全文HTML
--> --> --> 磷(P)是自然生态系统中生物生长的关键营养素,其过量存在会使水体出现富营养化,从而引发藻类过度生长、水质恶化、鱼类和植物死亡等严重的环境问题[1-3]。近年来,国家实施了严格的磷排放限值管理,并结合“五水共治”、“水十条”等相关政策法规的制定,目的是降低进入接收水体中的磷含量以减少其环境危害性[4-7]。工业废水的排放和农业径流是水生生态系统中磷含量增加的主要原因,因而针对它们的脱磷技术和工艺研究对磷污染控制具有关键作用。目前已被广泛采纳的磷去除方法包括生物法、化学法和物理法等。其中生物除磷方法[8-10],即常规的活性污泥法,成本低廉、磷去除效果好。但由于微生物的磷酸盐代谢能力会随着水中磷浓度降低而显著下降,因而对低磷水体的处理能力变弱,无法实现达标排放;化学法脱磷时[11-12],伴随着水中磷酸根的有效降解,常会有大量污泥产生,且目标排放浓度越低需要加入的药剂量就越大,二次污染严重;而以反渗透和电渗析为代表的物理方法[13-14],在实际应用中则存在着对进水水质要求高、处理费用昂贵和磷去除效率低等缺点。吸附法[15-17]与上述各类除磷方法相比,具有操作简单、去除磷效率高、吸附速度快和环境污染小等特征,因而在低磷废水处理中有重要作用。
对比常用的磷吸附剂如天然矿物、工业废渣、金属氧化物、生物质等,镧基吸附剂是化学稳定性较高的稀土金属吸附剂[18]。镧基吸附剂不但除磷效率高、磷重新释放程度小,而且还有磷亲和力强和饱和吸附量大的独特性质,因此,近年来在针对水和废水的磷去除材料研发中备受关注。典型的镧吸附剂包括镧化合物以及各种镧负载复合物等,如氢氧化镧、氧化镧纳米棒、氢氧化镧改性水凝胶、镧改性介孔二氧化硅等[19-22],其在应用中保持了良好的磷脱除能力和稳定性。然而研究人员在研究中也发现,上述这些镧基吸附剂在碱性条件下使用时,磷去除率与酸性和近中性下的结果相比能力变差,表现出在碱性条件下脱磷效率偏低的缺陷,因而限制了其在中性和碱性水体处理领域中的广泛应用[23-24]。
基于上述研究结果,本研究以碳酸镧作为吸附剂主体,采用在合成反应中加入晶型导向剂和控制反应温度的方法,获得了具有脱磷溶液pH适用范围宽、对水中磷组分平衡吸附量大、能被再生和重复进行脱磷的无定型纳米碳酸镧吸附剂,并初步探讨了该吸附剂在脱磷过程中的热力学和动力学特征。
