3.中国科学院大学,北京 100049
1.School of Resources and Environment, Fujian Agriculture and Forestry University, Fuzhou 350002, China
2.Key Laboratory of Urban Pollutant Conversion, Institute of Urban Environment, Chinese Academy of Sciences, Xiamen 361021, China
3.University of Chinese Academy of Sciences, Beijing 100049, China
为探究全程自养脱氮工艺(completely autotrophic nitrogen removal over nitrite,CANON)启动和高负荷运行过程中微生物响应特性并确定有效的调控策略,基于已稳定运行的厌氧氨氧化(anaerobic ammonium oxidation, anammox)系统,通过调控DO、pH和游离氨,并采取逐渐降低
在启动过程中始终为优势菌属。
To explore the microbial response characteristics during the start-up and the high-load operation phase and determine the effective regulation strategy of the completely autotrophic nitrogen removal over nitrite (CANON) process, a stable anaerobic ammonium oxidation (anammox) system was conducted by adjusting the environmental factors (DO, pH and free ammonia), gradually reducing the concentration of nitrite and increasing the concentration of ammonia, then its transformation to CANON process was completed. The results showed that when the free ammonia was 10~20 mg·L
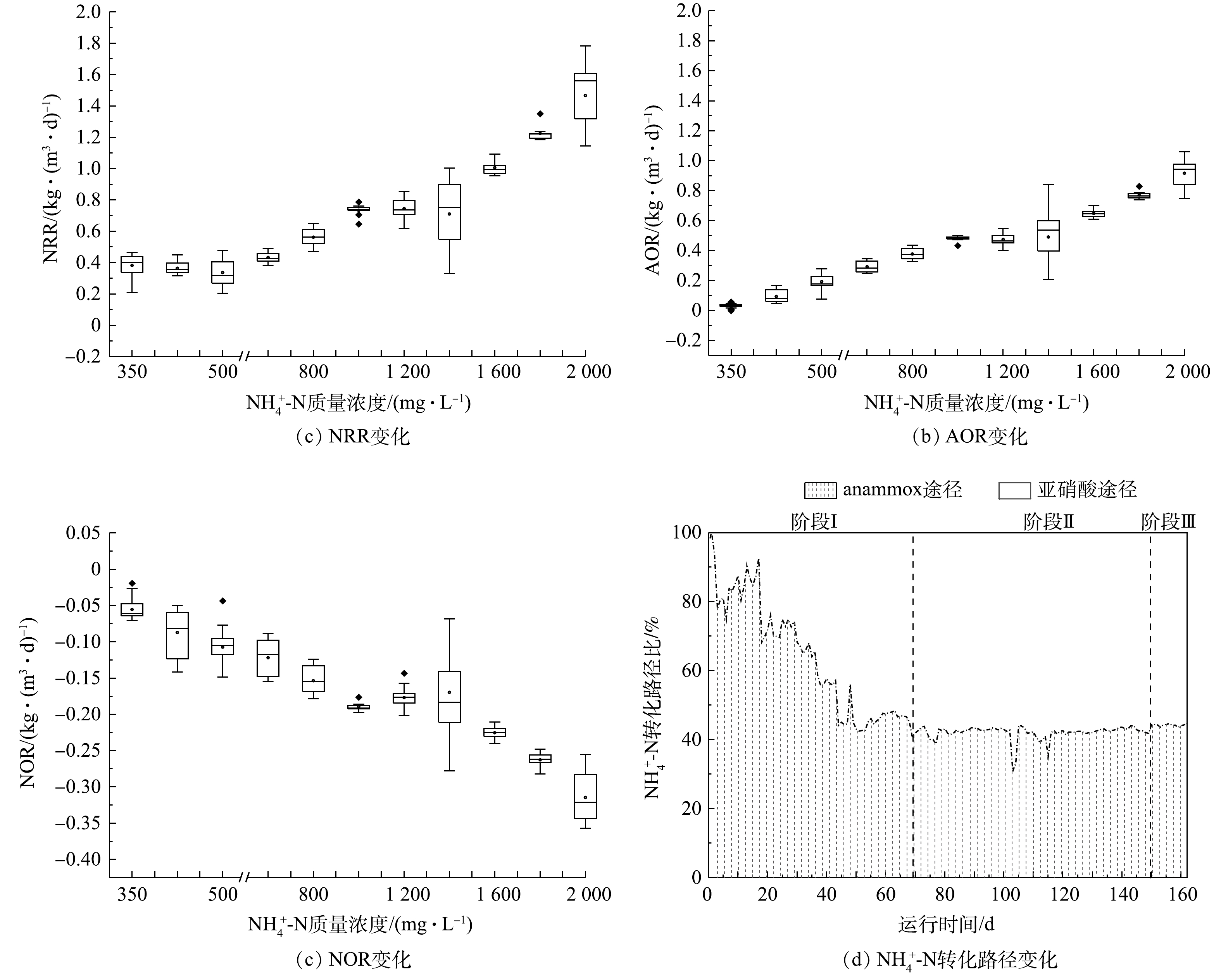
, and pH was 7.0~7.2, the growth of nitrite oxidation bacteria was inhibited, the ammonia oxidation rate and nitrogen removal rate could be gradually improved to 0.98 and 1.60 kg·(m
, respectively, and start-up of CANON was successfully completed. In addition, the
-N transformation ratio of anammox and nitrification pathway was finally stable at about 0.73. High concentration (>1 800 mg·L
) of ammonia increased the abundance of anammox, but had an opposite effect on the ammonium oxidizing bacteria. The
were the main genera of anammox bacteria under the early stage of the start-up and high-load condition, respectively. However, the
s, as a genus of AOB, were always the main genera during the start-up.
