全文HTML
--> --> --> 近10年来,我国的污水处理能力得到巨大提升,截至2018年底,我国建成运行的城镇污水处理厂处理能力已达1.95×108 m3·d?1[1]。由于我国城镇污水处理厂普遍采用传统活性污泥法工艺,随之产生的副产物—污泥的产量也急剧增加,目前,我国市政污泥年产量已超过4×107 t[1-2](含水率80%),因此,污泥的有效处理处置已成为亟待解决的问题。厌氧消化作为污泥减量化、稳定化和资源化的重要技术,在能源回收方面具有优势[3-4]。然而,由于停留时间较长、产甲烷速率较低等问题,污泥厌氧消化的实际应用效率仍有待提高。有研究表明,添加导电碳材料可显著提高有机物厌氧消化的甲烷产率。MUMME等[5]发现生物炭能有效缓解有机垃圾厌氧消化过程中的氨胁迫效应。WATANABE等[6]发现生物炭可加速废甘油厌氧消化产甲烷速率。已有研究[7]表明,生物炭能在其孔隙中定植甲烷八叠球菌和甲烷鬃菌,从而提高了VFAs的代谢转化速率。还有研究[8-11]表明,具有电化学活性的活性炭或生物炭可促进微生物直接种间电子传递(direct interspecies electron transfer, DIET),从而加速了产甲烷的过程。污泥的厌氧消化本质上是微生物在厌氧环境中逐步降解代谢有机物的生物化学过程,多糖、蛋白质和脂类等大分子有机物经水解、发酵后产生挥发性脂肪酸(VFAs),VFAs的代谢转化则需依靠厌氧细菌和产甲烷古菌之间的微生物种间电子传递来克服热力学能量壁垒[12-14]。因此,如何提高微生物种间电子传递效率,进而加速产甲烷反应,是强化污泥厌氧消化亟待解决的关键问题。然而,拥有不同电化学性质的导电碳材料如何影响污泥厌氧消化产甲烷过程,其作用机制仍有待进一步的探究。
碳材料的电化学性质主要包括电子供给能力(electron donating capacity, EDC)、电子接受能力(electron accepting capacity, EAC)和导电性(electrical conductivity, EC)[15]。EDC和EAC通常反映了碳材料表面具有氧化还原活性的官能团的数量,而EC则与碳材料内部的芳构化片层结构数量呈正比,反映的是其转移电子的能力[14-15]。通常,在较低热解温度条件下制备的生物炭表面具有数量较多的官能团,而石墨等高度芳构化的碳材料的导电性则较好[15]。
本研究以玉米秸秆为原料,在热解温度为300、500和700 ℃的条件下制备了3种具有不同电化学活性的生物炭(CS300、CS500和CS700),在厌氧消化批次实验中分别添加3种生物炭和导电型石墨(graphite),考察了不同电化学性质的生物炭和石墨对污泥厌氧消化过程的影响,通过建立电化学性质-污泥甲烷产率之间的相关性以及对体系微生物群落结构变化的分析,揭示了其影响机制,可为提高污泥厌氧消化甲烷产率以及具有电化学活性的导电碳材料在厌氧消化工艺中的实际应用提供一定的理论支撑。
1.1. 实验材料
