小鼠尿道感染试验 48 h的体内动态分布试验结果显示,与野生株相比,缺失株U17ΔrstA在尿液、膀胱、肾脏中的带菌量明显下降(*P < 0.05),表明rstA缺失致毒力显著降低,而回复株毒力恢复接近野生株水平(图 7)。
 |
| 图 7 野生株和缺失株小鼠体内动态分布试验 Figure 7 In vivo colonization in 6-week-old BALB/c mouse. A: urine; B: bladder; C: kidney. Data are presented as log10 CFU/mL of bacteria from urine or log10 CFU/g of bacteria from tissues. Horizontal bars represent mean values. Each data point represents a sample from an individual mouse and the detection limit is 100 CFU/mL or CFU/g. The data points that are below detection are on the line for limit of detection. Statistically significant differences are indicated with asterisks (*P < 0.05 as determined by the Mann-Whitney test). |
| 图选项 |
3 讨论 UPEC在入侵宿主建立尿道感染的过程中,要面对的一个主要宿主环境就是尿液。尿液是一种营养匮乏的环境,其中氨基酸、小分子多肽和核苷酸等含量很低,大量的是尿素成分[18]。UPEC能够适应尿液环境并在其中生长,主要利用多肽和氨基酸作为碳源[19],其生长还依赖于另外一个重要的营养元素—铁离子,但是游离的铁离子在尿液中的含量也是极低的[20]。UPEC对尿道的感染除了受限于营养代谢,还会面对其它一系列的环境压力,如活性氧、高浓度尿素,pH值,补体和其它的抗菌物质以及与其它微生物的竞争压力等等[21],因此处理与这些环境压力的关系对于UPEC能否成功建立感染至关重要。
TCSs在感受环境信号,调控细菌生理代谢以及致病性方面发挥着重要作用。在大肠杆菌中,通过对K-12菌株全基因组序列分析,发现存在29种组氨酸激酶感受元件、32种效应元件和1种组氨酸标签磷酸化转运子[6]。每种双组份系统在参与细菌环境适应性的过程中,往往能够识别特定的环境信号,换句话说,在某种特定的环境信号的刺激下,往往会激活相应的双组份系统参与应答,增强细菌的适应性。在众多的双组份系统中,已有部分系统被确认参与到UPEC的致病过程中,例如,TCS BarA/UvrY能够有效的控制碳源的糖分解和糖异生途径的转换,促进UPEC的致病作用[22];TCS QseC/QseB中,QseC激酶的缺失,能够异常调节核苷酸、氨基酸以及碳的代谢,并直接导致UPEC毒力的下降[23];另外,TCSs中PhoQ/PhoP和AirS系统也被认为与UPEC的致病性相关联[24-25]。目前,关于TCS RstA/RstB的环境刺激信号以及该系统是否参与UPEC的致病进程未见报道。
在TCS RstA/RstB系统中,RstA作为效应蛋白发挥着调控功能,本研究针对rstA效应基因,采用基因突变的方法,构建了rstA的缺失突变株,通过比较缺失株和野生株的生物学特性及致病性,以期了解rstA基因的生物学意义和功能,尤其对致病性方面的影响,进而明确其是否为UPEC的毒力基因。
通过体外模拟体内宿主环境试验,比较了rstA缺失株和野生株在强碱、强酸、高浓度尿素、高渗透压、氧化应激等环境条件下的生存能力,结果显示,缺失株在这些环境压力下的生存能力较野生株相比无明显差异,表明rstA可能对于UPEC应对上述几种环境压力过程中发挥的作用有限。但在贫铁环境中,rstA缺失株的生长速度较野生株相比明显下降,另外,qRT-PCR结果也显示,在贫铁条件下,rstA基因的表达量较正常条件下明显上调,这些结果提示,贫铁环境可能是rstA发挥效应的一种刺激信号。
此外,缺失株与野生株相比,在6周龄BALB/c小鼠尿道定殖能力显著下降,主要表现在尿液、膀胱和肾脏中的带菌量明显降低,表明rstA缺失导致UPEC U17的毒力下降,另外通过对缺失株中的rstA基因进行回复,显示回复株在尿道中的定殖能力接近野生株水平,说明缺失株致病力的下降主要是由于rstA缺失所致,进而说明rstA效应基因与UPEC的致病力相关联,由此可见TCS RstA/RstB在尿道致病性大肠杆菌的致病过程中发挥一定的作用。
References
| [1] | Russo TA, Johnson JR. Proposal for a new inclusive designation for extraintestinal pathogenic isolates of Escherichia coli:ExPEC. The Journal of Infectious Diseases, 2000, 181(5): 1753-1754. DOI:10.1086/jid.2000.181.issue-5 |
| [2] | Djori? D, Kristich CJ. Oxidative stress enhances cephalosporin resistance of Enterococcus faecalis through activation of a two-component signaling system. Antimicrobial Agents and Chemotherapy, 2015, 59(1): 159-169. DOI:10.1128/AAC.03984-14 |
| [3] | Li Z, Fu Q, Wang Z, Li T, Zhang H, Guo F, Wang Y, Zhang J, Chen C. TceSR two-component regulatory system of Brucella melitensis 16M is involved in invasion, intracellular survival and regulated cytotoxicity for macrophages. Letters in Applied Microbiology, 2015, 60(6): 565-571. DOI:10.1111/lam.2015.60.issue-6 |
