高明煜, 吴玉星, 朱百涛, 高小宁, 冯浩, 黄丽丽


西北农林科技大学植物保护学院, 旱区作物逆境生物学国家重点实验室, 陕西 杨凌 712100
收稿日期:2017-03-19;修回日期:2017-04-24;网络出版日期:2017-05-16
基金项目:国家自然科学基金(31471732,31671982)
*通信作者:黄丽丽, Tel:+86-29-87091312;E-mail:huanglili@nwsuaf.edu.cn
摘要:[目的]研究表明,细胞色素P450(CYP)在死体营养型真菌的毒素合成代谢中发挥重要作用,预测可能与病原菌致病相关。论文对苹果树腐烂病菌(Valsa mali)毒素合成基因簇中的1个上调表达的CYP基因Vmcyp5进行生物学功能研究,明确CYP基因对病原菌致病力影响,为细胞色素P450基因家族对苹果树腐烂病菌致病机理的进一步研究提供依据。[方法]通过Double-joint PCR和PEG介导的原生质体转化技术获得具有G418抗性的突变体,并对突变体进行PCR检测及Southern blotting验证得到单拷贝敲除突变体。将目的基因片段重新导入敲除突变体,筛选获得互补突变体。最终对野生型菌株及敲除突变体、互补突变体进行菌落、产孢及致病力观察,利用SPSS软件对数据进行差异显著性分析,并利用qRT-PCR技术分析突变体黑色素基因簇的表达水平。[结果]通过基因敲除技术获得1个Vmcyp5基因的敲除突变体。与野生型菌株相比,Vmcyp5基因的敲除突变体菌落呈白色,产孢量减少51.3%。qRT-PCR分析发现敲除突变体黑色素基因簇基因表达量降低。重要的是,敲除突变体致病力较野生型菌株降低24.5%。互补突变体菌落颜色、产孢及致病力近似恢复至野生型菌株水平。[结论]Vmcyp5基因与病原菌黑色素合成、子实体的产生和致病力相关。
关键词: 苹果树腐烂病菌 细胞色素P450 基因敲除 致病力
Characterization of cytochrome P450 gene Vmcyp5 in Valsa mali
Mingyu Gao , Yuxing Wu , Baitao Zhu , Xiaoning Gao , Hao Feng , Lili Huang


State Key Laboratory of Crop Stress Biology for Arid Areas, College of Plant Protection, Northwest A & F University, Yangling 712100, Shaanxi Province, China
Received 19 March 2017; Revised 24 April 2017; Published online 16 May 2017
*Corresponding author: Lili Huang, E-mail:huanglili@nwsuaf.edu.cn
Supported by the National Natural Science Foundation of China (31471732, 31671982)
Abstract: [Objective]Cytochrome P450 (CYP) plays a significant role in mycotoxin metabolism of necrotrophic fungus and may be related to pathogenicity. The objective of the study was to reveal the function of CYP genes in infection process.[Methods]The knockout cassette was constructed using Double-joint PCR and mutants were obtained and confirmed by PEG-mediated transformation of protoplasts, PCR and Southern blotting analysis. Gene complemented mutants were constructed by gap repair technology. The PDA routine culture was selected to analyze the vegetative growth of mutants. In vitro inoculation to apple twigs was used to detect the pathogenicity. Salient differences were analyzed by SPSS and qRT-PCR was used to detect the expression of melanin gene cluster.[Results]Compared with the original strain 03-8, the colony color of deletion mutant changed from light yellow to white, and the amount of propagulum reduced by 51.3%. The relative expression level of Vmcyp5-knockout mutant was assayed by qRT-PCR, and mutant's melanin gene cluster was down-regulated. More importantly, the pathogenicity of deletion mutant was decreased by 24.5%. Complementation mutant was almost back to the level of the original strain 03-8 in colony color, propagulum and pathogenicity.[Conclusion]Vmcyp5 may be related to melanin biosynthesis and propagulum formation, and participates in pathogen process of Valsa mali.