生物炭由玉米秸秆在无氧环境中慢速热解制成,制备方法如下:玉米秸秆烘干后破碎过筛,选择粒径为0.1~ 0.5 mm的颗粒。热解开始前,向马弗炉中通氮气(纯度99.99%,流量300 mL·min?1)15 min,然后将盛有玉米秸秆颗粒的坩埚置于马弗炉中热解2 h,期间保持氮气吹扫,热解温度分别设置为300、500和700 ℃。制得生物炭后,用去离子水洗涤,过滤后于60 ℃下烘干以备用,这3种碳材料分别记为CS300、CS500和CS700。导电型石墨为工业级产品(Sigma-Aldrich,美国)。厌氧消化批次实验的底物为市政污水处理厂的剩余污泥,取自上海市闵行第2污水处理厂的二沉池,经由孔径1 mm的钢筛过滤去除大颗粒杂质,静置24 h后,弃去上清液,将其保存在4 ℃冰箱中备用。总固体含量(TS)为3.42%,挥发性固体(VS)为2.01%,总化学需氧量(TCOD)为20 743 mg·L?1,可溶性化学需氧量(SCOD)为850.4 mg·L?1,氨氮(TAN)和总碱度(TA)分别为180.8 mg·L?1和880.6 mg·L?1。接种污泥取自实验室连续运行的剩余污泥中温厌氧消化反应装置,其中TS为2.09%,VS为1.09%。
1.2. 实验设计
采用批次实验,以2 L血清瓶为厌氧消化反应器,单个反应器工作体积为1.5 L。设置污泥厌氧消化实验与空白对照实验共10组(表1),每组设置3个平行,各组接种比例(接种物/底物,按TS计)均为20%。生物炭和石墨的投加量设为每1 g 总固体投加1 g生物炭或石墨,根据前期预实验结果[16],该投加量时取得的污泥厌氧消化甲烷产率最高,同时不影响污泥厌氧消化反应器的机械运行,因此,在本实验中继续采用该投加剂量。在对照实验中,使用与消化实验中底物等体积的去离子水,其目的是为了测定生物炭或石墨可能会被微生物降解产生的沼气量。在加入相应的材料后,使用氮气(纯度99.99%)对反应器内部吹脱5 min以保证厌氧环境,结束后,密封所有反应器。反应器顶空排气口接至MultiTalent 203全自动甲烷潜力测试系统(Nova Skantek,瑞典),该平台采用经典排水法测量气体体积,测量分辨率为3~5 mL。实验期间,反应器置于恒温(37 ℃)水浴中,搅拌速率为120 r·min?1。每24 h记录1次产气量,直到日产气体积低于累积产气体积的1%[17]为止。待消化实验终止,依次从消化实验组的反应器中提取消化污泥各1 mL,并将各实验组的样品混合后进行微生物群落结构分析。1.3. 分析方法
采用场发射扫描电镜(Sirion 200,美国)观察4种碳材料表面形态结构。利用快速表面积和孔隙分析仪(Quantachrome,美国)表征BET比表面积。样品在130 ℃、真空条件下脱气6 h,清除原料表面吸附的物质,以99.999% 的N2为吸附质,液氮温度77 K时测定吸附-脱附等温线,饱和蒸汽压为1.036 0×105 Pa,P/P0取点在0.05~0.35。碳材料的pH测定采用固液混合法,碳材料与去离子水按1∶10(g∶mL)比例混合,混合液于37 ℃、150 r·min?1下振荡24 h后,用pH电极(Mettler-Toledo,瑞士)测定pH。碳材料的灰分含量参照《木炭和木炭实验方法》(GB/T 17664-1999)进行测定,并使用电感耦合等离子体质谱(ICP-MS)分析其灰分组成。采用元素分析仪(Vario Macro Cube,德国)测定C、H、O的元素含量。碳基材料的EDC和EAC分别采用介导性电化学氧化和还原实验法[15]测得,EC则采用四探针法[18]测得。采用傅里叶红外光谱(FT-IR,Thermo Scientific,美国)分析表征碳材料的表面官能团。
沼气的气体组分(CH4、CO2)采用气相色谱(岛津GC-2014,日本)分析,检测器为热导检测器TCD,载气为氦气,色谱柱温度、检测器温度和进样口温度分别为80、80和120 ℃。选择Modified Gompertz 模型评估产甲烷动力学,用软件Origin 9.0拟合,模型方程如式(1)所示。