1.1. 实验材料
六水氯化镧(LaCl3·6H2O)购自阿拉丁生化科技股份有限公司;碳酸氢钠(NaHCO3)、七水合硫酸镁(MgSO4·7H2O)、乙醇(EtOH)、钼酸铵((NH4)2MoO4)、酒石酸锑钾、抗坏血酸、磷酸二氢钾(KH2PO4)、盐酸(HCl)、氢氧化钠(NaOH)等均为分析纯,购自国药集团化学试剂有限公司。1.2. 分析方法
X射线衍射仪(XRD,X`Pert PRO,荷兰帕纳科公司)分析得到样品的晶体衍射谱图;透射电镜(TEM,JEM-2100,日本电子株式会社)和冷场发射扫描电镜(SEM, SU8010,日本日立公司)表征分析样品的形貌;动态光散射仪(DLS,Zen-3600,英国Malvem公司)测定纳米复合物的Zeta电位;电感耦合等离子体质谱仪(ICP-MS,iCAP6300,美国赛默飞世尔公司)分析金属离子含量。1.3. 碳酸镧的制备
将160 mL的0.05 mol·L?1氯化镧溶液在25 ℃或85 ℃下磁力搅拌5 min,加入镁盐使c(Mg2+)∶c(La3+)=1∶1,继续搅拌10 min,按照1滴·s?1的速度将40 mL的 0.8 mol·L?1的碳酸氢钠溶液滴加到上述溶液中,继续搅拌30 min后,陈化30 min。待冷却后,抽滤并用蒸馏水清洗3次,45 ℃干燥12 h。将25 ℃和85 ℃下制备的碳酸镧分别标记为LC(25)和LC(85)。在相同合成条件下,没有镁盐存在时制备的碳酸镧分别标记为LC0(25)和LC0(85)。
1.4. 水中磷的吸附实验
称取一定质量的碳酸镧吸附剂置于250 mL锥形瓶中,加入100 mL不同质量浓度的磷溶液,在25 ℃恒温振荡器中振荡24 h后,取上清液经0.45 μm滤膜过滤,用钼酸铵比色法直接测定滤液中磷酸盐的质量浓度。吸附剂的平衡吸附量(qe)和磷去除率(η)按照式(1)和式(2)进行计算。式中:C0为磷溶液初始质量浓度,mg·L?1(以P计);qe为平衡时的磷吸附量,mg·g?1(以P计);Ce为平衡时磷溶液质量浓度,mg·L?1(以P计);V为溶液体积,L;m为吸附剂质量,g。
