 )
) 1南京师范大学心理学院, 南京 210097
2南京晓庄学院幼儿师范学院, 南京 211171
收稿日期:2017-06-06出版日期:2018-08-15发布日期:2018-07-02通讯作者:蔡厚德E-mail:caihoude@163.com基金资助:国家社科基金教育学一般项目(BHA170130)Neural mechanisms underlying dynamic changes of active maternal behavior in rodents
ZHANG Yifan1, QI Xingliang2, CAI Houde1,2( )
) 1 School of Psychology, Nanjing Normal University, Nanjing 210097, China
2 College of Preschool Education, Nanjing Xiaozhuang University, Nanjing 211171, China
Received:2017-06-06Online:2018-08-15Published:2018-07-02Contact:CAI Houde E-mail:caihoude@163.com摘要/Abstract
摘要: 主动母性行为是雌性哺乳动物在哺乳期内有效照料幼崽的一种动机行为, 对幼崽的生存和行为发展有重要影响。证据显示, 啮齿动物的主动母性行为会经历从产后早期的发动和维持到晚期衰退的动态改变, 反映了雌鼠对幼崽奖赏价值阶段性变化的适应; 这一过程不仅涉及分娩激素事件开启下丘脑内侧视前区(MPOA)-中脑腹侧被盖(VTA)-伏隔核(NA)-腹侧苍白球(VP)通路, 还需要杏仁核基底外侧核(BLA)和内侧前额皮层(MPFC)等脑区对上述通路进行实时调节。哺乳期主动母性行为动态改变及其神经机制的研究, 可以加深对行为进化和早期发展的认识, 也对人类母亲产后抑郁等临床问题的干预有借鉴意义。本文首先利用条件化位置偏好(CPP)任务的行为学证据分析幼崽奖赏价值与主动母性行为动态改变的关系; 然后系统阐述调控这一动态改变的神经机制; 最后对未来需要研究的一些重要问题或方向进行探讨。
图/表 1
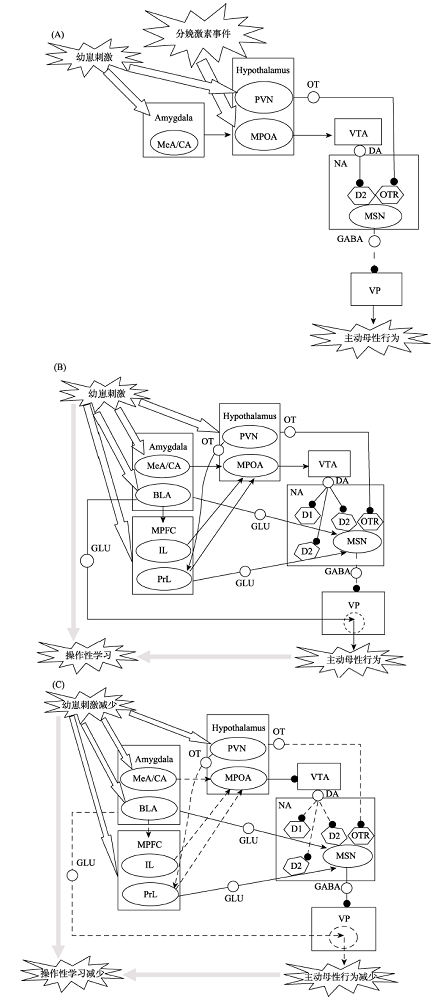
图1主动母性行为动态改变的神经通路机制注: (A) 产后早期 (发动); (B) 产后早期 (维持); (C) 产后晚期 (衰退)。实线段表示神经投射的功能增强, 虚线段表示神经投射的功能减弱; 末端为箭头的线段为兴奋性投射, 末端为圆点的线段为抑制性投射; 线段上的小圆圈处标注神经投射的递质类型; 虚线椭圆表示VP中可以被强化的神经回路。Amygdala=杏仁核; MeA/CA=杏仁核内侧核/皮质核; BLA=杏仁核基底外侧核; MPFC=内侧前额皮层; IL=边缘下区; PrL=边缘前区; Hypothalamus=下丘脑; PVN=室旁核; MPOA=内侧视前区; VTA=中脑腹侧被盖; NA=伏隔核; VP=腹侧苍白球; MSN=中型多棘神经元; DA=多巴胺; GLU=谷氨酸; GABA= γ-氨基丁酸; OT=催产素; OTR=催产素受体。
 图1主动母性行为动态改变的神经通路机制注: (A) 产后早期 (发动); (B) 产后早期 (维持); (C) 产后晚期 (衰退)。实线段表示神经投射的功能增强, 虚线段表示神经投射的功能减弱; 末端为箭头的线段为兴奋性投射, 末端为圆点的线段为抑制性投射; 线段上的小圆圈处标注神经投射的递质类型; 虚线椭圆表示VP中可以被强化的神经回路。Amygdala=杏仁核; MeA/CA=杏仁核内侧核/皮质核; BLA=杏仁核基底外侧核; MPFC=内侧前额皮层; IL=边缘下区; PrL=边缘前区; Hypothalamus=下丘脑; PVN=室旁核; MPOA=内侧视前区; VTA=中脑腹侧被盖; NA=伏隔核; VP=腹侧苍白球; MSN=中型多棘神经元; DA=多巴胺; GLU=谷氨酸; GABA= γ-氨基丁酸; OT=催产素; OTR=催产素受体。
图1主动母性行为动态改变的神经通路机制注: (A) 产后早期 (发动); (B) 产后早期 (维持); (C) 产后晚期 (衰退)。实线段表示神经投射的功能增强, 虚线段表示神经投射的功能减弱; 末端为箭头的线段为兴奋性投射, 末端为圆点的线段为抑制性投射; 线段上的小圆圈处标注神经投射的递质类型; 虚线椭圆表示VP中可以被强化的神经回路。Amygdala=杏仁核; MeA/CA=杏仁核内侧核/皮质核; BLA=杏仁核基底外侧核; MPFC=内侧前额皮层; IL=边缘下区; PrL=边缘前区; Hypothalamus=下丘脑; PVN=室旁核; MPOA=内侧视前区; VTA=中脑腹侧被盖; NA=伏隔核; VP=腹侧苍白球; MSN=中型多棘神经元; DA=多巴胺; GLU=谷氨酸; GABA= γ-氨基丁酸; OT=催产素; OTR=催产素受体。参考文献 63
| 1 | 陈磊磊, 聂莉娜, 李钰, 程鹏, 李鸣, 高军 . ( 2017). 五羟色胺系统对母性行为的调控及其机制. 心理科学进展, 25( 12), 2089-2098. |
| 2 | 刘飞, 蔡厚德 . ( 2010). 情绪生理机制研究的外周与中枢神经系统整合模型. 心理科学进展, 18( 4), 616-622. |
| 3 | Afonso, V. M, King, S., Chatterjee D., & Fleming A. S . ( 2009). Hormones that increase maternal responsiveness affect accumbal dopaminergic responses to pup- and food-stimuli in the female rat. Hormones and Behavior, 56( 1), 11-23. doi: 10.1016/j.yhbeh.2009.02.003URL |