.
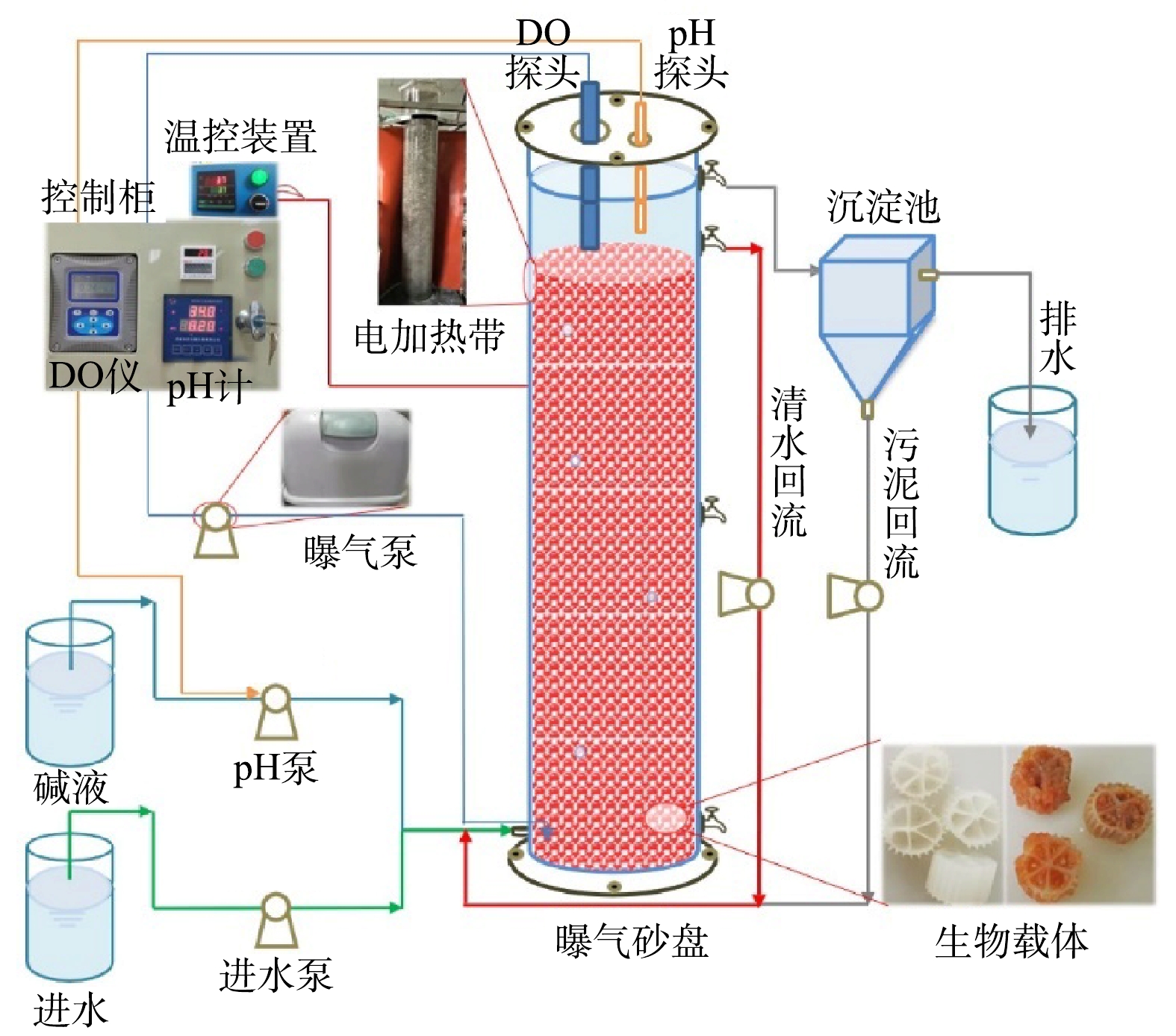
Schematic diagram of experimental device
Variation of SAA under different environmental factors
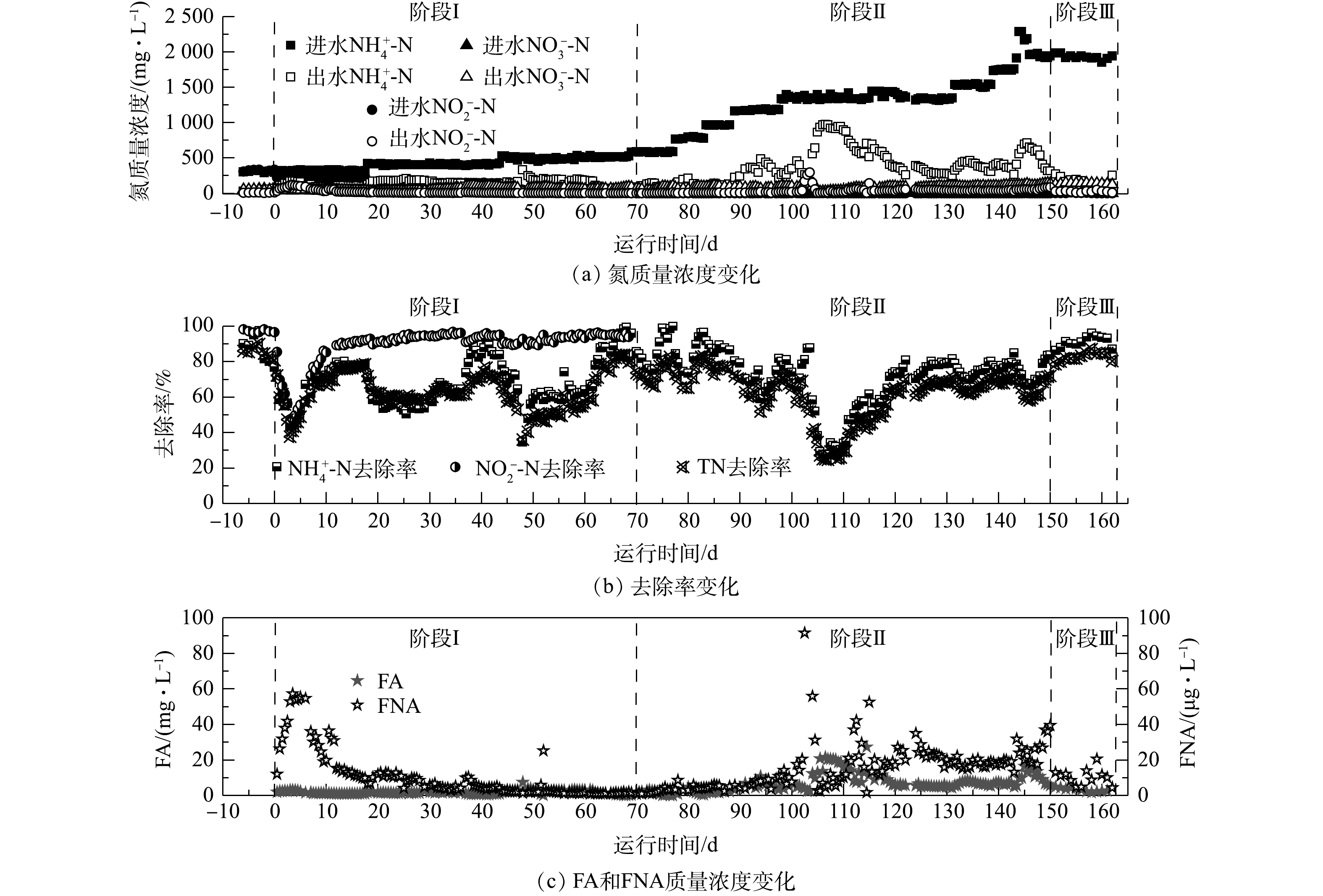
Variation of water quality during start-up of the CANON process
-N removal pathway during the start-up of CANON process
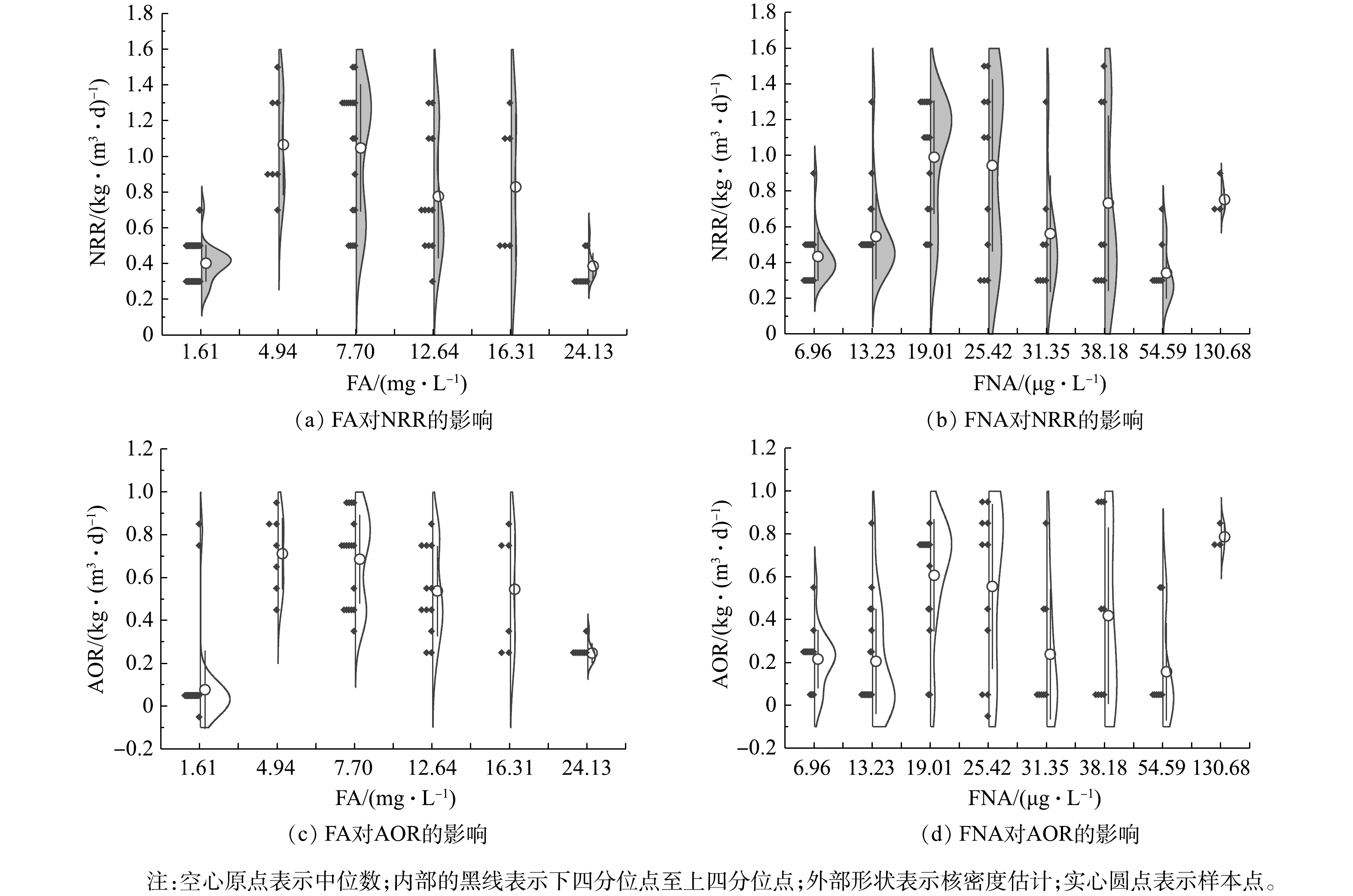
CANON启动过程中FA和FNA对氮转化速率的影响
Effect of FA and FNA on nitrogen conversion rate during the start-up of CANON process
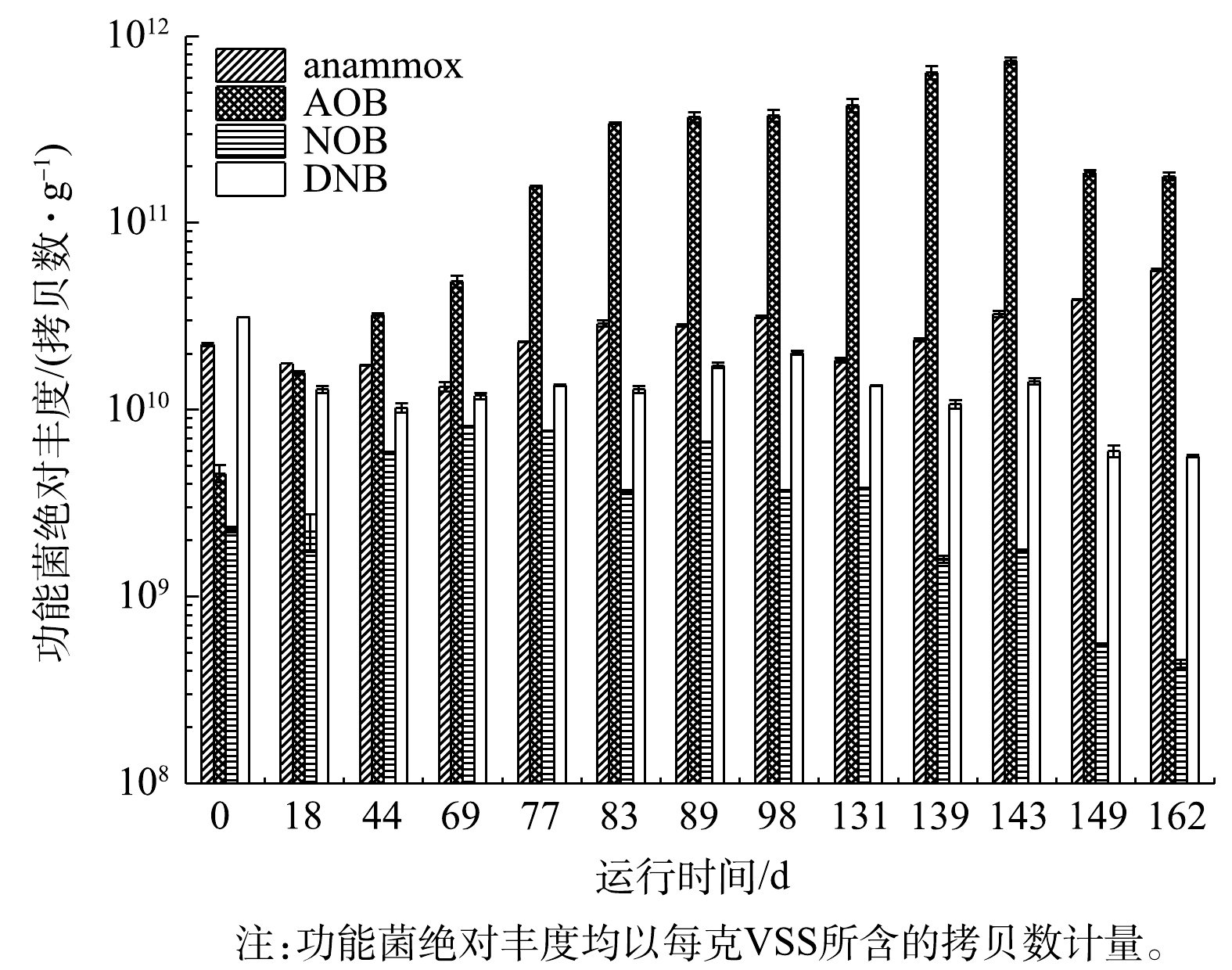
CANON工艺启动过程中4种功能菌丰度的变化
Changes in abundances of four functional bacterial groups during the start-up of CANON process
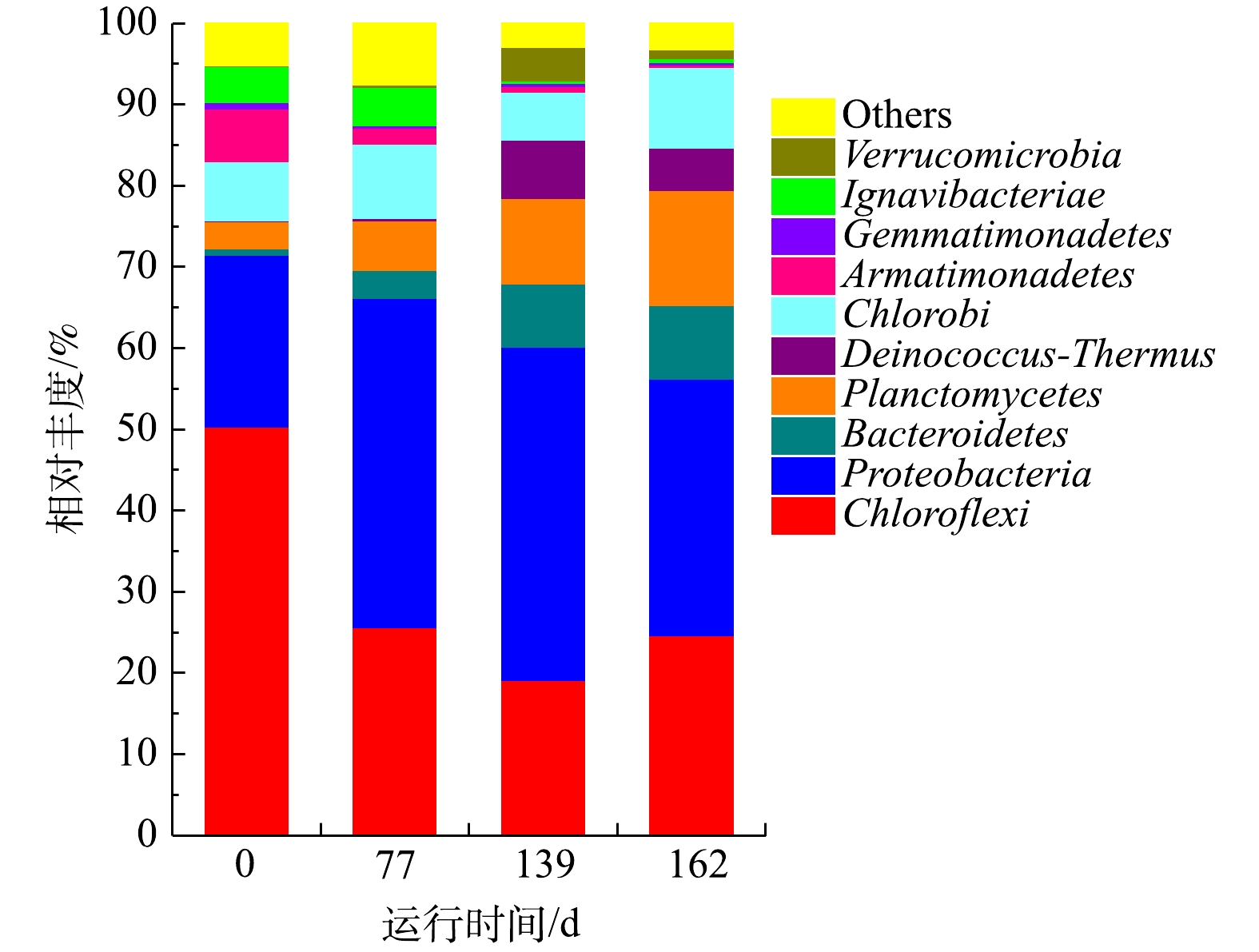
CANON工艺启动过程中在门水平上的菌群变化
Variation of microbial flora at a phylum level during the start-up of CANON process
| [1] | CONNAN R, DABERT P, MOYA-ESPINOSA M, et al. Coupling of partial nitritation and anammox in two- and one-stage systems: Process operation, N2O emission and microbial community[J]. Journal of Cleaner Production, 2018, 203: 559-573. |
| [2] | LI X, ZHANG J, ZHANG X, et al. Start-up and nitrogen removal performance of CANON and SNAD processes in a pilot-scale oxidation ditch reactor[J]. Process Biochemistry, 2019, 84: 134-142. doi: 10.1016/j.procbio.2019.06.010 |
| [3] | ZUO L, YAO H, LI H, et al. Modeling of completely autotrophic nitrogen removal process with salt and glycine betaine addition[J]. Chemosphere, 2021, 264(2): 128474. |
| [4] | HE S, ZHANG Y, NIU Q, et al. Operation stability and recovery performance in an Anammox EGSB reactor after pH shock[J]. Ecological Engineering, 2016, 90: 50-56. doi: 10.1016/j.ecoleng.2016.01.084 |
| [5] | SLIEKERS A, THIRD K, ABMA W, et al. CANON and Anammox in a gas-lift reactor[J]. FEMS Microbiology Letters, 2003, 218(2): 339-344. |
| [6] | YUE X, YU G, LIU Z, et al. Fast start-up of the CANON process with a SABF and the effects of pH and temperature on nitrogen removal and microbial activity[J]. Bioresource Technology, 2018, 254: 157-165. |