1) pH的影响。将0.02 g的吸附剂加入到100 mL、磷溶液质量浓度为 20 mg·L?1溶液中,用0.5、1 mol·L?1氢氧化钠和盐酸调节溶液pH分别为3.0、4.0、5.0、7.0、9.0、11.0。在25 ℃下,250 r·min?1的摇床中反应24 h,用0.45 μm滤膜过滤,测定溶液吸光度,计算去除率。
2)反应动力学。将0.04 g的LC(85)和LC(25)吸附剂分别加入到体积200 mL、质量浓度为20 mg·L?1的磷溶液中,在25 ℃、250 r·min?1的摇床中反应,间隔指定的时间后取样,用0.45 μm滤膜过滤。分别用准一级动力学方程(式(3))、准二级动力学方程(式(4))拟合实验结果。
式中:qt 为t时间的吸附量,mg·g?1;qe为平衡时的磷吸附量,mg·g?1;t为吸附时间,h;k1为拟一级动力学吸附速率常数,h?1;k2为拟二级动力学吸附速率常数,g·(mg·h)?1。
3)等温吸附。将0.15 g吸附剂,加入到100 mL、质量浓度分别为 15、18、20、25和30 mg·L?1的磷溶液中,在25 ℃下,250 r·min?1的摇床中反应24 h,用0.45 μm滤膜过滤。分别用Langmuir (式(5))和Freundlich(式(6))模型拟合实验数据。
式中:qe为平衡时吸附量,mg·g?1;qm为理论最大吸附量,mg·g?1;Ce是反应平衡时的磷溶液质量浓度,mg·L?1;KL为Langmuir常数,L·mg?1;KF 为Freundlich常数,mg·g?1;n是Freundlich模型中评价吸附强度的常数。
4)吸附剂加入量。在100 mL质量浓度为2 mg·L?1和20 mg·L?1磷溶液中分别加入一定量的吸附剂,使吸附剂的质量浓度分别为0.05、0.10、0.15、0.20、0.25、0.30和0.40 g·L?1,在25 ℃,250 r·min?1的摇床中反应24 h。
5)再生和循环吸附。称取0.1 g吸附饱和的LC(85),加入到浓度3 mol·L?1的50 mL氢氧化钠溶液中,在100 ℃油浴中回流反应5 h。反应结束后,抽滤、用蒸馏水清洗至中性,45 ℃干燥12 h。取再生后的LC(85)吸附剂0.02 g,置于体积100 mL、质量浓度为20 mg·L?1的磷溶液中,25 ℃下反应24 h。