| 4 | Afonso V. M., Shams W. M., Jin D., & Fleming A. S . ( 2013). Distal pup cues evoke dopamine responses in hormonally primed rats in the absence of pup experience or ongoing maternal behavior. Journal of Neuroscience, 33( 6), 2305-2312. doi: 10.1523/JNEUROSCI.2081-12.2013URL |
| 5 | Afonso V. M., Sison M., Lovic V., & Fleming A. S . ( 2007). Medial prefrontal cortex lesions in the female rat affect sexual and maternal behavior and their sequential organization. Behavioral Neuroscience, 121( 3), 515-526. doi: 10.1037/0735-7044.121.3.515URL |
| 6 | Atzil S., Hendler T., & Feldman R . ( 2011). Specifying the neurobiological basis of human attachment: Brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology, 36( 13), 2603-2615. doi: 10.1038/npp.2011.172URL |
| 7 | Balleine, B. W., & Dickinson, A . ( 1998). Goal-directed instrumental action: Contingency and incentive learning and their cortical substrates. Neuropharmacology, 37( 4-5), 407-419. doi: 10.1016/S0028-3908(98)00033-1URL |
| 8 | Banerjee S. B., & Liu R. C . ( 2013). Storing maternal memories: Hypothesizing an interaction of experience and estrogen on sensory cortical plasticity to learn infant cues. Frontiers in Neuroendocrinology, 34( 4), 300-314. doi: 10.1016/j.yfrne.2013.07.008URL |
| 9 | Benedetto L., Pereira M., Ferreira A., & Torterolo P . ( 2014). Melanin-concentrating hormone in the medial preoptic area reduces active components of maternal behavior in rats. Peptides, 58, 20-25. doi: 10.1016/j.peptides.2014.05.012URL |
| 10 | Cortés-Mendoza J., Díaz de León-Guerrero S., Pedraza-Alva G., & Pérez-Martínez L . ( 2013). Shaping synaptic plasticity: The role of activity-mediated epigenetic regulation on gene transcription. International Journal of Developmental Neuroscience, 31( 6), 359-369. |
| 11 | Dalley J. W., Cardinal R. N., & Robbins T. W . ( 2004). Prefrontal executive and cognitive functions in rodents: Neural and neurochemical substrates. Neuroscience and Biobehavioral Reviews, 28, 771-784. doi: 10.1016/j.neubiorev.2004.09.006URL |
| 12 | D'Cunha T. M., King S. J., Fleming A. S., & Lévy F . ( 2011). Oxytocin receptors in the nucleus accumbens shell are involved in the consolidation of maternal memory in postpartum rats. Hormones & Behavior, 59( 1), 14-21. |
| 13 | Dilgen J., Tejeda H. A., & O'Donnell P . ( 2013). Amygdala inputs drive feedforward inhibition in the medial prefrontal cortex. Journal of Neurophysiology, 110( 1), 221-229. doi: 10.1152/jn.00531.2012URL |
| 14 | Dobolyi A., Grattan D. R., & Stolzenberg D. S . ( 2014). Preoptic inputs and mechanisms that regulate maternal responsiveness. Journal of Neuroendocrinology, 26( 10), 627-640. doi: 10.1111/jne.12185URL |
