 ,, 王琰
,, 王琰 ,武汉大学生命科学学院,细胞稳态湖北省重点实验室,武汉 430072
,武汉大学生命科学学院,细胞稳态湖北省重点实验室,武汉 430072Progress on the molecular mechanisms of PCSK9-mediated degradation of low density lipoprotein receptor
Yuxian Wu ,, Yan Wang
,, Yan Wang ,Hubei Province Key Laboratory of Cell Homeostasis, College of Life Sciences, Wuhan University, Wuhan 430072, China
,Hubei Province Key Laboratory of Cell Homeostasis, College of Life Sciences, Wuhan University, Wuhan 430072, China通讯作者: 王琰,博士,教授,博士生导师,研究方向:脂类代谢。E-mail:Wang.y@whu.edu.cn
编委: 史岸冰
收稿日期:2020-04-6修回日期:2020-05-20网络出版日期:2020-10-20
| 基金资助: |
Received:2020-04-6Revised:2020-05-20Online:2020-10-20
| Fund supported: |
作者简介 About authors
吴玉娴,在读硕士研究生,专业方向:生物化学与分子生物学。E-mail:

摘要
血清低密度脂蛋白胆固醇(low density lipoprotein cholesterol, LDL-C)水平的升高是导致心血管疾病发生的主要危险因素。低密度脂蛋白受体(LDL receptor, LDLR)介导的低密度脂蛋白(low density lipoprotein, LDL)清除是决定循环中LDL-C水平的主要因素。LDL与细胞表面的LDLR结合后通过经典的网格蛋白小窝(clathrin-coated vesicles)内化进入细胞。在酸性核内体中,LDLR与LDL解离并循环回到细胞表面,释放的LDL将被运送到溶酶体中降解。前蛋白转化酶枯草溶菌素9 (proprotein convertase subtilisin kexin type 9, PCSK9)编码一种肝脏分泌型蛋白,其突变与LDL-C水平密切相关。前期研究已经证明,PCSK9直接与细胞表面的LDLR相互作用,二者一起通过网格蛋白小窝内化进入细胞。然而,在酸性核内体中,PCSK9和LDLR形成紧密的复合物,并进入溶酶体中进行降解,从而减少肝细胞表面LDLR的水平,降低肝脏对LDL-C的清除,该过程对于维持血浆中LDL在相对恒定的水平具有重要作用。因此,阻断PCSK9功能已成为治疗高胆固醇血症的新策略。本文综述了PCSK9的功能和机制研究的最新进展,并着重介绍了PCSK9抑制剂的研究进展,旨在为PCSK9-LDLR通路的研究和胆固醇代谢的调控提供参考。
关键词:
Abstract
Elevated serum level of low density lipoprotein cholesterol (LDL-C) is the leading risk factor for cardiovascular disease. LDL receptor (LDLR)-mediated LDL clearance is the major factor determining the LDL-C level in the circulation. LDL binds to the LDLR on the cell surface and enters the cells through classical clathrin-coated vesicles. In the acidic endosome, LDLR is uncoupled from LDL and recycles back to the cell surface. The released LDL is transported to the lysosome for degradation. The proprotein convertase subtilisin kexin type 9 (PCSK9) gene encodes a hepatic secretory protein, and its mutations are strongly associated with levels of LDL-C. We and others have shown that PCSK9 directly interacts with LDLR on the cell surface and both are internalized through the clathrin-coated vesicles. However, in the acidic endosome, PCSK9 and LDLR form a tight complex and are targeted to lysosome for degradation, thereby reducing the level of LDLR on the surface of hepatocytes and decreasing hepatic clearance of LDL-C, which plays an important role in maintaining a relatively constant level of LDL in the plasma. Thus, blocking PCSK9 function has become a new strategy to treat hypercholesterolemia.In this review, we will summarize the latest progress in the functional and mechanistic studies of PCSK9 and also highlight the research progress of PCSK9 inhibitors. It aims to provide a reference for the study of PCSK9-LDLR pathway and the regulation of cholesterol metabolism.