| [7] | PARK H, ROSENTHAL A, JEZEK R, et al. Impact of inocula and growth mode on the molecular microbial ecology of anaerobic ammonia oxidation (anammox) bioreactor communities[J]. Water Research, 2010, 44(17): 5005-5013. doi: 10.1016/j.watres.2010.07.022 |
| [8] | 刘竹寒, 岳秀, 于广平, 等. 单级全程自养脱氮工艺研究进展[J]. 水处理技术, 2017, 43(2): 8-13. |
| [9] | 李祥, 黄勇, 袁怡, 等. 不同泥源对厌氧氨氧化反应器启动的影响[J]. 环境工程学报, 2012, 6(7): 2143-2148. |
| [10] | FENG X, WANG X, WANG R, et al. Zeolite biofilm aeration filter plays a pre-nitritation role in the autotrophic nitrogen removal from iron oxide red wastewater[J]. Journal of Chemical Technology and Biotechnology, 2020, 95(12): ? 3261-3269. doi: 10.1002/jctb.6505 |
| [11] | FANG F, LI K, GUO J, et al. New insights into nitrous oxide emissions in a single-stage CANON process coupled with denitrification: thermodynamics and nitrogen transformation[J]. Water Science and Technology, 2020, 82(1): ? 157-169. |
| [12] | RUIZ G, JEISON D, RUBILAR O, et al. Nitrification-denitrification via nitrite accumulation for nitrogen removal from wastewaters[J]. Bioresource Technology, 2006, 97(2): 330-335. doi: 10.1016/j.biortech.2005.02.018 |
| [13] | XIAO P, AI S, ZHOU J, et al. N2O profiles in the enhanced CANON process via long-term N2H4 addition: Minimized N2O production and the influence of exogenous N2H4 on N2O sources[J]. Environmental Science and Pollution Research, 2020, 27(30): 37188-37198. |
| [14] | LI G, CARVAJAL-ARROYO J, SIERRA-ALVAREZ R, et al. Mechanisms and control of $ {\rm{N}}{{\rm{O}}^ -_2} $ inhibition of anaerobic ammonium oxidation (anammox)[J]. Water Environment Research, 2017, 89(4): 330-336. inhibition of anaerobic ammonium oxidation (anammox)[J]. Water Environment Research, 2017, 89(4): 330-336. |