2.1. 碳酸镧的结构和形貌表征
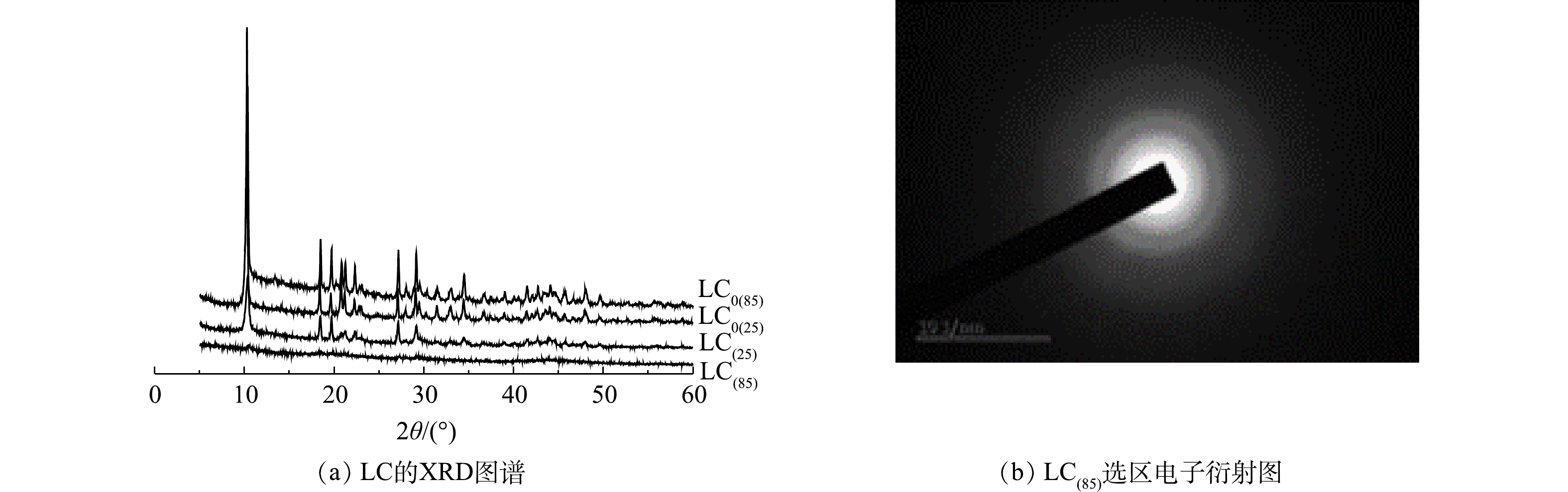
1) XRD和选区电子衍射分析。在晶型诱导剂Mg2+存在条件下,25 ℃和85 ℃下制备的碳酸镧LC(25)和LC(85)的XRD图谱如图1(a)所示。已知10.27°处的尖锐强衍射峰是La2(CO3)3·8H2O (PDF #25-1400) (002)晶面的特征衍射峰,18.51°、20.79°、21.24°、27.08°和29.06°处的衍射峰则分别对应La2(CO3)3·8H2O的(020)、(004)、(022)、(220)和(222) 晶面。比较晶型诱导剂Mg2+存在条件下制备的LC(85)和LC(25)图谱,发现合成温度的改变会影响产物的特征结晶峰大小,当制备温度由25 ℃升高到85 ℃时,碳酸镧(002)特征结晶峰明显变小到近乎消失,表明二者结晶状态发生了变化,即由晶体态的LC(25)转变到无定型的LC(85)。对比图1中同时给出的不使用晶型诱导剂Mg2+时,碳酸镧产物LC0(25)和LC0(85)的XRD图谱,发现他们也存在着(002)特征结晶衍射峰,由此可知,仅LC(85)具有独特的非晶态特征。另一方面,从LC(85)的选区电子衍射图(图1(b))中可知,其衍射图是多个弥散的衍射环,因而得出的结论也是LC(85)具有非结晶状态的特征。已知吸附剂的非晶态相比晶态相更有利于脱磷[25],因此,LC(85)的制备为获得高效脱磷剂提供了可能。2) SEM和TEM分析。加入晶型诱导剂Mg2+前后,不同温度下制备的碳酸镧SEM和TEM表征结果如图2所示。可以看出,随着合成温度的升高,碳酸镧颗粒变小且形貌发生显著变化。如碳酸镧在25 ℃合成时,无晶型诱导剂Mg2+存在下制备的LC0(25) (图2(a))为规则的六边形片状结构,大小介于几百纳米至几个微米之间;升高温度到85 ℃时,LC0(85) (图2(b))转变成不规整的片状结构,粒径变小。而在晶型诱导剂Mg2+存在时,25 ℃合成的LC (25) (图2(d))尽管保持了不规则片状多边形结构,但粒径与LC0(25)相比显著变小,多介于300到500 nm之间;而当制备温度升高至85 ℃时,LC(85) (图2(e))突变为粒径小于50 nm的均匀球形纳米颗粒,微观形貌与LC0(85)、LC0(25)和LC(25)的片状结构均不相同。