式中:Mt为时刻t累积的甲烷产量,mL·g?1;M0为累计产甲烷潜力,mL·g?1;Rmax为最大甲烷产率,mL·(g·d)?1;λ为停滞期,d;t为反应持续的时间,d;e取2.718 3。
1.4. 微生物群落分析
使用E.Z.N.A.? Soil试剂盒(Omega Bio-tek,Norcross,美国)抽提消化污泥样品DNA得到混合基因组。使用NanoDrop 2000分光光度计(Thermo Fisher Scientific,美国)检测DNA浓度和纯度,并用1%琼脂糖凝胶电泳检测DNA质量。高通量测序及统计分析委托上海美吉生物医药科技有限公司进行。2.1. 生物炭和石墨的理化性质表征
如图1所示,3种生物炭和石墨的表观形貌存在明显差异,CS300基本保持了生物质原料的表面形态,孔隙结构不发达,只存在少许疏松、大小不一的孔状结构。随热解温度的升高,生物质的化学键和大分子结构被破坏,挥发性有机成分进一步挥发[19],可看到CS500清晰的孔隙结构,表面形态更加整洁均匀,且有序性提高,CS700孔隙结构更加发达,孔数量明显增加,这说明适当增加热解温度有利于生物炭孔隙结构的形成。石墨表面平整,断面处呈现规则的层叠式片层结构。3种生物炭和石墨的理化性质如表2所示。3种生物炭均为碱性(pH>8),而石墨的pH为中性,且4种碳材料的pH与其灰分含量呈线性正相关关系(R2=0.939),与SHEN等[16]的研究结果一致。灰分的主要成分(表3)为硅元素以及碱金属和碱土金属元素(alkali and alkaline earth metals, AAEMs)。其中硅元素主要来源于农作物收获时掺杂其中的土壤沙石;AAEMs主要包括Na、K、Ca和Mg,由玉米秸秆等农作物热解制备的生物炭灰分中AAEMs的含量普遍较高[17, 19-21],本研究的结果与以上的研究结果相一致。农作物生长需施肥,而肥料中一般均含有包括N、P、K等主要常量营养元素以及Ca、Mg等中量营养元素。AAEMs不仅是生物质热解过程的原位催化剂,其氧化态化合物[22]也是导致生物炭呈碱性的主要原因。3种生物炭的BET比表面积为19.8~32.8 m2·g?1,石墨的BET比表面积最高(65.3 m2·g?1),与扫描电镜图展示的表观结构观察结果相符。O/C与H/C的摩尔比是反映碳材料化学稳定性和芳构化程度十分重要的2个指标,由其变化趋势可知,O/C与H/C的摩尔比与生物炭的热解温度呈反比;相较于3种生物炭,石墨的O/C摩尔比极低,而H/C摩尔比(0.27)则与CS700 (0.28)相近,这说明CS700的芳构化程度较高[17]。
由表2可知,3种生物炭和石墨呈现出不同的电化学性质。CS300的EDC值最高,CS500与CS700依次降低,而CS700的EAC值最高,CS300次之,CS500最低,石墨的EDC和EAC均为最低。EDC与EAC之和表示电子交换容量(electron exchange capacity, EEC),反映的是碳材料的氧化还原活性,因此,CS300和CS700的EEC最高,CS500次之,而石墨的EEC最低。从导电率(EC值)的变化来看,CS300和CS500的EC值极低,几乎不可导电,CS700的导电性较好(107.3 S·m?1),但仍然比石墨低了近200倍。热解温度是影响生物炭电化学性质的最重要的工艺参数之一。