Key words: Valsa mali Cytochrome P450 gene knockout pathogenicity
苹果树腐烂病(Apple tree valsa canker)由死体营养型真菌黑腐皮壳属Valsa mali Mayabe et Yamada引起[1]。该病原菌可致树势衰弱,严重时造成死树毁园,极大地影响和制约着苹果产量和品质,造成严重的经济损失。苹果树腐烂病的相关研究涉及病原学研究、病原菌侵染过程、发病规律及防治技术等[2-4],但致病机理尚不明确。很多的死体寄生菌如Alternaria、Botrytis、Cercospora、Fusarium、Sclerotinia等均可在病菌侵染过程中不断产生毒素杀死寄主细胞[5]。研究表明,细胞色素P450基因与病原真菌毒素的合成代谢相关[6]。因此,明确该类基因在苹果树腐烂病菌致病过程中的生物学功能,可为进一步解析致病机理奠定基础。
细胞色素P450 (Cytochrome P450,CYP)是广泛存在于自然界生物体中的一组结构和功能相关的超家族基因编码的同工酶。真菌细胞色素P450在许多次级代谢产物合成路径中具有重要的催化活性[7],可参与多种真菌毒素的生物合成[8-11]。而真菌毒素作为植物病原真菌产生的具有致病活性的小分子代谢产物,通常与植物病原菌致病过程密切相关[12]。前期研究表明,细胞色素P450可催化禾谷镰刀菌(Fusarium graminearum)、拟分枝孢镰刀菌(F. sporotrichioides)的单端孢霉烯族毒素合成中的某些羟基化反应[13];Wen等[14]研究发现,黄曲霉毒素基因簇上的CYP基因cypX参与了黄曲霉毒素的合成,该基因的缺失会影响中间产物的进一步转化;Siewers等[15]研究证实,CYP基因bcbot与灰霉菌葡双醛霉素的合成相关,该基因的缺失突变体引起致病力的显著降低。根据苹果树腐烂病菌全基因组及其转录组数据分析,获得了1个位于苹果树腐烂病菌V. mali的毒素代谢基因簇、在病原菌与寄主互作过程中上调表达的CYP基因Vmcyp5[16],推测该基因参与苹果树腐烂病菌毒素合成,与病原菌致病相关。
本研究以细胞色素P450基因Vmcyp5为研究对象,利用基因敲除技术和PEG介导的遗传转化技术获得敲除突变体,通过对敲除突变体的生物学观察(包括颜色、生长速度、子实体等)以及致病性的检测,明确该基因在苹果树腐烂病菌致病过程中的影响,进一步为细胞色素P450基因家族在苹果树腐烂病菌致病作用中的功能研究提供依据。
1 材料和方法 1.1 试验材料 苹果树腐烂病菌(V. mali)的分离株03-8,由西北农林科技大学植物保护学院植物病害综合治理研究室分离并保存。质粒pHIG2RHPH2-GFP-GUS (含潮霉素磷酸转移酶基因hph,Kana抗性),由浙江大学宋凤鸣教授惠赠。质粒pFL2 (含氨基糖苷磷酸转移酶基因neo,G418抗性)、酵母菌株XK1-25由西北农林科技大学许金荣教授惠赠。大肠杆菌菌株DH5α,由西北农林科技大学植物病害综合治理实验室保存。苹果枝条选取隔离温室培养的富士(Malus domestica Borkh. cv. Fuji)苹果树粗细均匀的1–2年生枝条。
1.2 主要试剂 Fastpfu (Transgene)、崩溃酶(Sigma)、溶壁酶(Sigma)、G418 (MP)、地高辛DNA标记检测试剂盒(Roche)、定量Mix (Genstar)、胶回收提取试剂盒(BioTeke)、质粒小量提取试剂盒(OMEGA)、核酸提取试剂盒(华越洋)及其他实验常规化学试剂及药品。引物合成自生工生物工程(上海)股份有限公司。
1.3 序列获得及引物设计 从苹果树腐烂病菌基因组序列中获得Vmcyp5基因序列,利用Premier 5.0软件设计引物。引物序列及其相关参数见表 1。
表 1. 试验所用引物序列 Table 1. Primers used in the test
| Primers | Sequences (5′→3′) |
| Vmcyp5-1F | GCTGAAATCTGGCTCTGGG |
| Vmcyp5-2R | CAGATACGGCAGAGAAATCGCAACCTCCGCTGCAAATTGACTTGGT |
| Vmcyp5-3F | GTTTAGATTCCAAGTGTCTACTGCTGGCACTACCACCATCCGAACCTC |
| Vmcyp5-4R | GCGCTATGTATGCAAGATAAGC |
| Vmcyp5-5F | AAGCCTGGCAGTGGTTTT |
| Vmcyp5-6R | GGATTGTTGGCAAGATGGA |
| Vmcyp5-7F | ATAGCCTGGAGTGTCGGTAGA |
| Vmcyp5-8R | TTTCAAAGGGGCAGCATT |
| Vmcyp5-CF | ACCGGAAATGAAGAGTAACGA |
| Vmcyp5-CR | TTGCTTTGATGACCCTACGA |
| Vmcyp5-qF | CGTTGAGTACAAGGGCCATATC |
| Vmcyp5-qR | TTATGACCCAACACGCTTCG |
| Vmcyp5-cF | CGACTCACTATAGGGCGAATTGGGTACTCAAATTGGTGTAGGTCGTTGGGAATCAA |
| Vmcyp5-cR | CACCACCCCGGTGAACAGCTCTCCGCCCTTGCTCACGTACGACACCATACCGTTCA |
| neo-F | GAGGTTGCGATTTCTCTGCCGTATCTG |