另一方面,从LC(85)和LC(25)的TEM分析结果可以看出,LC(85) (图2(f))为球型,粒径约为50 nm;而LC(25) (图2(c))的TEM图谱则为多边形的片状形貌,径向尺寸大于100 nm。显然,LC(85)和LC(25)的TEM表征结果与上述的SEM结论相吻合,即LC(85)与其他LC样品相比具有更小的粒径和特征的球形微观结构。
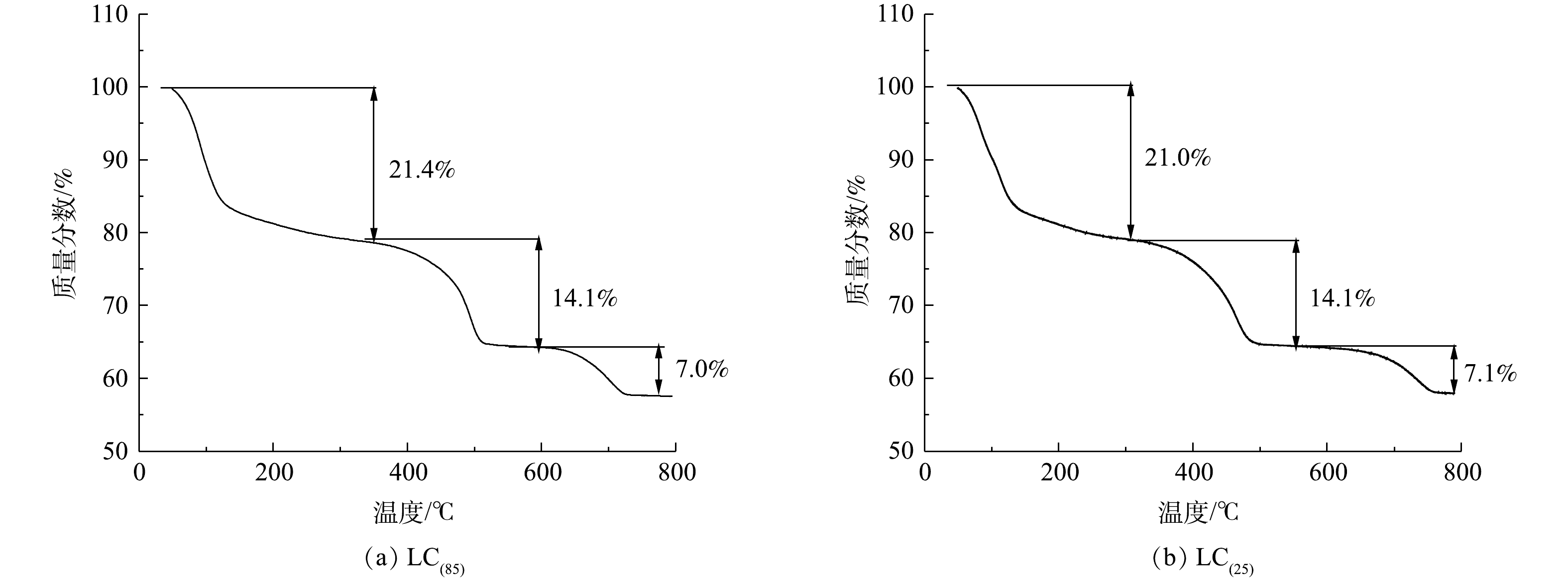
3) TGA和ICP分析。图3是不同温度下制备的LC(85) (图3(a))和LC(25) (图3(b))的TG图谱。可以看出,二者的热重曲线均可分为3个阶段:第1阶段在350 ℃之前(质量损失21.0%~21.4%);第2阶段在350~600 ℃(质量损失14.1%);第3阶段在600~800 ℃(质量损失7.0%~7.1%),总质量损失为42.1%~42.5%。由于LC(85)和LC(25)在每个阶段失重百分比差别较小,因而可以认为,制备温度的改变对碳酸镧所含结晶水的数量影响小,LC(85)和LC(25)均是含有8个结晶水的碳酸镧。
取LC(85)和LC(25)样品用16 mol·L?1的浓硝酸溶解,然后定容稀释至指定浓度,经滤膜过滤后,用电感耦合等离子体质谱仪进行检测。得到的ICP分析结果是,纳米吸附剂LC(85)和LC(25)中镧含量分别为45.82%和45.28%,镁含量分别为0.09%和0.02%。已知La2(CO3)3·8H2O的理论镧含量是46.16%,2个样品的ICP的表征结果与其相差很小,同时考虑到Mg2+在LC(85)和LC(25)中含量很低,因此,有理由认为Mg2+在碳酸镧的合成过程中,没有随着反应的进行以碳酸镁或氢氧化镁的形式转变为产物而共存于LC(85)中,而仅作为晶型控制剂存在于反应液中,且本身质量在反应中几乎无损耗。本研究中Mg2+在晶体制备中的这种作用,与马俊等[26]得出的Mg2+能作为晶型控制剂对碳酸钙进行晶型调控的结论相符。
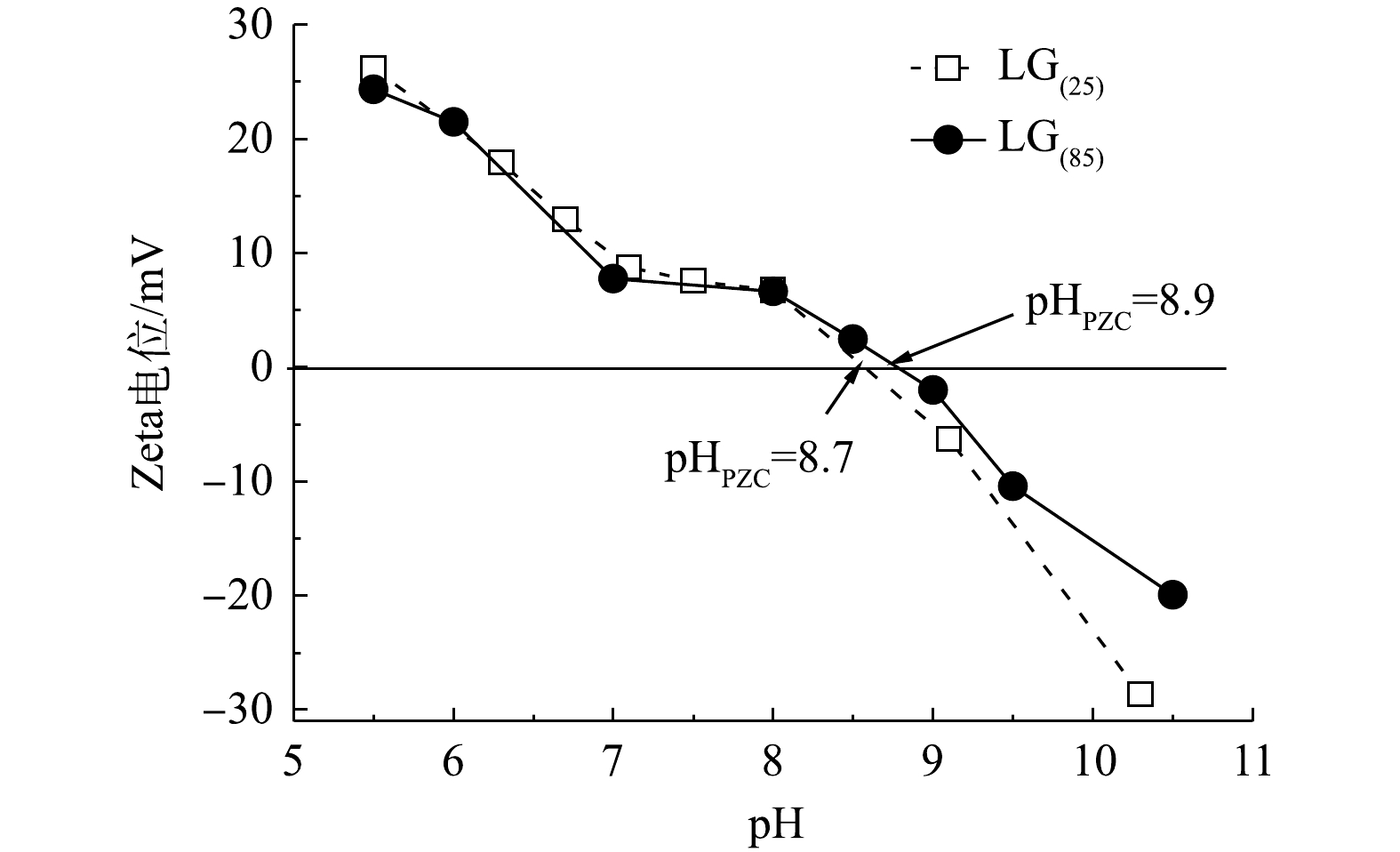