| [15] | YANG R, MAO W, WANG X, et al. Response and adaptation of microbial community in a CANON reactor exposed to an extreme alkaline shock[J]. Archaea-An International Microbiological Journal, 2020, 2020: 8888615. |
| [16] | HE S, NIU Q, MA H, et al. The treatment performance and the bacteria preservation of anammox: A review[J]. Water, Air and Soil Pollution, 2015, 226(5): 163. |
| [17] | LI S, CHEN Y, LI C, et al. Influence of free ammonia on completely autotrophic nitrogen removal over nitrite (CANON) process[J]. Applied Biochemistry and Biotechnology, 2012, 167(4): 694-704. |
| [18] | VADIVELU V, YUAN Z, FUX C, et al. The inhibitory effects of free nitrous acid on the energy generation and growth processes of an enriched Nitrobacter culture[J]. Environmental Science & Technology, 2006, 40(14): 4442-4448. |
| [19] | PUYOL D, CARVAJAL-ARROYO J, SIERRA-ALVAREZ R, et al. Nitrite (not free nitrous acid) is the main inhibitor of the anammox process at common pH conditions[J]. Biotechnology Letters, 2014, 36(3): 547-551. |
| [20] | ZHANG Y, HE S, NIU Q, et al. Characterization of three types of inhibition and their recovery processes in an anammox UASB reactor[J]. Biochemical Engineering Journal, 2016, 109: 212-221. |
| [21] | WAKI M, TOKUTOMI T, YOKOYAMA H, et al. Nitrogen removal from animal waste treatment water by anammox enrichment[J]. Bioresource Technology, 2007, 98(14): 2775-2780. |
| [22] | YANG R, WANG X, GUO Y, et al. Evaluation of anammox pathway recovery after high COD loading using water quality, molecular biology and isotope labelling analysis[J]. Bioprocess and Biosystem Engineering, 2020, 43(4): ? 625-636. doi: 10.1007/s00449-019-02260-0 |
| [23] | HENDRICKX T, KAMPMAN C, ZEEMAN G, et al. High specific activity for anammox bacteria enriched from activated sludge at 10 ℃[J]. Bioresource Technology, 2014, 163: 214-221. doi: 10.1016/j.biortech.2014.04.025 |