| neo -R | GCCAGCAGTAGACACTTGGAATCTAAAC |
| G852 | TCGGCTATGACTGGGCACAACA |
| G850 | GAGCGGCGATACCGTAAAGCAC |
| G856F | GAATGGTCAAATCAAACTGCTAGATAT |
| G855R | TGTTGGGTTTGAGCTAGGTGGG |
| pFL2-F | TAACGCCAGGGTTTTCCCAGTAC |
| pFL2-R | CGTGCTGCTTCATGTGGTCGG |
| VM00287-F | ATGATAGCCGCAATGAGC |
| VM00287-R | CCAGGCACCCAGTAAAGTA |
| VM00288-F | GCTCATCCTCACCTCGTCCAT |
| VM00288-R | TGCCAGGCGTTCTCATCG |
| VM00289-F | AGTCCATCACGGAGTCAATC |
| VM00289-R | GCAGAACTGAACGAGGGTG |
| VM00290-F | GGATGGCAGGGCTCTAC |
| VM00290-R | TGTCATTGCGAAAGGTGT |
| VM00291-F | ACGGGAGCGAGCAGGATA |
| VM00291-R | GTCACTGGCGGACAGAACAC |
| VM00292-F | TTTCTTCCGCCTATCCCA |
| VM00292-R | CGTCATACCGTTGAGCAGC |
| G6PDH-F | TCAGAACAAGTTCGAGGGCGACAA |
| G6PDH-R | TAGGGGCAATAGAGGGCTTGTTCA |
| Italics represented homologous sequences. | |
表选项
1.4 敲除载体的构建 以苹果树腐烂病菌野生型菌株03-8的基因组DNA为模板,分别利用引物Vmcyp5-1F/2R和Vmcyp5-3F/4R扩增目的基因的上游和下游片段,再以质粒pFL2作为模板,利用引物neo-F/R扩增得到neo片段。回收各片段,并通过Double-joint PCR方法构建基因敲除载体[17]。
1.5 PEG介导的遗传转化 对构建完成的基因敲除载体进行PCR产物浓缩,随后制备苹果树腐烂病菌原生质体并进行PEG介导的原生质体转化[18]。将复生的原生质体与含100μg/mL G418的Bottom Agar培养基均匀混合后倒入培养皿(Φ=9 cm,下同)中,25℃倒置培养12 h后,用冷却至60℃、含150μg/mL G418的Top Agar培养基覆盖,继续置于25 ℃培养4–5 d后挑取转化子。
1.6 突变体检测
1.6.1 PCR检测阳性转化子: 提取转化子DNA,以此为模板,利用引物Vmcyp5-5F/6R、G856F/ Vmcyp5-8R、Vmcyp5-7F/G855R、G852/G850进行PCR检测[19]。通过是否扩增出片段大小正确的条带判断该转化子是否为阳性转化子。
1.6.2 Southern blotting验证敲除突变体: 利用CTAB法提取阳性转化子和野生型菌株03-8的基因组DNA,使用内切酶Cla Ⅰ对基因组DNA进行酶切,经电泳、转膜后,以neo基因片段为探针进行Southern blotting杂交,最后利用蛋白照相系统照相,确定敲除突变体。具体试验方法参考地高辛DNA标记检测试剂盒(Roche)说明书。
1.7 基因敲除回复 以基因组DNA为模板,利用互补引物Vmcyp5-cF/cR扩增Vmcyp5基因片段。回收PCR产物,以质粒pDL2为载体,构建Vmcyp5基因互补载体。试验方法参考郑大伟[20],略有改动。通过PEG介导的原生质体转化法将回复载体转入敲除突变体Vmcyp5-5,以潮霉素作为筛选抗生素,最终获得互补敲除突变体。遗传转化步骤及互补突变体筛选方法同1.5。
1.8 苹果树腐烂病菌生物学性状测定
1.8.1 营养生长观察: 将敲除突变体、互补敲除突变体及野生型菌株03-8在PDA培养基上培养2 d后,挑取菌落边缘处的菌饼(Φ=5 mm,下同)分别接到含有10 mL PDA培养基的培养皿的正中,于25℃避光倒置培养48 h后观察菌落颜色、形状及菌丝生长等情况,运用十字交叉法测量菌落直径。继续培养7 d后,将培养皿转至25℃光暗交替条件(光照:黑暗=12 h:12 h),观察子实体产生情况并于40 d后统计子实体数量。每个菌株设置3个重复,试验重复3次。利用SPSS软件对测量所得数据进行差异性分析(P=0.05)。
1.8.2 致病性分析: 致病力检测方法参照臧睿等[21],略有改动。对消毒灭菌的健康离体苹果枝条进行烫伤处理后,分别取敲除突变体、互补敲除突变体和野生型菌株的菌饼以及无菌PDA块接种于伤口处,并将枝条于25℃保湿静置培养5 d后测量病斑大小。每个菌株设置9个重复,试验重复3次。利用SPSS软件对测量所得数据进行差异性分析(P=0.05)。
1.9 qRT-PCR分析黑色素基因簇表达量
1.9.1 样品制备: 挑取野生型菌株03-8和敲除突变体的菌饼置于含100 mL灭菌马铃薯葡萄糖培养基(PDB)的三角瓶中,25℃、100 r/min摇培3 d后用灭菌纱布收集菌丝。具体方法参照许春景等[22]。菌丝样品装入锡箔袋–80℃保存备用。