Keywords:
PDF (707KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
吴玉娴, 王琰. PCSK9降解低密度脂蛋白受体分子机制研究进展. 遗传[J], 2020, 42(10): 965-978 doi:10.16288/j.yczz.20-065
Yuxian Wu.
胆固醇是人体生命活动必不可少的脂类物质[1,2]。血浆中胆固醇水平的升高会引发动脉粥样硬化性心血管疾病,同时大大增加心肌梗死和卒中的风险[3,4]。人体中60%~70%的血浆胆固醇由低密度脂蛋白(low density lipoprotein, LDL)运输[5],而血浆中75%左右的LDL通过肝脏细胞的低密度脂蛋白受体(low- density lipoprotein receptor, LDLR)内吞进入肝脏细胞被清除[6],其余25%左右被外周组织器官吸收利用。LDLR介导的LDL内吞途径被认为是机体清除血浆LDL最为重要和高效的方式[7]。
前蛋白转化酶枯草溶菌素9 (proprotein convertase subtilise kexin 9, PCSK9)可结合LDLR并介导其降解,从而调节血浆低密度脂蛋白胆固醇水平(low density lipoprotein cholesterol, LDL-C)[7,8]。研究发现,PCSK9的一些突变体会造成常染色体显性高胆固醇血症(autosomal dominant hypercholesterolemia, ADH)[9,10,11,12]。PCSK9介导LDLR降解通路的发现使得PCSK9成为心血管疾病治疗的新靶点,也促使人们迅速研究PCSK9的生物学机制[13]。本文主要对LDLR和PCSK9的蛋白结构、生物学功能及PCSK9降解LDLR的分子机制及相关抑制剂的开发等方面的研究进展进行了综述,为胆固醇代谢调控的分子机制的研究和高血脂症的治疗提供参考。
1 LDLR结构与功能
美国西南医学中心的两位科学家Brown和Goldstein等[14,15]于1974年在研究家族性高胆固醇血症(familial hypercholesterolemia, FH)时发现了LDLR的存在,这一发现对脂蛋白、载脂蛋白的深入研究有巨大的推进作用。LDLR是一个单链单次跨膜糖蛋白, 广泛分布于肝脏、动脉壁平滑肌、血管内皮细胞和白细胞。LDLR成熟体由839个氨基酸组成,在结构上分为5个功能区,包括:配体结合结构域(ligand binding domain)、表皮生长因子前体结构域(EGF precursor structure domain)、含O-连接糖链结构域(O-linked sugars structure domain)、跨膜结构域(transmembrane domain)和胞浆结构域(cytoplasmic structure domain)[16,17],其中配体结合结构域由7个配体结合重复序列LR1-LR7 (ligand binding repeat)组成,EGF前体结构域包括3个EGF样重复序列(EGF-A、B、C)和一个6个YWTD模体组成的β 螺旋结构[18]。