| [24] | 国家环境保护总局. 水和废水监测分析方法[M]. 4版. 北京: 中国环境科学出版社, 2002. |
| [25] | LIU W, YANG Q, MA B, et al. Rapid achievement of nitritation using aerobic starvation[J]. Environmental Science & Technology, 2017, 51(7): 4001-4008. |
| [26] | YU R, CHANDRAN K. Strategies of Nitrosomonas europaea 19718 to counter low dissolved oxygen and high nitrite concentrations[J]. BMC Microbiology, 2010, 10(1): 70. |
| [27] | BRAKER G, FESEFELDT A, WITZEL K. Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples[J]. Applied and Environmental Microbiology, 1998, 64(10): 3769-3775. |
| [28] | GRAVE R, DA SILVEIRA NICOLOSO R, CASSOL P, et al. Determining the effects of tillage and nitrogen sources on soil N2O emission[J]. Soil and Tillage Research, 2018, 175: 1-12. |
| [29] | WANG Y, MA X, ZHOU S, et al. Expression of the nirS, hzsA, and hdh genes in response to nitrite shock and recovery in Candidatus Kuenenia stuttgartiensis[J]. Environmental Science & Technology, 2016, 50(13): 6940-6947. |
| [30] | MIAO Y, ZHANG L, LI B, et al. Enhancing ammonium oxidizing bacteria activity was key to single-stage partial nitrification-anammox system treating low-strength sewage under intermittent aeration condition[J]. Bioresource Technology, 2017, 231: 36-44. |
| [31] | PARK M, PARK H, CHANDRAN K. Molecular and kinetic characterization of planktonic Nitrospira spp. selectively enriched from activated sludge[J]. Environmental Science & Technology, 2017, 51(5): 2720-2728. |
| [32] | ZHANG Z, CHEN S, WU P, et al. Start-up of the Canon process from activated sludge under salt stress in a sequencing batch biofilm reactor (SBBR)[J]. Bioresource Technology, 2010, 101(16): 6309-6314. doi: 10.1016/j.biortech.2010.03.040 |
| [33] | VDZQUEZ-PADIN J, MOSQUERA-CORRAL A, CAMPOS J, et al. Microbial community distribution and activity dynamics of granular biomass in a CANON reactor[J]. Water Research, 2010, 44(15): 4359-4370. |