4) Zeta(ζ)电位的变化。由LC(85)和LC(25)的Zeta电位图(图4)可知,LC(85)和LC(25)的等电点pHpzc分别为8.9和8.7,与不加入诱导剂Mg2+制备的LC0(25)的Zeta电位相比(pHpzc=7.4),不但在任一特定pH条件下LC(85)的Zeta电位高于LC(25),且其对应的pHpzc也均大于LC0(25)。由于当溶液的pH低于pHpzc,碳酸镧纳米吸附剂会发生质子化和正电荷化,因而能强化同带负电荷磷酸根离子间的静电相互作用,导致碳酸镧吸附磷酸根的能力增强;而当溶液的pH高于pHpzc时,碳酸镧表面脱质子化而显示出负电性,造成纳米吸附剂与磷酸根之间的静电斥力,不利于磷酸根在碳酸镧表面的吸附。晶型诱导剂Mg2+加入后,LC(85)和LC(25)的pHpzc均变大,因而为增强碳酸镧吸附磷酸根的效果提供了较好的界面性质。
2.2. 吸附性能分析
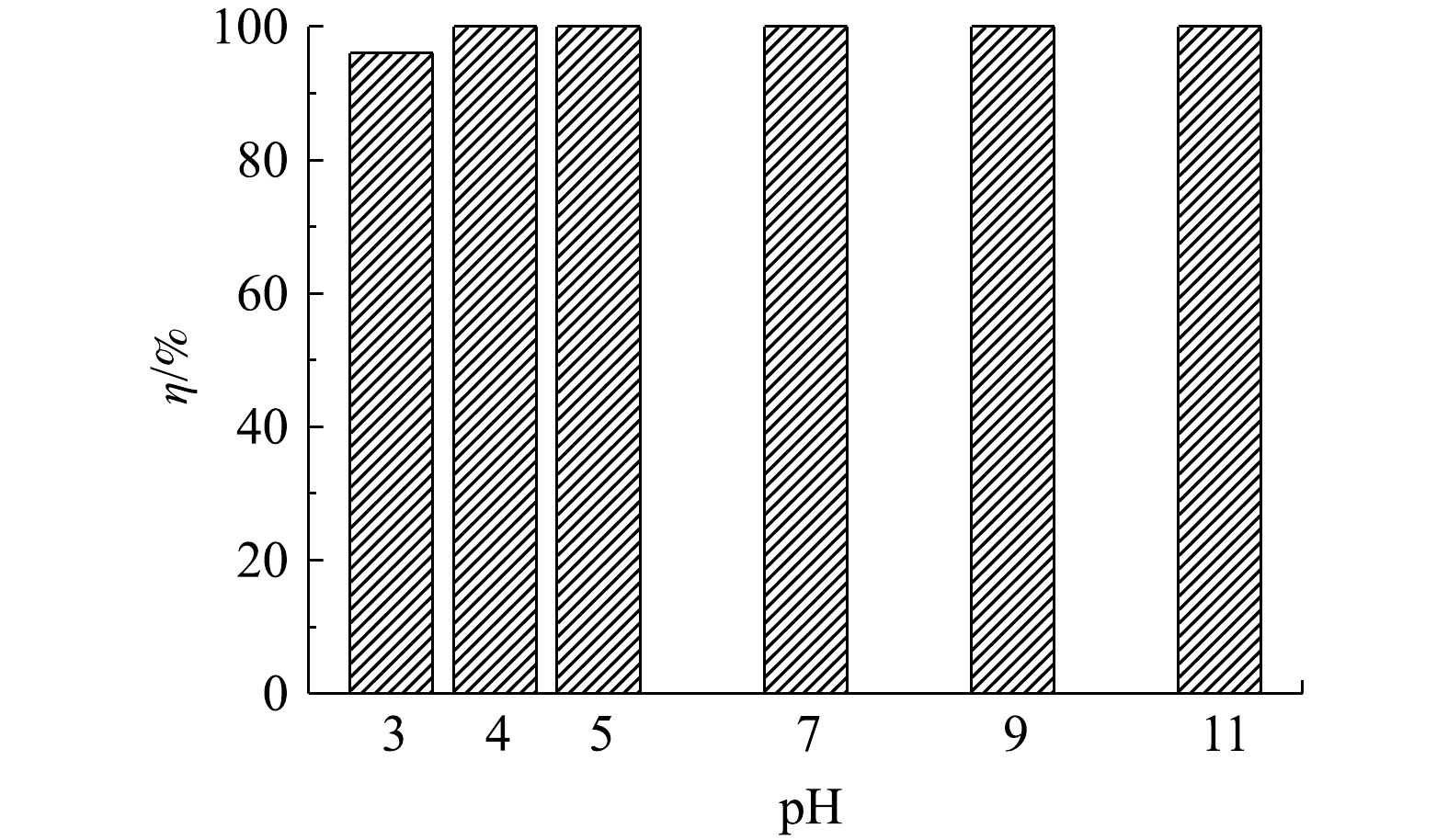
1) pH对吸附效果的影响。溶液pH会影响吸附剂的表面电位以及磷酸根在溶液中的价态和电离度,因而是评价脱磷剂性能的重要因素之一。为了考察pH对LC(85)吸附磷能力的影响,将溶液的pH调节在3.0~11.0,研究其对初始质量浓度为100 mg·L?1磷溶液的脱磷效果。由图5可以看出,LC(85)不但脱磷能力强而且在宽pH条件内保持了高脱磷稳定性,即随着溶液pH由3.0增大到11.0,磷去除率一直保持在95%以上。已知在酸性条件下,由于溶液中磷酸根主要以







2)吸附动力学。在脱磷过程中LC(85)的吸附量随时间变化曲线如图6(a)所示。可以看出,该吸附剂对磷酸根的去除过程可分为2个阶段:第1阶段为快速吸附过程,LC(85)的磷吸附量在1 h内迅速达到97.6 mg·g?1;第2阶段为缓慢增加直至达到平衡值,即继续延长吸附时间到12 h,LC(85)对磷的吸附量仅增大为100.5 mg·g?1。在相同条件下,对比LC(25)吸附磷酸根的动力学曲线,发现LC(85)和LC(25)的磷平衡吸附量尽管近似相等,但由于LC(85)的快速吸附过程仅需1 h,而LC(25)的快速吸附过程则延长到4 h,因此,LC(85)在第1阶段的吸附过程中具备了更快的脱磷能力。