1.9.2 总RNA提取和第一链cDNA合成: 按照华越洋核酸提取试剂盒的方法说明,提取菌丝样品。随后以5μg RNA为模板,使用引物Oligo d(T)18反转录合成第一链cDNA,方法参照Thermo反转录试剂盒说明书。
1.9.3 实时荧光定量PCR: 分别以稀释10倍的野生型菌株和敲除突变体的菌丝样品的cDNA为模板,以苹果树腐烂病菌G6PDH为内参基因[23],利用特异性引物VM00287-F/R、VM00288-F/R、VM00289-F/R、VM00290-F/R、VM00291-F/R和VM00292-F/R,通过CFX ConnectTM实时定量PCR仪(Bio-Rad)对苹果树腐烂病黑色素基因簇6个基因(VM00287、VM00288、VM00289、VM00290、VM00291和VM00292)[24]进行定量PCR检测。每次3个平行试验,重复3次。采用2–△△CT方法计算目的基因相对表达量。具体PCR体系及程序见表 2。
表 2. qRT-PCR体系和程序 Table 2. qRT-PCR reaction system and amplification procedure
| PCR reaction system (20 μL) | V/μL | T/℃ | Time |
| 2×SYBR green fluorescent dye (GenStar) | 10.0 | 95 | 10 min |
| ddH2O | 7.4 | 95 |  |
| 55 | |||
| cDNA template | 1.0 | 95 | |
| 65 | 5 s | ||
| Vmcyp5-qF/qR(G6PDH-F/R) | 0.8 | 95 | –0.5 ℃ gradient cooling |
表选项
2 结果和分析 2.1 敲除载体的构建 分别扩增Vmcyp5的上下游和抗性基因neo,片段大小分别为1227、1488、1433 bp (图 1-A)。利用Double-joint PCR对上游片段、下游片段和neo片段进行融合,得到大小为4148 bp的融合片段(图 1-B)。再以Vmcyp5-CF/CR为引物进行巢式PCR,获得大小为3868 bp的目的条带(图 1-B),即基因敲除载体构建完成。
 |
| 图 1 敲除载体构建的PCR检测 Figure 1 PCR detections of the gene knockout vector. A: PCR amplification of upstream, downstream fragments and neo. Lane L: Target upstream; lane R: Target downstream; M1: DL2000. B: Construction of the gene knockout vector. Lane 1: Fusion fragment of Double-joint PCR; Lane 2: Nested PCR fragment; M2: DL5000. |
| 图选项 |
2.2 敲除突变体鉴定 以转化子DNA为模板,利用4对引物进行PCR检测。对鉴定为阳性的突变体进行Southern blotting验证,最终得到1个单拷贝敲除突变体ΔVmcyp5-5,目的条带为2436 bp (图 2-A、B)。用引物Vmcyp5-5F/6R对基因回复转化子进行PCR检测,获得3个互补敲除突变体,分别为ΔVmcyp5-A、ΔVmcyp5-B和ΔVmcyp5-C (图 2-C)。
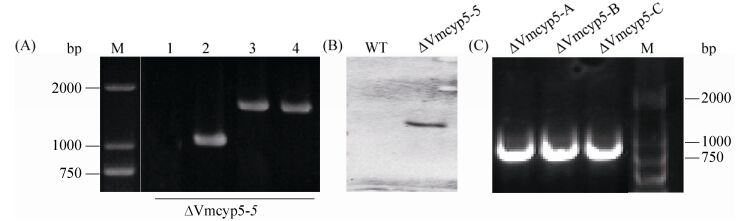 |
| 图 2 突变体的检测和验证 Figure 2 PCR and Southern blotting detections of the mutants. A: PCR detection of the putative deletion mutant using four pairs of primers. Lane 1: Primer pairs Vmcyp5-5F/6R (targeted gene); lane 2: Primer pairs G850/G852 (neo cassette); lane 3: Primer pairs Vmcyp5-7F/G855R (upstream region); lane 4: Primer pairs G856F/ Vmcyp5-8R (downstream region); M: DL2000. B: Southern blotting detection of the putative deletion mutant. C: PCR screening of the complementation mutants. M: DL2000. |
| 图选项 |
2.3 突变体营养生长观察 在PDA培养基上恒温静置培养的敲除突变体ΔVmcyp5-5与野生型菌株03-8相比,菌落颜色变白(图 3-A、B),菌落形态及生长速率等并无显著性差异(图 3-C)。继续光暗交替条件下培养,ΔVmcyp5-5与03-8皆于14–16 d产生黑色子实体。统计培养40 d后的成熟子实体数量发现,ΔVmcyp5-5产孢量相较于03-8减少51.3% (P=0.05) (图 3-D)。互补突变体在营养生长和产孢方面近似恢复至野生型菌株水平。表明Vmcyp5对苹果树腐烂病营养生长及子实体产生有一定的影响。
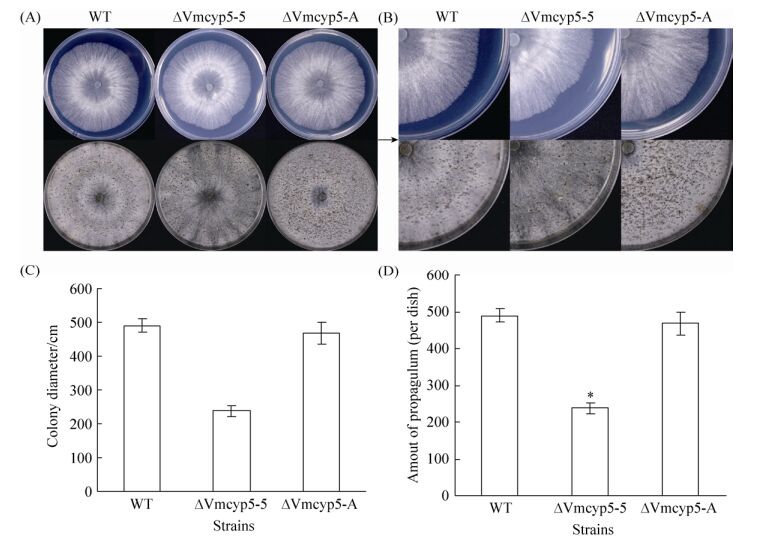 |