通常认为:在中温热解(300~500 ℃)环境中,生物炭表面可形成数量众多的以氢醌为主的还原性官能团,具有较高的EDC;当热解温度升至500~700 ℃时,生物炭的氧化还原活性主要由EAC所主导[15];当热解温度高达700 ℃或以上时,生物炭表面几乎无法形成官能团,导致其氧化还原活性急剧下降,但与此同时,高温热解可促进芳构化,从而在材料内部形成类似片层石墨的结构,因此,所形成的生物炭具有一定的导电性。从4种碳材料的红外光谱(图2)可看出,CS300和CS500在1 640 cm?1处的吸收峰较明显,该吸收峰主要是由碳氧双键(C=O)和碳碳双键(C=C)的伸缩振动引起的。相比之下,CS700和石墨的谱图未出现特征性振动,说明它们的表面官能团数量极少[23]。氧化还原能力高的碳材料可持续供给或接受电子,发挥着“电池”的作用,而导电能力高的碳材料则可充当“电导体”的作用。SUN等[17]的研究表明,中低温热解环境中制备的生物炭(H/C>0.35, O/C>0.09)主要通过表面具有氧化还原活性的官能团持续为微生物体系供给或接受电子,而导电能力高的碳材料(H/C<0.35, O/C<0.09)则主要通过内部的共轭π-电子体系直接传递电子。
2.2. 不同电化学性质的生物炭和石墨对污泥厌氧消化产甲烷的影响
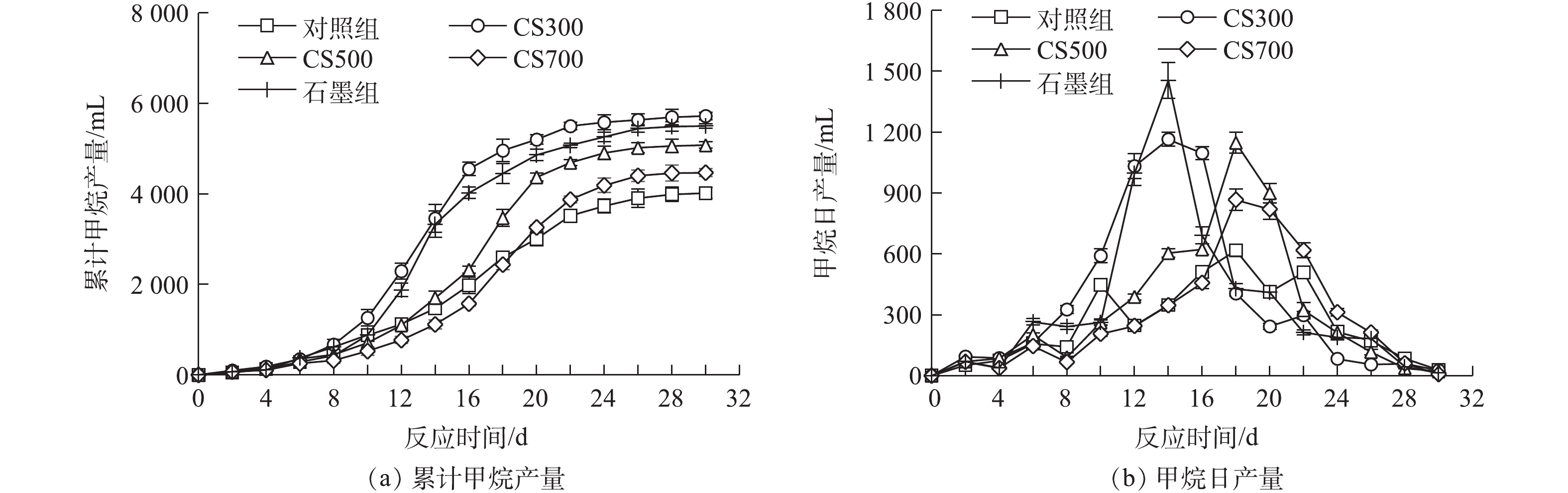
图3为各实验组的累积甲烷产量随时间的变化。由图3(a)可知,添加不同电化学性质的生物炭和石墨对污泥厌氧消化的总甲烷产量均有不同程度的促进作用。在消化实验结束时,对照组、CS300组、石墨组、CS500组和CS700组的累积甲烷产量分别为3 293、5 720、5 579、5 177和4 466 mL,而对照实验的相应实验组均无甲烷产生。相较于对照组,添加生物炭CS300、石墨、生物炭CS500和CS700实验组的累积甲烷产量分别提高了42.4%、38.9%、28.9%和11.2%。图3(b)为各实验组的甲烷日产量随时间变化,可见CS300组的甲烷日产量从第8天后开始迅速增加,在第12~16天期间,达到峰值(为1 146 mL·d?1),消化反应进行至第22天时达到总甲烷产量的95%;石墨组的甲烷日产量从第10天后快速增加,在第14天达到最大产甲烷速率(1 454 mL·d?1),之后甲烷日产量急剧降低;而CS500组和CS700组在反应进行的前半程产甲烷速率呈现出缓慢上升趋势,其最大产甲烷速率均出现在第18天,与对照组的产甲烷速率随时间变化的趋势相似,但CS500组(1 146 mL·d?1)和CS700组(866 mL·d?1)的甲烷日产量峰值比对照组(616 mL·d?1)分别高出86.04%和40.58%。产甲烷动力学过程用修正Gompertz模型拟合,结果如表4所示。各实验组的拟合系数R2均接近或大于0.99,这表明该模型较为准确地反映了各实验组的产甲烷过程。相较于对照组,添加生物炭和石墨均不同程度地延长了产甲烷的停滞期,CS300组和石墨组的停滞期较短且两者在数值上较为接近,而CS700组的停滞期最长。由此可见,氧化还原活性较高的生物炭抑或导电性优秀的石墨均无法加速污泥的水解或酸化过程。此外,尽管CS500和CS700为碱性(pH>10),但由于污泥体系本身的缓冲能力很强,投加碱性生物炭至消化反应器中未能有效提升环境pH来促进污泥水解,从而缩短产甲烷的停滞期。相反地,CS500组和CS700组的停滞期相较于对照组分别延长了27.4%和39.7%,这可能是由生物炭灰分中的Ca、Mg、Al、Fe等有机结合金属(organic-binding metal, OBM)阳离子释放所引起的,CS500和CS700的灰分含量较高且Ca和Al为其主要灰分元素组成。