| [34] | YUE X, YU G, LIU Z, et al. Start-up of the completely autotrophic nitrogen removal over nitrite process with a submerged aerated biological filter and the effect of inorganic carbon on nitrogen removal and microbial activity[J]. Bioresource Technology, 2018, 254: 347. doi: 10.1016/j.biortech.2018.01.107 |
| [35] | YUE X, YU G, LU Y, et al. Effect of dissolved oxygen on nitrogen removal and the microbial community of the completely autotrophic nitrogen removal over nitrite process in a submerged aerated biological filter[J]. Bioresource Technology, 2018, 254: 67-74. doi: 10.1016/j.biortech.2018.01.044 |
| [36] | HUYNH T, NGUYEN P, PHAN T, et al. Application of CANON process for nitrogen removal from anaerobically pretreated husbandry wastewater[J]. International Biodeterioration & Biodegradation, 2019, 136: 15-23. |
| [37] | 李亚峰, 秦亚敏, 谢新立, 等. 间歇曝气条件下短程硝化的实现及影响因素研究[J]. 环境工程学报, 2011, 5(7): 1518-1521. |
| [38] | YONG M, PENG Y, WANG S, et al. Achieving nitrogen removal via nitrite in a pilot-scale continuous pre-denitrification plant[J]. Water Research, 2009, 43(3): 563-572. |
| [39] | CEMA G, SZATKOWSKA B, PLAZA E, et al. Nitrogen removal rates at a technical-scale pilot plant with the one-stage partial nitritation/Anammox process[J]. Water Science & Technology, 2006, 54(8): 209. |
| [40] | MAO N, REN H, GENG J, et al. Engineering application of anaerobic ammonium oxidation process in wastewater treatment[J]. World Journal of Microbiology & Biotechnology, 2017, 33(8): 153. |
| [41] | ZHANG F, PENG Y, WANG S, et al. Efficient step-feed partial nitrification, simultaneous Anammox and denitrification (SPNAD) equipped with real-time control parameters treating raw mature landfill leachate[J]. Journal of Hazardous Materials, 2019, 364: 163-172. doi: 10.1016/j.jhazmat.2018.09.066 |