| 图 3 野生型菌株03-8和突变体在PDA培养基上的菌落形态(25 ℃恒温静置48 h)和产孢情况(25 ℃恒温静置40 d) Figure 3 Colony morphology (After 48 hours' standing at 25 ℃) and propagulum formation (After 40 days' standing at 25 ℃) of the wild-type strain 03-8 (WT) and the mutants inoculate on PDA medium. A: Colony morphology and propagulum formation of WT, ΔVmcyp5-5 and ΔVmcyp5-A; B: 4 times magnified image of A; C: Colony diameter of WT, ΔVmcyp5-5 and ΔVmcyp5-A; D: Propagulum analysis of WT, ΔVmcyp5-5 and ΔVmcyp5-A. The asterisk indicates a significant difference from the wild-type (P=0.05). |
| 图选项 |
2.4 突变体致病力检测 将接种完成的苹果树枝条于25 ℃保湿培养5 d后,测量病斑直径发现(图 4-A),ΔVmcyp5-5的致病力较野生型菌株03-8显著下降24.5% (P=0.05)。同时,互补突变体的致病力与野生型菌株相比无差异(图 4-B)。表明该基因与苹果树腐烂病菌致病过程相关。
 |
| 图 4 野生型菌株03-8和突变体的致病力测定和分析(5 d) Figure 4 Virulence experiments and analysis of the wild-type strain 03-8 and the mutants (5 d). A: Virulence experiments of WT, ΔVmcyp5-5 and ΔVmcyp5-A; B: Virulence analysis of WT, ΔVmcyp5-5 and ΔVmcyp5-A. The asterisk indicates a significant difference from the wild-type (P=0.05). |
| 图选项 |
2.5 黑色素基因簇表达量的实时定量PCR分析 qRT-PCR结果显示,与野生型菌株03-8相比,黑色素基因簇上的6个基因在突变体ΔVmcyp5-5中的表达量均下调,下调倍数分别为4.0、8.9、1.8、2.2、4.8和2.9倍(图 5)。表明Vmcyp5的缺失引起黑色素基因簇表达水平的变化,该基因可能与黑色素合成相关。
 |
| 图 5 野生型菌株03-8和ΔVmcyp5-5的黑色素基因簇基因相对表达量 Figure 5 Relative expression levels of melanin gene cluster in the wild-type strain 03-8 and ΔVmcyp5-5. The expression levels of melanin gene cluster in the wild-type strain 03-8 were treated as one fold, then the relative expression levels of the genes in melanin gene cluster of ΔVmcyp5-5 were obviously down-regulated as shown. |
| 图选项 |
3 讨论 真菌毒素是一类由病原菌产生、在一定浓度下可以引起寄主植物发病的致病因子。前期研究表明,真菌细胞色素P450可催化真菌毒素代谢途径中疏水中间体的转化[25-27],参与病原菌致病过程,对致病力产生影响[15, 28]。本研究基于前期研究,证实Vmcyp5基因在病原菌侵染初期上调表达,推测其与病原菌致病相关。为确定该基因生物学功能,本研究利用基因敲除技术及PEG介导的遗传转化获得目的基因的敲除突变体和回复突变体,通过一系列生物学试验研究基因Vmcyp5对苹果树腐烂病菌营养生长及致病力的影响。结果表明,Vmcyp5与病原菌子实体的产生以及黑色素的合成代谢相关,并参与病原菌的致病过程。
本研究所选取的CYP基因Vmcyp5来自于V. mali毒素代谢基因簇,虽经试验证实该基因与病原菌致病相关,但并非决定性因素。究其原因,可能在于真菌细胞色素P450数量丰富,因其酶家族的多样性而拥有多种功能[29],但在现有研究背景下,仅有极少量的CYP基因显示为病原菌致病关键基因。此外,CYP基因通常以基因簇的形式编码细胞色素P450单加氧酶,因其多基因家族特征导致各基因间功能冗余现象的存在,从而通过协同作用引起功能互补。
本研究为细胞色素P450基因对苹果树腐烂病菌致病力作用的研究提供了一定的基础研究依据。而病原真菌毒素的合成代谢是一个相当复杂的过程,对于苹果树腐烂病菌而言,仍有诸多问题未解,亟待在未来的研究中进一步探索苹果树腐烂病菌基因调控毒素合成代谢的机理,为揭示病原菌致病机制奠定基础。
References
| [1] | Wang XL, Wei JL, Huang LL, Kang ZS. Re-evaluation of pathogens causing Valsa canker on apple in China. Mycologia, 2011, 103(2): 317-324. DOI:10.3852/09-165 |