| [4] | Zhang SM, Li XF, Wang X, Li Z, He J. The two-component signal transduction system YvcPQ regulates the bacterial resistance to bacitracin in Bacillus thuringiensis. Archives of Microbiology, 2016, 198(8): 773-784. DOI:10.1007/s00203-016-1239-z |
| [5] | Hoch JA. Two-component and phosphorelay signal transduction. Current Opinion in Microbiology, 2000, 3(2): 165-170. DOI:10.1016/S1369-5274(00)00070-9 |
| [6] | Yamamoto K, Hirao K, Oshima T, Aiba H, Utsumi R, Ishihama A. Functional characterization in vitro of all two-component signal transduction systems from Escherichia coli. Journal of Biological Chemistry, 2005, 280(2): 1448-1456. DOI:10.1074/jbc.M410104200 |
| [7] | Zhou L, Lei XH, Bochner BR, Wanner BL. Phenotype microarray analysis of Escherichia coli K-12 mutants with deletions of all two-component systems. Journal of Bacteriology, 2003, 185(16): 4956-4972. DOI:10.1128/JB.185.16.4956-4972.2003 |
| [8] | Campbell TL, Ederer CS, Allali-Hassani A, Brown ED. Isolation of the rstA gene as a multicopy suppressor of YjeE, an essential ATPase of unknown function in Escherichia coli. Journal of Bacteriology, 2007, 189(8): 3318-3321. DOI:10.1128/JB.00131-06 |
| [9] | Ogasawara H, Hasegawa A, Kanda E, Miki T, Yamamoto K, Ishihama A. Genomic SELEX search for target promoters under the control of the PhoQP-RstBA signal relay cascade. Journal of Bacteriology, 2007, 189(13): 4791-4799. DOI:10.1128/JB.00319-07 |
| [10] | Cabeza ML, Aguirre A, Soncini FC, Véscovi EG. Induction of RpoS degradation by the two-component system regulator RstA in Salmonella enterica. Journal of Bacteriology, 2007, 189(20): 7335-7342. DOI:10.1128/JB.00801-07 |
| [11] | Tran TK, Han QQ, Shi YX, Guo L. A comparative proteomic analysis of Salmonella typhimurium under the regulation of the RstA/RstB and PhoP/PhoQ systems. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 2016, 1864(12): 1686-1695. DOI:10.1016/j.bbapap.2016.09.003 |
| [12] | Jeon J, Kim H, Yun J, Ryu S, Groisman EA, Shin D. RstA-promoted expression of the ferrous iron transporter FeoB under iron-replete conditions enhances Fur activity in Salmonella enterica. Journal of Bacteriology, 2008, 190(22): 7326-7334. DOI:10.1128/JB.00903-08 |
| [13] | Zhao LX, Gao S, Huan HX, Xu XJ, Zhu XP, Yang WX, Gao QQ, Liu XF. Comparison of virulence factors and expression of specific genes between uropathogenic Escherichia coli and avian pathogenic E.coli in a murine urinary tract infection model and a chicken challenge model. Microbiology, 2009, 155(5): 1634-1644. DOI:10.1099/mic.0.024869-0 |