XU等[24]的研究指出,厌氧消化体系中OBM浓度的增加会大幅增加污泥水解所需的表观活化能,同时会降低表面水解酶结合位点的数量。在甲烷的产率和产量方面,所有添加了碳材料的实验组相较于对照组均有所提高。石墨组的最大甲烷产率为最高,比对照组的相应数值提高了136.0%;CS300组的最大甲烷产量为最高,比对照组的相应数值提高了30.0%。
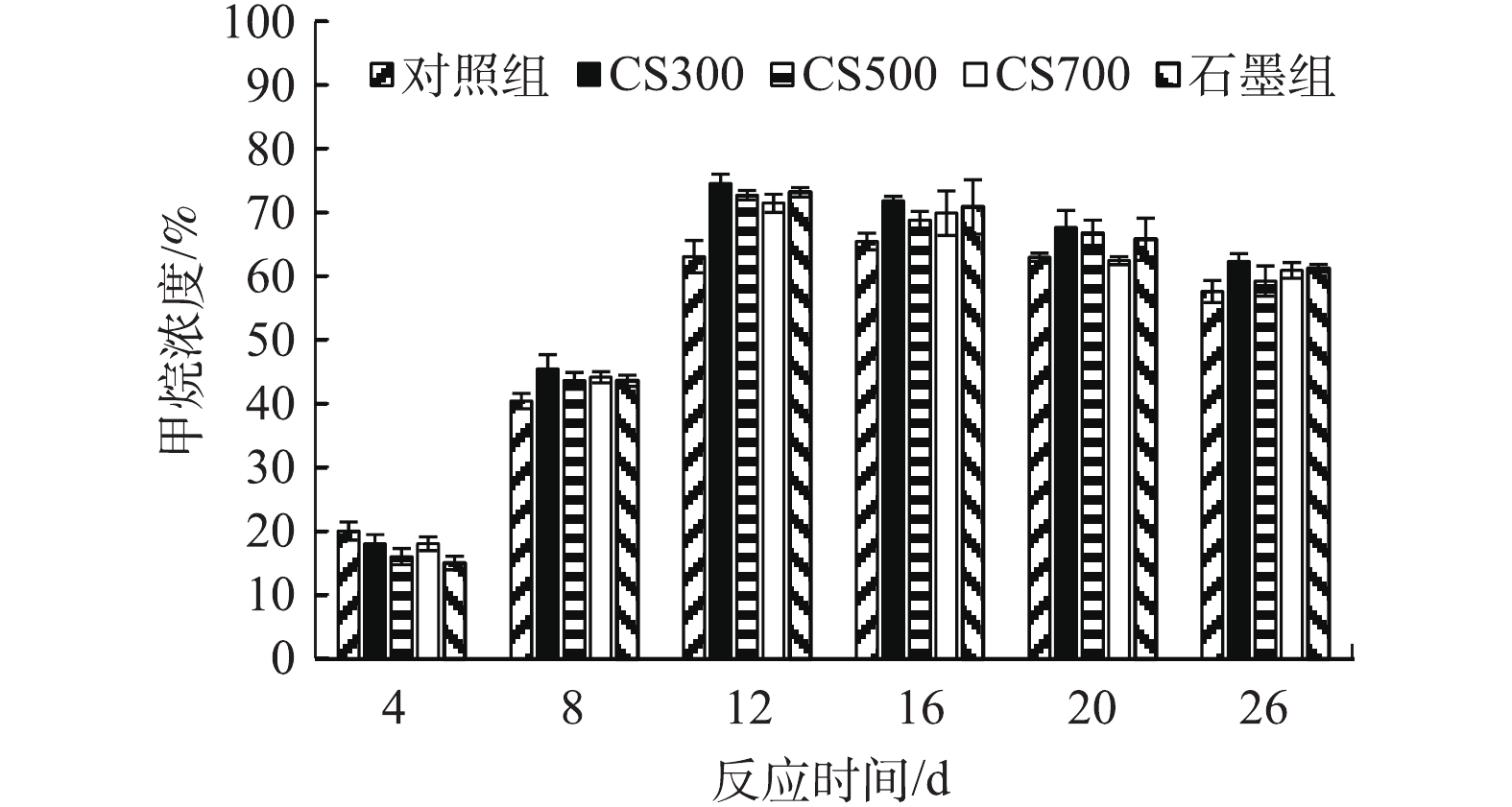
图4为各实验组在不同反应时间的沼气甲烷含量。由图4可知,添加碳材料的4个实验组与对照组呈现出相似的变化趋势,在第8天之前沼气的主要组分为CO2,甲烷产量较低,从相应的累积甲烷产量的变化(图3(a))来看,各实验组的产甲烷速率显著增长均出现在第8天或之后,与沼气甲烷含量的变化趋势相一致。这主要是因为污泥在水解、酸化、产乙酸和产甲烷4个阶段中均有CO2产生,而甲烷仅在厌氧消化的产甲烷阶段形成。添加导电碳材料的4个实验组沼气甲烷含量均在第12天达到峰值,且均超过70%,而对照组的沼气甲烷含量则在第16天达到峰值(65%)。对照组、CS300组、CS500组、CS700组和石墨组的沼气平均甲烷含量分别为59.6%、64.2%、62.5%、61.3%和62.9%,由此可知,添加生物炭和石墨均可提升沼气甲烷含量,但提升幅度则十分有限,说明生物炭和石墨对CO2并无显著的吸附或吸收作用,这可能是因为生物炭和石墨的比表面积过低,且无法提高环境pH, 从而使碳酸大量转化为碳酸氢盐或碳酸盐。
2.3. 不同电化学性质的生物炭和石墨对污泥厌氧消化微生物群落结构影响
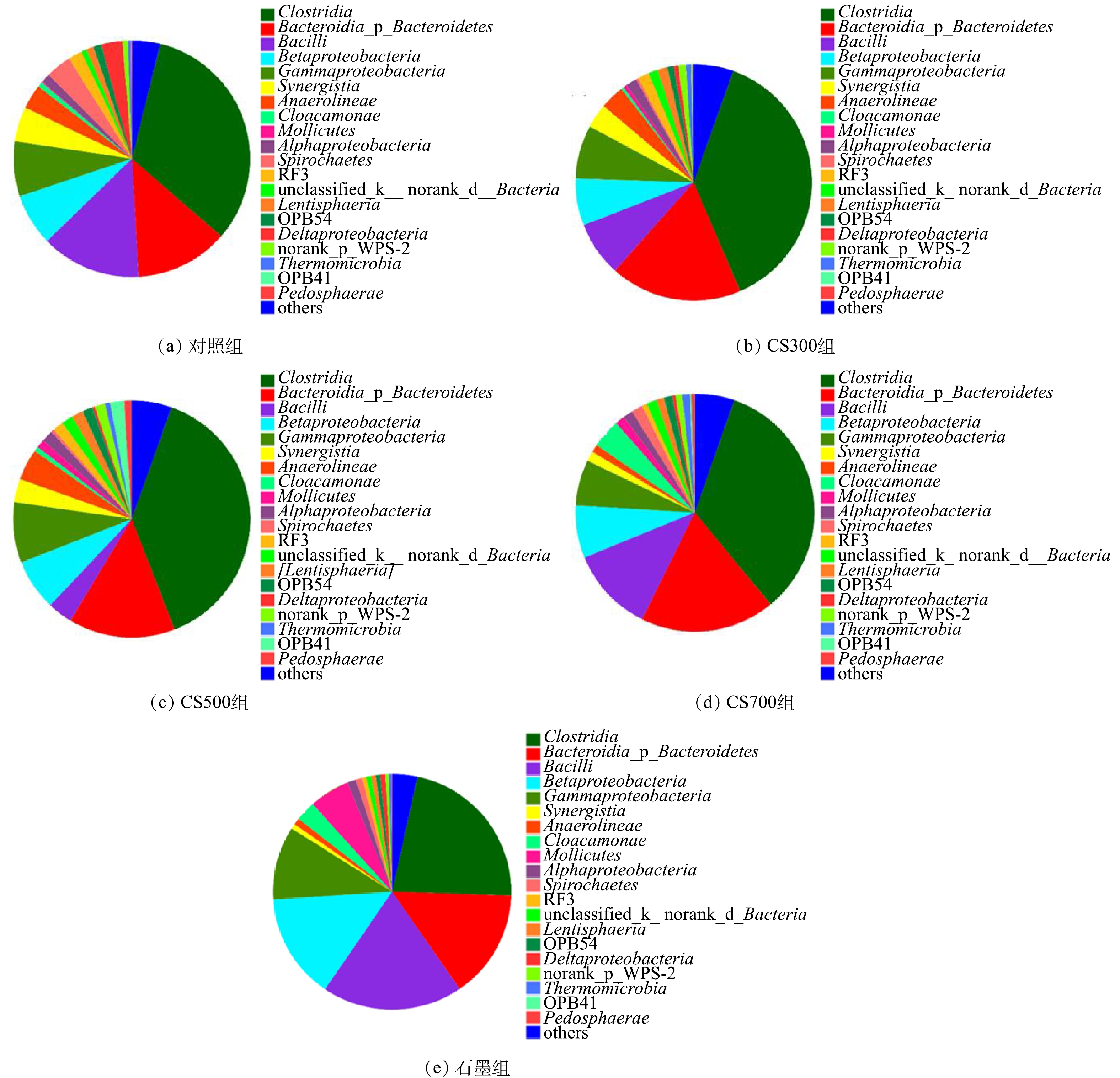
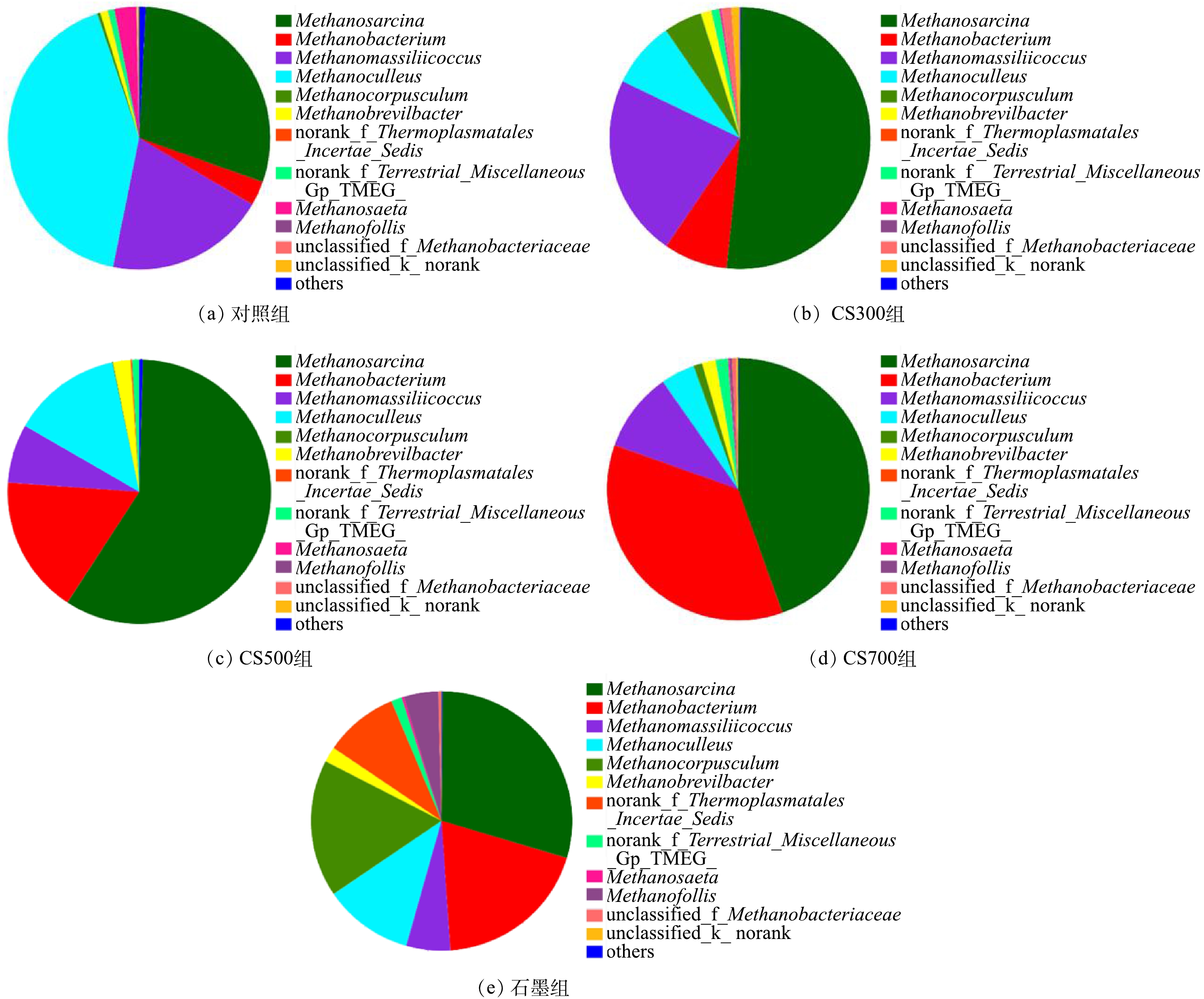
5个实验组的细菌群落结构在门级别上的结构相似,主要包括厚壁菌门Firmicutes、变形菌门Proteobacteria、拟杆菌门Bacteroidetes以及绿弯菌门Chloroflexi。图5展示了各实验组的纲级别细菌群落结构,结果显示梭菌Clostridia、拟杆菌Bacteroidia、芽胞杆菌Bacilli、乙型变形菌Betaproteobacteria以及丙型变形菌Gammaproteobacteria为主要的细菌群落。相较于对照组,添加CS300或CS500生物炭可显著提高Clostridia的相对丰度,但添加导电型石墨则导致其相对丰度明显下降。Clostridia能够水解多糖等大分子有机物产生相应的小分子单体,并进一步将它们转化为VFAs,是污泥水解酸化阶段的重要功能微生物之一[25]。Bacteroidia的相对丰度由对照组的12.7%分别上升至18.0%(CS300组)、14.6%(CS500组)、18.3%(CS700组)以及14.9%(石墨组)。与Clostridia的功能性相类似,属于Bacteroidia纲的大部分细菌对多糖和蛋白质等大分子有机物均有较好的水解能力,并可产生乙酸和其他短链脂肪酸[26-28]。此外,互养菌Synergistia也受碳材料的影响,它们的相对丰度由对照组的4.8%分别下降至3.3%(CS300组)、3.2%(CS500组)、1.3%(CS700组)以及0.7%(石墨组)。我们注意到,所添加的碳材料导电性越强,它们的相对丰度越低。