| [2] | Ke XW, Huang LL, Han QM, Gao XN, Kang ZS. Histological and cytological investigations of the infection and colonization of apple bark by Valsa malivar. mali. Australasian Plant Pathology, 2013, 42(1): 85-93. |
| [3] | Li ZP, Gao XN, Du ZT, Hu Y, Kang ZS, Huang LL. Survey of apple Valsa canker in Weibei area of Shaanxi province. Acta Agriculturae Boreali-Occidentalis Sinica, 2013, 22(1): 174-178. (in Chinese) 李正鹏, 高小宁, 杜战涛, 胡杨, 康振生, 黄丽丽. 陕西渭北地区苹果树腐烂病发生情况调查. 西北农业学报, 2013, 22(1): 174-178. DOI:10.7606/j.issn.1004-1389.2013.01.029 |
| [4] | Li ZP. Study on the biological control of Saccharothrix yanglinggensis Hhs.015 on apple Valsa canker. Master Dissertation of Northwest A&F University, 2012. (in Chinese) 李正鹏. 杨凌糖丝菌Hhs. 015对苹果树腐烂病的生物防治研究. 西北农林科技大学硕士学位论文, 2012. |
| [5] | Qi GF, Yang B, Ye JR. Research advances on the toxin of the plant pathogenic fungus. Journal of Nanjing Forestry University, 2000, 24(2): 66-70. (in Chinese) 祁高富, 杨斌, 叶建仁. 植物病原真菌毒素研究进展. 南京林业大学学报, 2000, 24(2): 66-70. |
| [6] | McLean KJ, Leys D, Munro AW. Microbial cytochromes P450//de Montellano PRO. Cytochrome P450: Structure, Mechanism, and Biochemistry. Switzerland: Springer International Publishing, 2015: 261-407. |
| [7] | ?re?nar B, Petri? ?. Cytochrome P450 enzymes in the fungal kingdom. Biochimica et Biophysica Acta (BBA)-Proteins and Proteomics, 2011, 1814(1): 29-35. DOI:10.1016/j.bbapap.2010.06.020 |
| [8] | Ehrlich KC, Chang PK, Yu JJ, Cotty PJ. Aflatoxin biosynthesis cluster gene cypA is required for G aflatoxin formation. Applied and Environmental Microbiology, 2004, 70(11): 6518-6524. DOI:10.1128/AEM.70.11.6518-6524.2004 |
| [9] | Proctor RH, Plattner RD, Desjardins AE, Busman M, Butchko RAE. Fumonisin production in the maize pathogen Fusarium verticillioides:genetic basis of naturally occurring chemical variation. Journal of Agricultural and Food Chemistry, 2006, 54(6): 2424-2430. DOI:10.1021/jf0527706 |
| [10] | Bhatnagar D, Ehrlich KC, Cleveland TE. Molecular genetic analysis and regulation of aflatoxin biosynthesis. Applied Microbiology and Biotechnology, 2003, 61(2): 83-93. DOI:10.1007/s00253-002-1199-x |
| [11] | Yu JJ, Chang PK, Ehrlich KC, Cary JW, Bhatnagar D, Cleveland TE, Payne GA, Linz JE, Woloshuk CP, Bennett JW. Clustered pathway genes in aflatoxin biosynthesis. Applied and Environmental Microbiology, 2004, 70(3): 1253-1262. DOI:10.1128/AEM.70.3.1253-1262.2004 |