| 15 | Febo M., Numan M., & Ferris C. F . ( 2005). Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. Journal of Neuroscience, 25( 50), 11637-11644. doi: 10.1523/JNEUROSCI.3604-05.2005URL |
| 16 | Fleming A. S., Ruble D., Krieger H., & Wong P. Y . ( 1997). Hormonal and experiential correlates of maternal responsiveness during pregnancy and the puerperium in human mothers. Hormones & Behavior, 31( 2), 145-158. |
| 17 | Gagnidze K., Weil Z. M., Faustino L. C., Schaafsma S. M., & Pfaff D. W . ( 2013). Early histone modifications in the ventromedial hypothalamus and preoptic area following oestradiol administration. Journal of Neuroendocrinology, 25( 10), 939-955. doi: 10.1111/jne.2013.25.issue-10URL |
| 18 | Jin S. H., Blendy J. A., & Thomas S. A . ( 2005). Cyclic AMP response element-binding protein is required for normal maternal nurturing behavior. Neuroscience, 133( 3), 647-655. doi: 10.1016/j.neuroscience.2005.03.017URL |
| 19 | Kesner R. P . ( 2000). Subregional analysis of mnemonic functions of the prefrontal cortex in the rat. Psychobiology, 28( 2), 219-228. |
| 20 | Killcross, S., & Coutureau, E . ( 2003). Coordination of actions and habits in the medial prefrontal cortex of rats. Cerebral Cortex, 13( 4), 400-408. doi: 10.1093/cercor/13.4.400URL |
| 21 | Kim P., Strathearn L., & Swain J. E . ( 2016). The maternal brain and its plasticity in humans. Hormones & Behavior, 77, 113-123. |
| 22 | Kuroda K. O., Meaney M. J., Uetani N., Fortin Y., Ponton A., & Kato T . ( 2007). ERK-fosB signaling in dorsal MPOA neurons plays a major role in the initiation of parental behavior in mice. Molecular and Cellular Neuroscience, 36( 2), 121-131. doi: 10.1016/j.mcn.2007.05.010URL |
| 23 | Laurent, H. K., & Ablow, J. C . ( 2012). A cry in the dark: Depressed mothers show reduced neural activation to their own infant’s cry. Social Cognitive & Affective Neuroscience, 7( 2), 125-134. |
| 24 | Lee A., Clancy S., & Fleming A. S . ( 1999). Mother rats bar-press for pups: Effects of lesions of the MPOA and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behavioural Brain Research, 100( 1-2), 15-31. doi: 10.1016/S0166-4328(98)00109-0URL |
| 25 | Li, M., & Fleming, A. S . ( 2003). The nucleus accumbens shell is critical for normal expression of pup-retrieval in postpartum female rats. Behavioural Brain Research, 145( 1-2), 99-111. doi: 10.1016/S0166-4328(03)00135-9URL |