| [14] | Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proceedings of the National Academy of Sciences of the United States of America, 2000, 97(12): 6640-6645. DOI:10.1073/pnas.120163297 |
| [15] | Ve?erek B, Moll I, Bl?si U. Control of Fur synthesis by the non-coding RNA RyhB and iron-responsive decoding. The EMBO Journal, 2007, 26(4): 965-975. DOI:10.1038/sj.emboj.7601553 |
| [16] | Stepanovi? S, Vukovi? D, Daki? I, Savi? B, ?vabi?-Vlahovi? M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. Journal of Microbiological Methods, 2000, 40(2): 175-179. DOI:10.1016/S0167-7012(00)00122-6 |
| [17] | Hagberg L, Engberg I, Freter R, Lam J, Olling S, Edén CS. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infection and Immunity, 1983, 40(1): 273-283. |
| [18] | Haugen BJ, Pellett S, Redford P, Hamilton HL, Roesch PL, Welch RA. In vivo gene expression analysis identifies genes required for enhanced colonization of the mouse urinary tract by uropathogenic Escherichia coli strain CFT073dsdA. Infection and Immunity, 2007, 75(1): 278-289. DOI:10.1128/IAI.01319-06 |
| [19] | Alteri CJ, Smith SN, Mobley HLT. Fitness of Escherichia coli during urinary tract infection requires gluconeogenesis and the TCA cycle. PLoS Pathogens, 2009, 5(5): e1000448. DOI:10.1371/journal.ppat.1000448 |
| [20] | Su Q, Guan TB, He Y, Lv HT. Siderophore biosynthesis governs the virulence of uropathogenic Escherichia coli by coordinately modulating the differential metabolism. Journal of Proteome Research, 2016, 15(4): 1323-1332. DOI:10.1021/acs.jproteome.6b00061 |
| [21] | Kurabayashi K, Agata T, Asano H, Tomita H, Hirakawa H. Fur represses adhesion to, invasion of, and intracellular bacterial community formation within bladder epithelial cells and motility in uropathogenic Escherichia coli. Infection and Immunity, 2016, 84(22): 3220-3231. |
| [22] | Tomenius H, Pernestig AK, Jonas K, Georgellis D, M?llby R, Normark S, Melefors ?. The Escherichia coli BarA-UvrY two-component system is a virulence determinant in the urinary tract. BMC Microbiology, 2006, 6: 27. DOI:10.1186/1471-2180-6-27 |
| [23] | Hadjifrangiskou M, Kostakioti M, Chen SL, Henderson JP, Greene SE, Hultgren SJ. A central metabolic circuit controlled by QseC in pathogenic Escherichia coli. Molecular Microbiology, 2011, 80(6): 1516-1529. DOI:10.1111/mmi.2011.80.issue-6 |
| [24] | Alteri CJ, Lindner JR, Reiss DJ, Smith SN, Mobley HLT. The broadly conserved regulator PhoP links pathogen virulence and membrane potential in Escherichia coli. Molecular Microbiology, 2011, 82(1): 145-163. DOI:10.1111/mmi.2011.82.issue-1 |
| [25] | Pernestig AK, Normark SJ, Georgellis D, Melefors O. The role of the AirS two-component system in uropathogenic Escherichia coli. Advances in Experimental Medicine and Biology, 2000, 485: 137-142. |