对LC(85)和LC(25)吸附数据进行拟一级和拟二级动力学模型的拟合(图6(b)和图6(c))。由表1所示的拟合结果可知,LC(85)和LC(25)吸附曲线经动力学拟合后得到的磷平衡吸附量均接近实测值。但由于LC(85)和LC(25)的拟二级动力学(R2分别为0.999 9 和0.999 9)比拟一级动力学(R2分别为0.364 1 和 0.985 2)更好地模拟了磷酸根在LC上的吸附过程,所以溶液中磷的去除过程主要应是通过碳酸镧进行化学吸附或化学键合来实现的。此外,对比表1中给出的二级动力学常数k2,发现当制备纳米碳酸镧的反应温度从25 ℃升高到85 ℃时,对应的k2也从LC(25)的0.078 8增大到了LC(85)的0.345 6,因而证明了LC(85)相比于LC(25)具有更快脱磷反应速度。显然,LC(85)所特有的球状微观结构和较小的颗粒尺度,使其在脱磷过程中能表现出较佳的动力学特征。
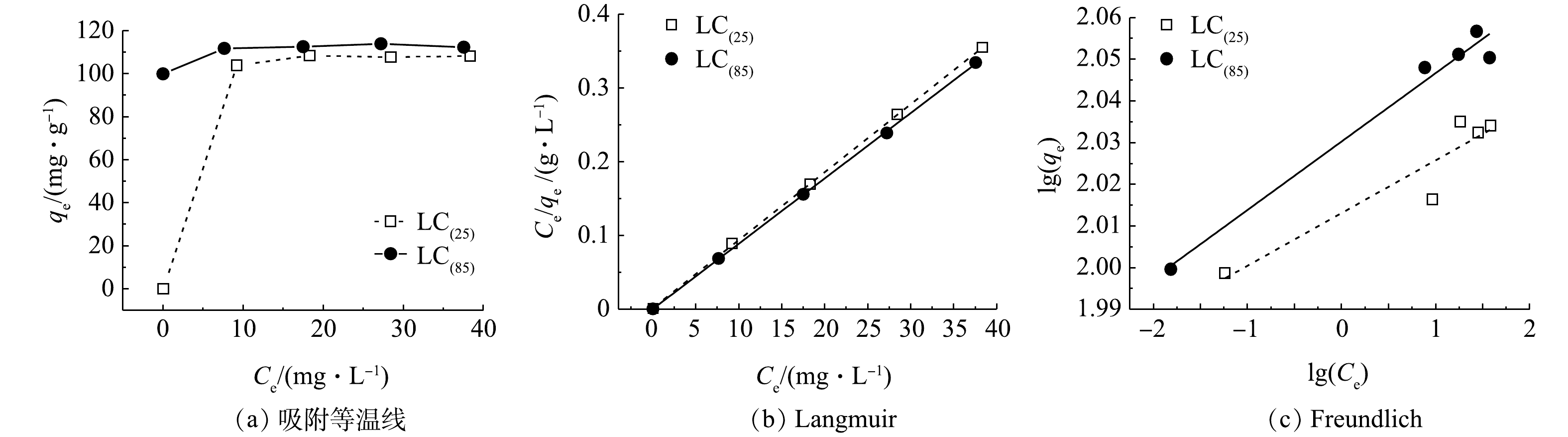
3)吸附等温线。吸附等温线体现了材料吸附能力的强弱,是反应体系中平衡浓度与表面吸附量之间的关系。Langmuir吸附等温式基于吸附剂表面是均质的以及吸附质分子间无相互作用两个基本假设,其主要用于单分子层吸附的拟合;Freundlich吸附等温式则适用于非均质或多层吸附的拟合。将LC(85)和LC(25)在室温下的吸附等温线数据(图7(a))分别用Langmuir和 Freundlich 模型进行拟合(图7(b)和图7(c)),拟合结果见表2。由于LC(85)和LC(25) 的Langmuir吸附等温式R2分别为0.999 8和0.999 8,均高于Freundlich吸附等温式R2的0.967 9和0.840 4,表明其吸附过程更符合Langmuir模型,因而可以认为溶液中的磷酸根是以单分子层方式分别吸附在LC(85)和LC(25)上。再根据Langmuir模型计算出LC(85)和LC(25)最大饱和吸附量,可知分别为112.8 mg·g?1和108.5 mg·g?1。
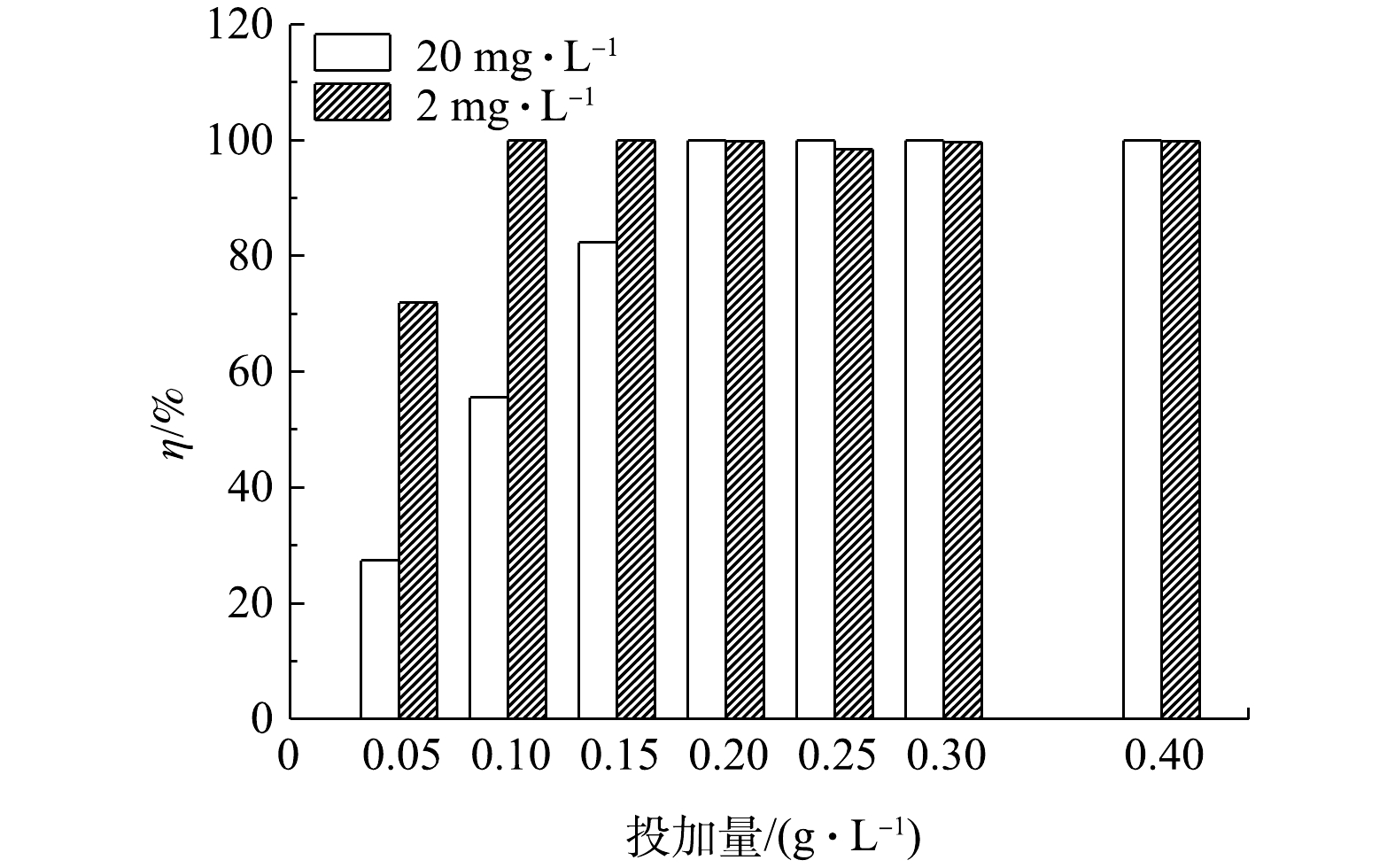