| 26 | Lonstein J. S., Lévy F., & Fleming A. S . ( 2015). Common and divergent psychobiological mechanisms underlying maternal behaviors in non-human and human mammals. Hormones and Behavior, 73, 156-185. doi: 10.1016/j.yhbeh.2015.06.011URL |
| 27 | Marlin B. J., Mitre M., D'Amour J. A., Chao M. V., & Froemke R. C . ( 2015). Oxytocin enables maternal behaviour by balancing cortical inhibition. Nature, 520( 7548), 499-504. doi: 10.1038/nature14402URL |
| 28 | Mattson, B. J., & Morrell, J. I . ( 2005). Preference for cocaine- versus pup-associated cues differentially activates neurons expressing either Fos or cocaine- and amphetamine- regulated transcript in lactating, maternal rodents. Neuroscience, 135( 2), 315-328. doi: 10.1016/j.neuroscience.2005.06.045URL |
| 29 | Mattson B. J., Williams S., Rosenblatt J. S., & Morrell J. I . ( 2001). Comparison of two positive reinforcing stimuli: Pups and cocaine throughout the postpartum period. Behavioral Neuroscience, 115( 3), 683-694. doi: 10.1037/0735-7044.115.3.683URL |
| 30 | Moltz, H., & Wiener, E . ( 1966). Effects of ovariectomy on maternal behavior of primiparous and multiparous rats. Journal of Comparative & Physiological Psychology, 62( 3), 382-387. |
| 31 | Nicola, S. M . ( 2007). The nucleus accumbens as part of a basal ganglia action selection circuit. Psychopharmacology, 191( 3), 521-550. doi: 10.1007/s00213-006-0510-4URL |
| 32 | Numan, M . ( 2006). Hypothalamic neural circuits regulating maternal responsiveness toward infants. Behavioral and Cognitive Neuroscience Reviews, 5( 4), 163-190. doi: 10.1177/1534582306288790URL |
| 33 | Numan M., Bress J. A., Ranker L. R., Gary A. J., Denicola A. L., Bettis J. K., & Knapp S. E . ( 2010). The importance of the basolateral/basomedial amygdala for goal-directed maternal responses in postpartum rats. Behavioural Brain Research, 214( 2), 368-376. doi: 10.1016/j.bbr.2010.06.006URL |
| 34 | Numan M., Rosenblatt J. S., & Komisaruk B. R . ( 1977). Medial preoptic area and onset of maternal behavior in the rat. Journal of Comparative & Physiological Psychology, 91( 1), 146-164. |
| 35 | Numan, M., & Stolzenberg, D. S . ( 2009). Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Frontiers in Neuroendocrinology, 30( 1), 46-64. doi: 10.1016/j.yfrne.2008.10.002URL |
| 36 | Numan, M., & Young, L. J . ( 2016). Neural mechanisms of mother-infant bonding and pair bonding: Similarities, differences, and broader implications. Hormones and Behavior, 77, 98-112. doi: 10.1016/j.yhbeh.2015.05.015URL |
| 37 | Olazábal D., Pereira M., Agrati D., Ferreira A., Fleming A. S., González-Mariscal G.,.. Uriarte N . ( 2013 a). New theoretical and experimental approaches on maternal motivation in mammals. Neuroscience and Biobehavioral Reviews, 37, 1860-1874. doi: 10.1016/j.neubiorev.2013.04.003URL |