| [12] | Desjardins AE, Hohn TM. Mycotoxins in plant pathogenesis. Molecular Plant-Microbe Interactions, 1997, 10(2): 147-152. DOI:10.1094/MPMI.1997.10.2.147 |
| [13] | Kimura M, Tokai T, Takahashi-Ando N, Ohsato S, Fujimura M. Molecular and genetic studies of Fusarium trichothecene biosynthesis:pathways, genes, and evolution. Bioscience, Biotechnology, and Biochemistry, 2007, 71(9): 2105-2123. DOI:10.1271/bbb.70183 |
| [14] | Wen Y, Hatabayashi H, Arai H, Kitamoto HK, Yabe K. Function of the cypX and moxY genes in aflatoxin biosynthesis in Aspergillus parasiticus. Applied and Environmental Microbiology, 2005, 71(6): 3192-3198. DOI:10.1128/AEM.71.6.3192-3198.2005 |
| [15] | Siewers V, Viaud M, Jimenez-Teja D, Collado IG, Gronover CS, Pradier JM, Tudzynsk B, Tudzynski P. Functional analysis of the cytochrome P450 monooxygenase gene bcbot1 of Botrytis cinerea indicates that botrydial is a strain-specific virulence factor. Molecular Plant-Microbe Interactions, 2005, 18(6): 602-612. DOI:10.1094/MPMI-18-0602 |
| [16] | Yin ZY, Liu HQ, Li ZP, Ke XW, Dou DL, Gao XN, Song N, Dai QQ, Wu YX, Xu JR, Kang ZS, Huang LL. Genome sequence of Valsa canker pathogens uncovers a potential adaptation of colonization of woody bark. New Phytologist, 2015, 208(4): 1202-1216. DOI:10.1111/nph.13544 |
| [17] | Yu JH, Hamari Z, Han KH, Seo JA, Reyes-Domínguez Y, Scazzocchio C. Double-joint PCR:a PCR-based molecular tool for gene manipulations in filamentous fungi. Fungal Genetics and Biology, 2004, 41(11): 973-981. DOI:10.1016/j.fgb.2004.08.001 |
| [18] | Gao J, Li YB, Ke XW, Kang ZS, Huang LL. Development of genetic transformation system of Valsa mali of apple mediated by PEG. Acta Microbiologica Sinica, 2011, 51(9): 1194-1199. (in Chinese) 高静, 李艳波, 柯希望, 康振生, 黄丽丽. PEG介导的苹果腐烂病菌原生质体转化. 微生物学报, 2011, 51(9): 1194-1199. |
| [19] | Song N, Dai QQ, Huang LL, Han QM. Construction of knockout vector of GTP cyclohydrolase Ⅱ gene and mutant's biological characteristics of Valsa mali. Scientia Agricultura Sinica, 2014, 47(15): 2980-2989. (in Chinese) 宋娜, 戴青青, 黄丽丽, 韩青梅. 苹果树腐烂病菌GTP-环化水解酶Ⅱ基因敲除载体构建及其突变体的表型分析. 中国农业科学, 2014, 47(15): 2980-2989. DOI:10.3864/j.issn.0578-1752.2014.15.008 |
| [20] | Zheng DW. Construction of gene knock-out approach for Ustilaginoidea virens and the function analysis of HOG1 in Ustilaginoidea virens and Fusarium graminearum. Doctor Dissertation of Northwest A&F University, 2015. (in Chinese) |