Synergistia是典型的互养型发酵细菌,通常与氢营养型产甲烷古菌共生以克服热力学能量壁垒,促进VFAs的降解代谢生成甲烷[26, 29]。以上结果表明,4种碳材料并无法富集Synergistia这类互养型发酵细菌。近期已有研究[30-32]指出,导电性优秀的碳材料可促进厌氧发酵细菌与产甲烷古菌之间的直接电子传递(DIET)机制,从而加速VFAs的代谢和甲烷的生成。目前,已知的能够与产甲烷古菌建立DIET机制的互养型发酵细菌包括地杆菌Geobacter、互营单胞菌Syntrophomonas以及陶厄氏菌Thauera[33-35],分别隶属于Deltaproteobacteria、 Clostridia和Betaproteobacteria,其在本研究的各实验组污泥消化反应器中均有被检测到。鉴于石墨组的高产甲烷速率,而互养共生体系的微生物种间电子传递主要是以氢分子(H2)为电子载体,由此推测,Synergistia相对丰度的显著下降可能与DIET机制的建立存在相关性。值得注意的是,虽然3种生物炭未能富集Betaproteobacteria,但其在石墨组的相对丰度(13.04%)相较于对照组(7.14%)获得了显著的提升,这可能也与DIET机制有关。图6为各实验组古菌在属级别的菌群结构。由图6可知,优势产甲烷古菌主要包括Methanosarcina、Methanobacterium、Methanocullues和Methanomassiliicoccus,氢营养型产甲烷古菌整体上更占优势。甲烷八叠球菌Methanosarcina的相对丰度在CS300组、CS500组和CS700组中较高,分别为51.5%、44.0%和44.4%,在对照组和石墨组中则较低,分别为29.1%和29.9%。Methanosarcina的代谢灵活性高,可利用3种代谢途径(乙酸裂解、H2还原CO2和甲基途径)产甲烷,其通常是污泥厌氧消化系统中的优势菌属。甲烷囊菌Methanoculleus在对照组中的相对丰度高达41.4%,但导电碳材料的投加可使其相对丰度显著下降。作为典型的氢营养性产甲烷古菌,Methanoculleus除H2之外还能利用乙醇或某些仲醇作为电子供体以产甲烷[36]。相较于对照组(19.8%),另一氢营养型产甲烷古菌Methanomassiliicoccus的相对丰度仅在CS300组中略有上升(22.6%),而在其余实验组中均出现明显下降。从甲烷杆菌Methanobacterium的相对丰度变化情况来看,4种碳材料均能起到富集作用,其相对丰度的增幅高达12.3倍(CS700组)。甲烷粒菌Methanocorpusculum能利用H2或甲酸作为电子供体还原CO2产甲烷,其在对照组和CS700组中的相对丰度极低,而在CS300组和石墨组中的相对丰度则较高,分别为4.78%和25.44%。此外,甲烷鬃菌Methanosaeta在所有实验组中的相对丰度都是极低,无论是氧化还原活性最高的生物炭CS300或是导电性最好的石墨均未能富集。近期有研究[37]证实了Methanosaeta可通过DIET机制将CO2转化为甲烷(CO2+8H++8e? → CH4+2H2O),但代谢底物仅限于乙醇。Methanosaeta是专性乙酸营养型产甲烷古菌,通过乙酸裂解途径生成甲烷(CH3COOH → CH4+CO2)。其在某一厌氧体系中是否能成为优势产甲烷古菌主要取决于该体系的乙酸浓度水平[38-39]。由于其对乙酸具有较高的亲和性,Methanosaeta更容易在低乙酸浓度环境中大量富集[40]。通常认为,在缺乏Methanosaeta的厌氧体系中,乙酸代谢主要依靠乙酸氧化细菌(syntrophic acetate oxidizing bacteria, SAOB)与氢营养型产甲烷古菌所构成的互养共生菌群[41]。鉴于此,在本研究中,各实验组的乙酸代谢主要途径为乙酸氧化,而非乙酸裂解。