| 38 | Olazábal D., Pereira M., Agrati D., Ferreira A., Fleming A. S., González-Mariscal G.,.. Uriarte N . ( 2013 b). Flexibility and adaptation of the neural substrate that supports maternal behavior in mammals. Neuroscience and Biobehavioral Reviews, 37, 1875-1892. doi: 10.1016/j.neubiorev.2013.04.004URL |
| 39 | Parada M., King S., Li M., & Fleming A. S . ( 2008). The roles of accumbal dopamine D1 and D2 receptors in maternal memory in rats. Behavioral Neuroscience, 122( 2), 368-376. doi: 10.1037/0735-7044.122.2.368URL |
| 40 | Peña, C. J., & Champagne, F. A . ( 2015). Neonatal overexpression of estrogen receptor-α alters midbrain dopamine neuron development and reverses the effects of low maternal care in female offspring. Developmental Neurobiology, 75( 10), 1114-1124. doi: 10.1002/dneu.v75.10URL |
| 41 | Pereira, M . ( 2016). Structural and functional plasticity in the maternal brain circuitry. In H. J. V. Rutherford & L. C. Mayes (Eds.), Maternal brain plasticity: Preclinical and human research and implications for intervention. New Directions for Child and Adolescent Development (no. 153, pp. 23-46). Wiley Periodicals, Inc. |
| 42 | Pereira, M., & Ferreira, A . ( 2016). Neuroanatomical and neurochemical basis of parenting: Dynamic coordination of motivational, affective and cognitive processes. Hormones and Behavior, 77, 72-85. doi: 10.1016/j.yhbeh.2015.08.005URL |
| 43 | Pereira, M., & Morrell, J. I . ( 2009). The changing role of the medial preoptic area in the regulation of maternal behavior across the postpartum period: Facilitation followed by inhibition. Behavioural Brain Research, 205( 1), 238-248. doi: 10.1016/j.bbr.2009.06.026URL |
| 44 | Pereira, M., & Morrell, J. I . ( 2010). The medial preoptic area is necessary for motivated choice of pup- over cocaine- associated environments by early postpartum rats. Neuroscience, 167( 2), 216-231. doi: 10.1016/j.neuroscience.2010.02.015URL |
| 45 | Pereira, M., & Morrell, J. I . ( 2011). Functional mapping of the neural circuitry of rat maternal motivation: Effects of site-specific transient neural inactivation. Journal of Neuroendocrinology, 23( 11), 1020-1035. doi: 10.1111/j.1365-2826.2011.02200.xURL |
| 46 | Reisbick S., Rosenblatt J. S., & Mayer A. D . ( 1975). Decline of maternal behavior in the virgin and lactating rat. Journal of Comparative & Physiological Psychology, 89( 7), 722-732. |
| 47 | Riccio, A . ( 2010). Dynamic epigenetic regulation in neurons: Enzymes, stimuli and signaling pathways. Nature Neuroscience, 13( 11), 1330-1337. doi: 10.1038/nn.2671URL |