4)吸附剂加入量的影响。图8是在磷溶液质量浓度分别为2 mg·L?1或20 mg·L?1时,水样的磷去除率随LC(85)投加量改变的情况。可以看出,随着LC(85)投加量的增加,水样中的磷去除率上升。如当LC(85)的投加量由0.05 g·L?1增加到0.2 g·L?1时,2 mg·L?1和20 mg·L?1水样的磷去除率分别由71.9%和27.4%增大到接近100%。再继续增加LC(85)到0.4 g·L?1时,两模拟水样的磷去除率接近饱和,所以选择0.2 g·L?1的LC(85)投加量作为磷溶液质量浓度为2~20 mg·L?1水样的最佳加入量。
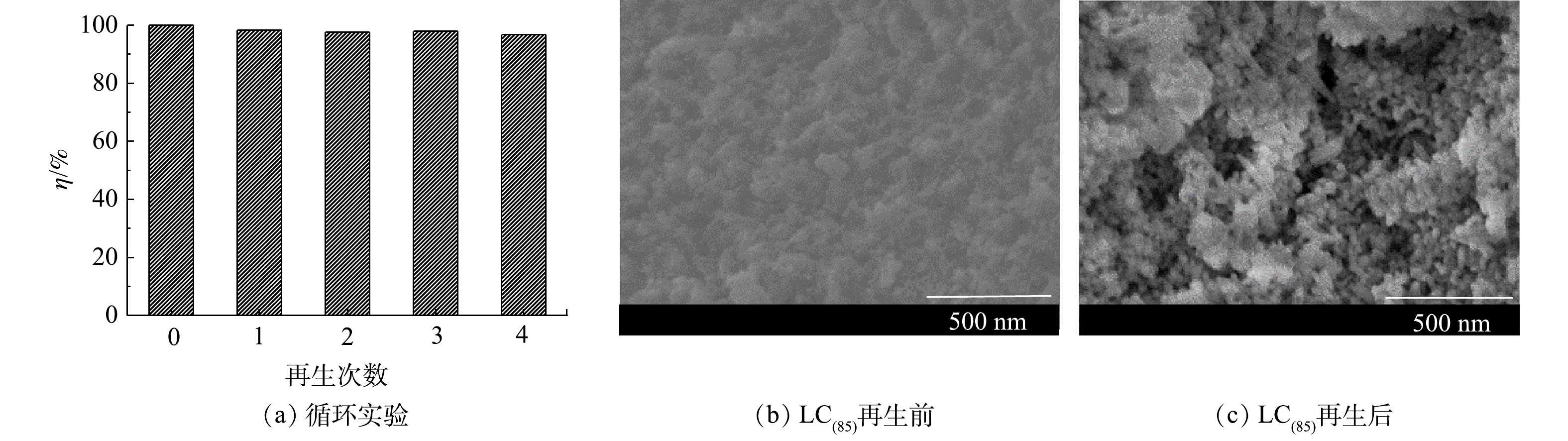
5)循环吸附性能。将吸附脱磷后的LC(85)样品进行再生,考察LC(85)作为脱磷剂使用时的稳定性和可重复性。经4次再生/吸附后的脱磷效果如图9所示。由此可知,LC(85)在连续5个脱磷周期内均保持了良好的磷酸根去除能力,对于磷溶液质量浓度为20 mg·L?1的水样,脱磷率由99.8%下降到96.8%,表明LC(85)经多次吸附再生后仍能保持良好的脱磷性能,因而在工业化方面具备较好的应用前景。
比较LC(85)经碱再生前后的SEM图谱(图9(b)和图9(c)),可以看出LC(85)吸附水中的磷酸根之后,其颗粒表面变粗糙且有黏连;而经碱再生的LC(85),虽然粒子间出现了少量团聚,但大部分颗粒能独立存在且表面光滑,基本保持了吸附前LC(85)的纳米尺度和微观形貌,因而为LC(85)在多次循环脱磷过程中保持高脱磷性提供了结构上的保证。
2) Langmuir吸附比Freundlich吸附更好地模拟了脱磷过程,表明溶液中磷酸根的吸附过程主要通过单分子层吸附方式进行,其最大饱和吸附量可达112.8 mg·g?1;另一方面,LC(85)的拟二级模型的系数(R2=0.999 9)比拟一级模型的系数(R2=0.364 1)高,说明LC的脱磷主要是通过化学吸附或化学键合来实现的。
3)当模拟水样的磷质量浓度为20 mg·g?1时,脱磷后产生的LC(85)经碱再生后可继续保持高脱磷能力,4次再生操作后磷去除能力仍可达96.8%。
参考文献

 下载:
下载: 

 点击查看大图
点击查看大图