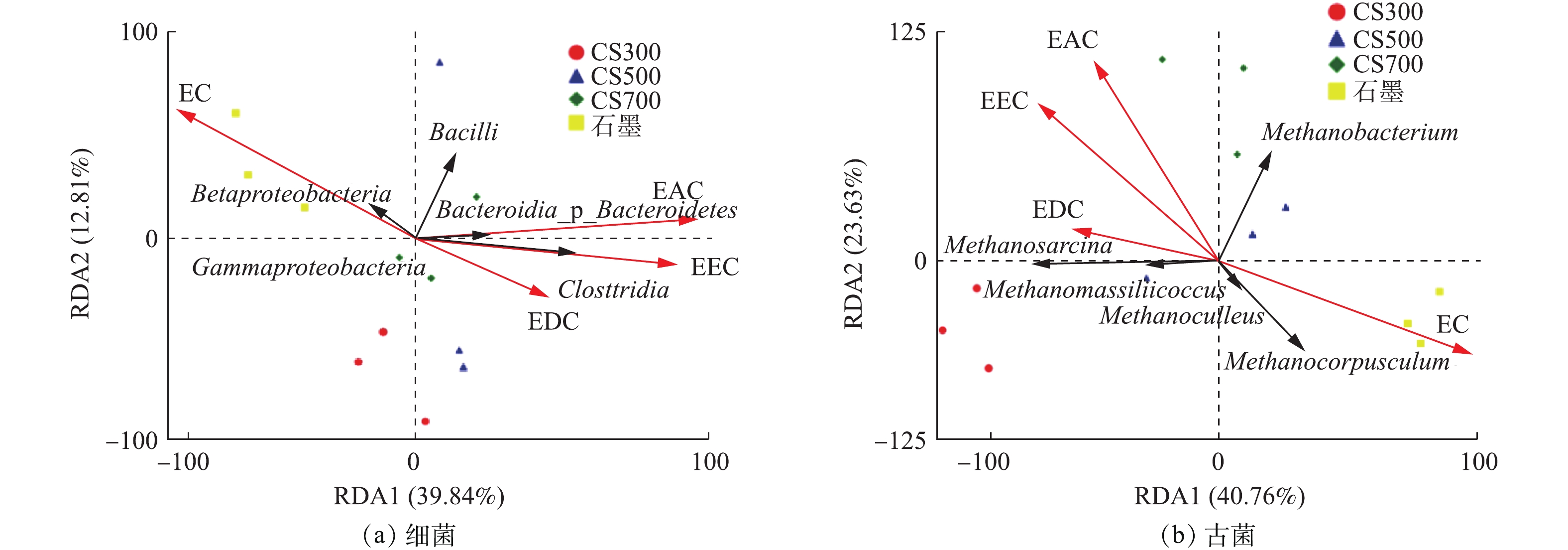
冗余分析(RDA)是环境因子约束化的主成分分析法,可揭示微生物群落结构与厌氧消化过程中特定环境因子之间的关联性,分别选择细菌与古菌的相对丰度为物种变量、碳材料的电化学性质参数(EDC、EAC、EEC、EC)为解释变量,RDA分析结果如图7所示。由图7可知,碳材料的电化学性质对细菌和古菌的群落结构影响程度分别达到52.65%和64.39%。图7(a)显示,石墨组的细菌群落结构变化主要受EC影响,CS300组、CS700组与电化学指标的相关性很小。图7(b)显示,石墨组的古菌群落结构变化也主要受EC影响,CS300组、CS500组和CS700组古菌群落结构变化的主要影响因子则分别为EDC、EEC和EAC。Methanosarcina的相对丰度与碳材料的EDC、EAC、EEC均正相关,与EDC的正关联性最强,Methanomassiliicoccus观察到与Methanosarcina相同的规律;Methanoculleus和Methanocorpusculum主要与EC正相关,而Methanobacterium则主要与EAC正相关。整体而言,从细菌和古菌的RDA象限分布来看,反映碳材料导电能力的参数EC与其氧化还原活性的参数(EDC、EAC、EEC)矢量方向相背,表明这2种电化学性质改变所引起的厌氧消化微生物群落结构变化有显著差异。这可能是因为,氧化还原活性高但几乎无法导电的生物炭和导电能力强但表面几乎无官能团的石墨,二者对厌氧消化产甲烷微生物的互养共生体系(必须依靠种间电子传递)的促进机制是不同的。
2.4. 不同电化学性质的生物炭和石墨促进污泥厌氧消化产甲烷的机理解析
在本研究中,CS300生物炭与导电型石墨的电化学性质完全不同:前者拥有很高的氧化还原活性(EEC=1.007 mmol·g?1),但其几乎没有导电性(EC=3.5×10?8 S·m?1);后者的表面官能团数量极少,但却是优秀的电导体(EC=2.0×104 S·m?1)。然而,二者对污泥厌氧消化产甲烷过程的提高能力却很相近,前者的累积甲烷产量仅比后者高出2.5%,差异不显著(P=0.07),这表明不同电化学性质的碳材料提高污泥厌氧消化甲烷产率的促进机制是有所不同的。CHEN等[8]的研究结果表明,尽管生物炭比颗粒活性炭的导电性低了1 000多倍,但二者对Geobacter metallireducens-Methanosarcina barkeri共培养体系代谢乙醇产甲烷的促进效率几乎完全相同,这说明由生物炭所产生的促进作用可能与其导电性无关。本研究的结果与他们的研究结果相似。基于循环伏安曲线的测定结果,目前,已有大量研究证实,生物炭表面形成的以醌-氢醌氧化还原对为代表的官能团具有反复接受和供给电子的能力,可作为电子传输的载体介导微生物种间电子传递以加速反应的进程。例如,生物炭可强化地杆菌Geobacter metallireducens GS-15和希瓦氏菌Shewanella oneidensis MR-1的微生物胞外电子传递机制,加速这些微生物利用呼吸作用降解小分子有机物(乙醇、乙酸、乳酸等)、同时还原Fe(Ⅲ)矿物质的过程[42-44]。