| [21] | Zang R, Huang LL, Kang ZS, Wang XL. Biological characteristics and pathogenicity of different isolates of Cytospora spp. isolated from apple trees in Shaanxi province. Acta Phytopathologica Sinica, 2007, 37(4): 343-351. (in Chinese) 臧睿, 黄丽丽, 康振生, 王旭丽. 陕西苹果树腐烂病菌(Cytospora spp.)不同分离株的生物学特性与致病性研究. 植物病理学报, 2007, 37(4): 343-351. |
| [22] | Xu CJ, Wu YX, Dai QQ, Li ZP, Gao XN, Huang LL. Function of polygalacturonase genes Vmpg7 and Vmpg8 of Valsa mali. Scientia Agricultura Sinica, 2016, 49(8): 1489-1498. (in Chinese) 许春景, 吴玉星, 戴青青, 李正鹏, 高小宁, 黄丽丽. 苹果树腐烂病菌多聚半乳糖醛酸酶基因Vmpg7和Vmpg8的功能. 中国农业科学, 2016, 49(8): 1489-1498. DOI:10.3864/j.issn.0578-1752.2016.08.006 |
| [23] | Yin ZY, Ke XW, Huang DX, Gao XN, Voegele RT, Kang ZS, Huang LL. Validation of reference genes for gene expression analysis in Valsa mali var. mali using real-time quantitative PCR. World Journal of Microbiology and Biotechnology, 2013, 29(9): 1563-1571. |
| [24] | Bashyal BM, Chand R, Kushwaha C, Sen D, Prasad LC, Joshi AK. Association of melanin content with conidiogenesis in Bipolaris sorokiniana of barley (Hordeum vulgare L.).World Journal of Microbiology and Biotechnology, 2010, 26(2): 309-316. |
| [25] | Farkas J, Schricker R, Briza P, Eckerstorfer M, Breitenbach M. The enzymatic properties of Dit2p (CYP56) from Saccharomyces cerevisiae. FASEB Journal, 1997, 11(9): A827. |
| [26] | Tsitsigiannis DI, Keller NP. Oxylipins as developmental and host-fungal communication signals. Trends in Microbiology, 2007, 15(3): 109-118. DOI:10.1016/j.tim.2007.01.005 |
| [27] | B?mke C, Rojas MC, Gong F, Hedden P, Tudzynski B. Isolation and characterization of the gibberellin biosynthetic gene cluster in Sphaceloma manihoticola. Applied and Environmental Microbiology, 2008, 74(17): 5325-5339. DOI:10.1128/AEM.00694-08 |
| [28] | Koch A, Kumar N, Weber L, Keller H, Imani J, Kogel KH. Host-induced gene silencing of cytochrome P450 lanosterol C14α-demethylase-encoding genes confers strong resistance to Fusarium species. Proceedings of the National Academy of Sciences of the United States of America, 2013, 110(48): 19324-19329. DOI:10.1073/pnas.1306373110 |
| [29] | Ichinose H. Molecular and functional diversity of fungal cytochrome P450s. Biological and Pharmaceutical Bulletin, 2012, 35(6): 833-837. DOI:10.1248/bpb.35.833 |