| [42] | CHU Z, WANG K, LI X, et al. Microbial characterization of aggregates within a one-stage nitritation-anammox system using high-throughput amplicon sequencing[J]. Chemical Engineering Journal, 2015, 262: 41-48. |
| [43] | KOMPANTSEVA E, KUBLANOV I, PEREVALOVA A, et al. Calorithrix insularis gen. nov., sp. nov., a novel representative of the phylum Calditrichaeota[J]. International Journal of Systematic and Evolutionary Microbiology, 2017, 67(5): 1486-1490. doi: 10.1099/ijsem.0.001744 |
| [44] | TIAN S, TIAN Z, YANG H, et al. Detection of viable bacteria during sludge ozonation by the combination of ATP assay with PMA-Miseq sequencing[J]. Water, 2017, 9(3): 166. |
| [45] | LEAL C, PEREIRA A, NUNES F, et al. Anammox for nitrogen removal from anaerobically pre-treated municipal wastewater: Effect of COD/N ratios on process performance and bacterial community structure[J]. Bioresource Technology, 2016, 211: 257-266. doi: 10.1016/j.biortech.2016.03.107 |
| [46] | KONG Q, HE X, FENG Y, et al. Pollutant removal and microorganism evolution of activated sludge under ofloxacin selection pressure[J]. Bioresource Technology, 2017, 241: 849-856. doi: 10.1016/j.biortech.2017.06.019 |
| [47] | WANG Y, CHEN J, ZHOU S, et al. 16S rRNA gene high-throughput sequencing reveals shift in nitrogen conversion related microorganisms in a CANON system in response to salt stress[J]. Chemical Engineering Journal, 2017, 317: 512-521. doi: 10.1016/j.cej.2017.02.096 |
| [48] | CONNAN R, DABERT P, KHALIL H, et al. Batch enrichment of anammox bacteria and study of the underlying microbial community dynamics[J]. Chemical Engineering Journal, 2016, 297: 217-228. doi: 10.1016/j.cej.2016.03.154 |
| [49] | SHU D, HE Y, YUE H, et al. Metagenomic insights into the effects of volatile fatty acids on microbial community structures and functional genes in organotrophic anammox process[J]. Bioresource Technology, 2015, 196: 621-633. doi: 10.1016/j.biortech.2015.07.107 |