| 48 | Romero-Fernandez W., Borroto-Escuela D. O., Agnati L. F., & Fuxe K . ( 2013). Evidence for the existence of dopamine D2-oxytocin receptor heteromers in the ventral and dorsal striatum with facilitatory receptor-receptor interactions. Molecular Psychiatry, 18( 8), 849-850. doi: 10.1038/mp.2012.103URL |
| 49 | Root D. H., Melendez R. I., Zaborszky L., & Napier T. C . ( 2015). The ventral pallidum: Subregion-specific functional anatomy and roles in motivated behaviors. Progress in Neurobiology, 130, 29-70. doi: 10.1016/j.pneurobio.2015.03.005URL |
| 50 | Rosenblatt, J. S . ( 1967). Nonhormonal basis of maternal behavior in the rat. Science, 156( 3781), 1512-1513. doi: 10.1126/science.156.3781.1512URL |
| 51 | Rosenblatt, J. S., & Siegel, H. I . ( 1981). Factors governing the onset and maintenance of maternal behavior among nonprimate mammals. In D. J. Gubernick & P. H. Klopfer (Eds.), Parental care in mammals ( pp. 13-76). Boston, MA: Springer. |
| 52 | Sabihi S., Dong S. M., Durosko N. E., & Leuner B . ( 2014). Oxytocin in the medial prefrontal cortex regulates maternal care, maternal aggression and anxiety during the postpartum period. Frontiers in Behavioral Neuroscience, 8, 258. |
| 53 | Seifritz E., Esposito F., Neuhoff J. G., Lüthi A., Mustovic H., Dammann G.,.. Di Salle F . ( 2003). Differential sex-independent amygdala response to infant crying and laughing in parents versus nonparents. Biological Psychiatry, 54( 12), 1367-1375. doi: 10.1016/S0006-3223(03)00697-8URL |
| 54 | Seip, K. M., & Morrell, J. I . ( 2009). Transient inactivation of the ventral tegmental area selectively disrupts the expression of conditioned place preference for pup- but not cocaine- paired contexts. Behavioral Neuroscience, 123( 6), 1325-1338. doi: 10.1037/a0017666URL |
| 55 | Seip K. M., Pereira M., Wansaw M. P., Reiss J. I., Dziopa E. I., & Morrell J. I . ( 2008). Incentive salience of cocaine across the postpartum period of the female rat. Psychopharmacology, 199( 1), 119-130. doi: 10.1007/s00213-008-1140-9URL |
| 56 | Sesack, S. R., & Grace, A. A . ( 2010). Cortico-basal ganglia reward network: Microcircuitry. Neuropsychopharmacology, 35( 1), 27-47. doi: 10.1038/npp.2009.93URL |
| 57 | Stolzenberg, D. S., & Champagne, F. A . ( 2016). Hormonal and non-hormonal bases of maternal behavior: The role of experience and epigenetic mechanisms. Hormones and Behavior, 77, 204-210. doi: 10.1016/j.yhbeh.2015.07.005URL |