在本研究中,CS300表面存在的醌类物质可替代H2或甲酸,作为电子载体介导乙酸/VFAs氧化细菌与氢营养型产甲烷古菌的种间电子传递;并且CS300主要作为电子供体(EDC>EAC),可大幅增加厌氧消化体系中终端电子供体的数量。PREVOTEAU等[45]的研究表明,生物炭连续供给电子的时间长达66 d。虽然CS300 (EEC=1.007 mmol ·g?1)与CS700 (EEC=0.968 mmol ·g?1)的氧化还原活性很相近,但是CS300实验组的累积甲烷产量却比CS700实验组高出28.1% (P<0.000 1),这表明相较于EEC,EDC与污泥厌氧消化甲烷产量的相关性更高。对于低EDC、高EAC的碳材料(例如CS700)而言,其在厌氧消化体系中主要作为电子受体,可能会与CO2的还原反应竞争电子,由于电子受体参与数千次的氧化还原循环,这可能会大幅减少原本理应提供给CO2还原反应的电子数量,从而抑制氢营养型产甲烷途径的代谢效率。
近年来,DIET机制引起了研究人员的广泛关注,它的发现让人们认识到除了以H2或甲酸作为电子载体促进厌氧消化体系互养共生微生物种间电子传递这一途径以外,还存在另一种间电子传递机制,即DIET。当导电性能优秀的碳材料被投加至厌氧消化体系后,互养共生微生物菌群附着在其表面,此时导电碳材料作为“电导媒介”即可在微生物菌群之间进行直接电子传递。目前,已有大量研究[30-33, 46]表明,导电性能优秀的碳材料,如石墨、石墨烯、碳纤维布、碳纳米管等,可提高有机物厌氧消化体系的甲烷产量。人们推测,这些能起到“电导体”作用的碳材料可促进互养共生微生物的直接种间电子传递(DIET),可能是强化厌氧消化产甲烷的主要机制。但需要注意的是,大多数研究仅依靠间接实验证据(如碳基材料能富集Geobacter等已被证实可进行DIET的微生物)来推测DIET是主要的促进机制,直接证据则相当缺乏。目前基于直接实验证据被证实主要通过DIET进行产甲烷的微生物仅有Geobacter metallireducens、Methanothrix harundinacea以及Methanosarcina barkeri[9-10, 37]。
2) 4种碳材料对污泥厌氧消化产甲烷过程均有促进作用,但促进幅度的差异性较大:具有高氧化还原活性(EEC和EDC最高)的生物炭CS300组的促进效果最好,其累积甲烷产量较对照组提高了42.4%;其次为导电性最强(EC最高)的石墨,优于生物炭CS500组和CS700组。
3) 3种生物炭提高了氢营养型产甲烷古菌的相对丰度,CS300对甲烷八叠球菌Methanosarcina的富集能力最强,专性乙酸营养型产甲烷古菌Methanosaeta的丰度极低,这表明互养乙酸/VFA氧化细菌与氢营养型产甲烷古菌的共生代谢是污泥厌氧消化产甲烷的主要代谢途径。
4)不同电化学性质的生物炭和石墨强化污泥厌氧消化产甲烷的作用机制不同:生物炭表面含有的氧化活性官能团(醌/氢醌类物质)可通过反复供给、接受电子大幅地增加厌氧体系中的微生物可用电子数量,从而提高了互养微生物种间电子传递效率;导电性高的碳材料(石墨)则主要通过促进微生物群落的直接种间电子传递DIET来提高产甲烷效率。
参考文献


 下载:
下载: 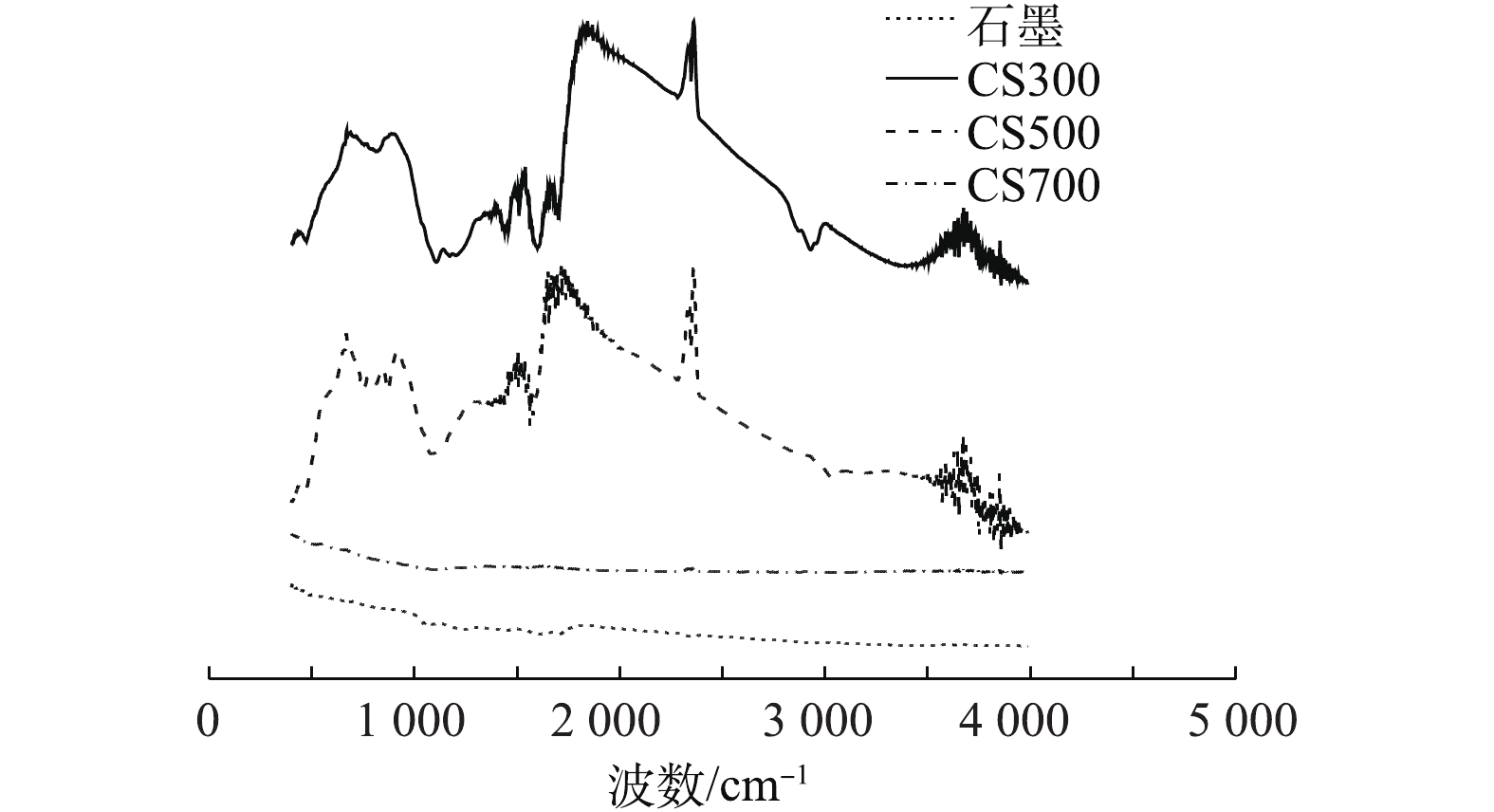
 点击查看大图
点击查看大图