| [50] | SPETH D, GUERRERO-CRUZ S, DUTILH B, et al. Genome-based microbial ecology of anammox granules in a full-scale wastewater treatment system[J]. Nature Communications, 2016, 7: 11172. |
| [51] | WANG Z, ZHANG X, HUANG K, et al. Metagenomic profiling of antibiotic resistance genes and mobile genetic elements in a tannery wastewater treatment plant[J]. PloS One, 2013, 8(10): e76079. |
| [52] | KINDAICHI T, YURI S, OZAKI N, et al. Ecophysiological role and function of uncultured Chloroflexi in an anammox reactor[J]. Water Science and Technology, 2012, 66(12): 2556-2561. doi: 10.2166/wst.2012.479 |
| [53] | ALAGELY A, KREDIET C, RITCHIE K, et al. Signaling-mediated cross-talk modulates swarming and biofilm formation in a coral pathogen Serratia marcescens[J]. ISME Journal, 2011, 5(10): 1609-1620. doi: 10.1038/ismej.2011.45 |
| [54] | WANG C, LIU S, XU X, et al. Potential coupling effects of ammonia-oxidizing and anaerobic ammonium-oxidizing bacteria on completely autotrophic nitrogen removal over nitrite biofilm formation induced by the second messenger cyclic diguanylate[J]. Applied Microbiology & Biotechnology, 2017, 101(9): 3821-3828. |
| [55] | CHEN C, SUN F, ZHANG H, et al. Evaluation of COD effect on anammox process and microbial communities in the anaerobic baffled reactor (ABR)[J]. Bioresource Technology, 2016, 216: 571-578. doi: 10.1016/j.biortech.2016.05.115 |
| [56] | CUI Y, ZHANG H, LU P, et al. Effects of carbon sources on the enrichment of halophilic polyhydroxyalkanoate-storing mixed microbial culture in an aerobic dynamic feeding process[J]. Scientific Reports, 2016, 6: 30766. doi: 10.1038/srep30766 |
















































































































































 下载:
下载: 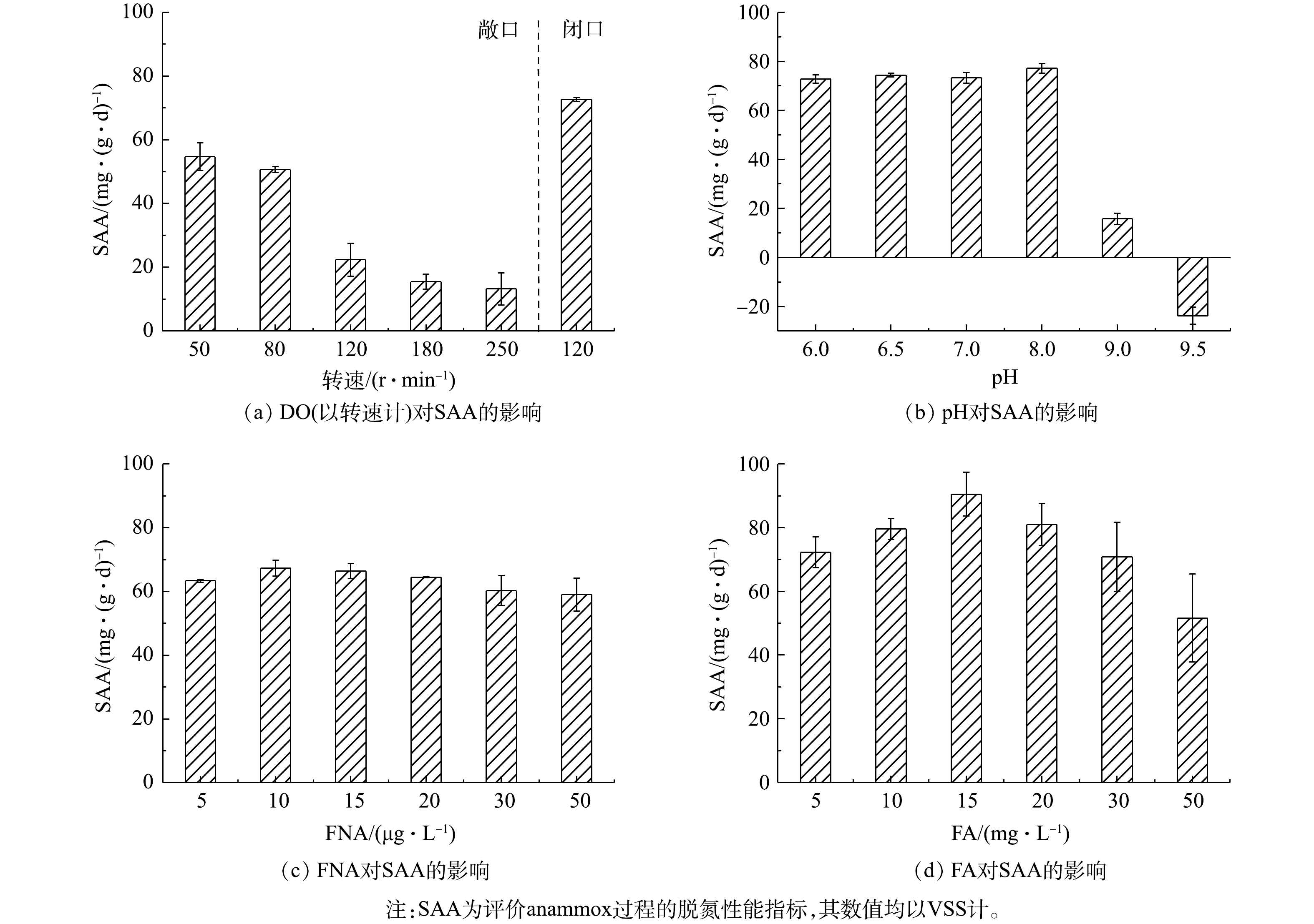









 点击查看大图
点击查看大图