| 58 | Strathearn, L . ( 2011). Maternal neglect: Oxytocin, dopamine and the neurobiology of attachment. Journal of Neuroendocrinology, 23( 11), 1054-1065. doi: 10.1111/j.1365-2826.2011.02228.xURL |
| 59 | Swain J. E., Tasgin E., Mayes L. C., Feldman R., Constable R. T., & Leckman J. F . ( 2008). Maternal brain response to own baby-cry is affected by cesarean section delivery. Journal of Child Psychology & Psychiatry, 49( 10), 1042-1052. |
| 60 | Tzschentke T. M . ( 2007). Measuring reward with the conditioned place preference (CPP) paradigm: Update of the last decade. Addiction Biology, 12( 3-4), 227-462. doi: 10.1111/adb.2007.12.issue-3-4URL |
| 61 | Wansaw M. P., Pereira M., & Morrell J. I . ( 2008). Characterization of maternal motivation in the lactating rat: Contrasts between early and late postpartum responses. Hormones and Behavior, 54( 2), 294-301. doi: 10.1016/j.yhbeh.2008.03.005URL |
| 62 | Wu Z., Autry A. E., Bergan J. F., Watabe-Uchida M., & Dulac C. G . ( 2014). Galanin neurons in the medial preoptic area govern parental behaviour. Nature, 509( 7500), 325-330. doi: 10.1038/nature13307URL |
| 63 | Zha, X., & Xu, X. H . ( 2015). Dissecting the hypothalamic pathways that underlie innate behaviors. Neuroscience Bulletin, 31( 6), 629-648. doi: 10.1007/s12264-015-1564-2URL |
相关文章 15
| [1] | 王琳, 王志丹, 王泓婧. 孤独症儿童动作发展障碍的神经机制[J]. 心理科学进展, 2021, 29(7): 1239-1250. |
| [2] | 张照, 张力为, 龚然. 视觉工作记忆的过滤效能[J]. 心理科学进展, 2021, 29(4): 635-651. |
| [3] | 周爱保, 胡砚冰, 周滢鑫, 李玉, 李文一, 张号博, 郭彦麟, 胡国庆. 听而不“闻”?人声失认症的神经机制[J]. 心理科学进展, 2021, 29(3): 414-424. |
| [4] | 赵小红, 童薇, 陈桃林, 吴冬梅, 张蕾, 陈正举, 方晓义, 龚启勇, 唐小蓉. 敬畏的心理模型及其认知神经机制[J]. 心理科学进展, 2021, 29(3): 520-530. |
| [5] | 魏真瑜, 邓湘树, 赵治瀛. 亲社会行为中的从众效应[J]. 心理科学进展, 2021, 29(3): 531-539. |
| [6] | 岳童, 黄希庭, 傅安国. 人们何以能够“舍生取义”?基于保护性价值观认知神经机制的解释[J]. 心理科学进展, 2021, 29(3): 540-548. |
| [7] | 王葛彤, 席洁, 陈霓虹, 黄昌兵. 双眼视差的神经机制与知觉学习效应[J]. 心理科学进展, 2021, 29(1): 56-69. |
| [8] | 郭滢, 龚先旻, 王大华. 错误记忆产生的认知与神经机制:信息加工视角[J]. 心理科学进展, 2021, 29(1): 79-92. |
| [9] | 刘启鹏, 赵小云, 王翠艳, 徐艺雅, 王淑燕. 反刍思维与注意脱离损坏的关系及其神经机制[J]. 心理科学进展, 2021, 29(1): 102-111. |
| [10] | 翁纯纯, 王宁. 时距知觉的动物研究范式及相关神经机制[J]. 心理科学进展, 2020, 28(9): 1478-1492. |
| [11] | 杨晓梦, 王福兴, 王燕青, 赵婷婷, 高春颍, 胡祥恩. 瞳孔是心灵的窗口吗?——瞳孔在心理学研究中的应用及测量[J]. 心理科学进展, 2020, 28(7): 1029-1041. |
| [12] | 程士静, 何文广. 语义认知的习得、发展和老化及其神经机制[J]. 心理科学进展, 2020, 28(7): 1156-1163. |
| [13] | 张晶晶, 梁啸岳, 陈伊笛, 陈庆荣. 音乐句法加工的认知机制与音乐结构的影响模式[J]. 心理科学进展, 2020, 28(6): 883-892. |
| [14] | 杨国春, 伍海燕, 齐玥, 刘勋. 人类性别加工的认知神经机制[J]. 心理科学进展, 2020, 28(12): 2008-2017. |
| [15] | 李灵, 侯晓旭, 张亚, 隋雪. 食物线索注意偏向及其神经机制[J]. 心理科学进展, 2020, 28(12): 2040-2051. |
PDF全文下载地址:
http://journal.psych.ac.cn/xlkxjz/CN/article/downloadArticleFile.do?attachType=PDF&id=4403