LDLR的主要功能是摄入血浆胆固醇,人体血浆中大约75%的LDL是通过介导的内吞作用进入各组织细胞所清除(图1),其余由清道夫受体(scavenger receptor)摄取、氧化以及由周围组织进行非受体介导途径所摄取[16,19]。在LDLR介导LDL内吞的过程中,LDL中的载脂蛋白ApoB100与LDLR结合形成复合体,同时,在常染色体隐性高胆固醇血症衔接蛋白(autosomal dominant hypercholesterolemia, ADH)和AP-2 (adaptin protein 2)以及网格蛋白介导下启动内吞作用[20]。随着核内体中pH值的降低,LDLR从开放构象转变为封闭构象,释放结合的LDL,LDLR循环回到细胞膜表面[21,22],而LDL被运送到溶酶体水解[16,23]。由此可见,LDLR介导的LDL内吞途径对于维持血浆LDL在相对恒定的水平有重要作用。
图1
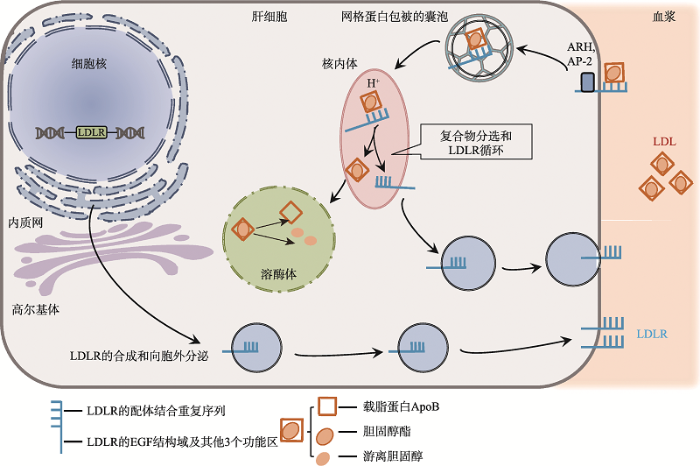 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1LDLR介导LDL的内吞及LDLR循环
LDLR与血液中的LDL在膜上结合成LDL/LDLR复合物,后被合并到包合网格蛋白的囊泡中,囊泡在核内体内解离,LDLR自由地返回到细胞表面(循环),而LDL则被运送到溶酶体,在溶酶体中被降解。
Fig. 1LDLR mediates LDL endocytosis and LDLR circulation
2 PCSK9蛋白结构与功能
2003年,Marianne等[9]在两个常染色体显性形式的FH的法国家庭中发现两种PCSK9基因突变——S127R和F216L可导致严重的家族性高胆固醇血症。这是继LDLR和ApoB之后第3个被发现的与高胆固醇血症有关的基因[24,25]。2.1 PCSK9蛋白的结构
PCSK9是枯草溶菌素蛋白酶K亚家族的第9个成员,该基因编码的蛋白质由692个氨基酸残基组成,包含N端信号肽序列、前结构域、催化结构域、枢纽域和富含半胱氨酸的C端结构域[26,27]。PCSK9蛋白主要表达于肝脏,也在小肠、肾脏、胰腺β细胞、巨噬细胞和血管平滑肌细胞甚至脂肪细胞中表达[28,29],以分泌性蛋白的形式随血液循环。不同于原蛋白转化酶家族的其他成员,PCSK9在合成后被导向内质网,其中信号肽在内质网上被剪切,在152位残基处经历一次自催化剪切激活,形成一个14 kDa的前结构片段和一个60 kDa的糖基化成熟体。剪切后的前结构域仍然以非共价键结合在成熟体上,与成熟片段形成一个复合体[30],前结构域通过结合在催化结构域上来抑制蛋白酶催化活性,这一步骤是PCSK9转运至高尔基体和以不活跃的二聚体复合物分泌所必需的[31]。随后复合体离开内质网转运到高尔基体中,在高尔基体中经过乙酰化等一系列修饰后最终分泌到血液中[32,33]。2.2 PCSK9降解LDLR的分子机制
自2003年PCSK9被发现与家族性高胆固醇血症有关联后,时隔2年后人们确立了PCSK9中两种相对常见的“功能缺失型”突变和低血浆LDL-C水平之间的因果关系[34]。此后的数年间,研究人员开始研究PCSK9和LDLR的互作。随着实验方法和技术的更新换代,人们对PCSK9介导LDLR降解的分子机制有了更多的发现。2012,Wang等[35]发现结合PCSK9的LDLR依然通过经典的网格蛋白(clathrin)内吞囊泡途径进入细胞,最终在溶酶体被降解,而这一过程完全不需要泛素化与蛋白酶体途径、细胞自噬途径和ESCRT途径,这一发现对理解PCSK9的作用机制提供了新的思路。目前发现PCSK9介导LDLR的降解有两种机制:胞内途径和胞外途径(图2)。因此,PCKS9-LDLR复合物的结合和形成既可以发生在细胞内,也可以发生在细胞表面。由于这些里程碑式的观察和发现,PCSK9已经成为一个非常有吸引力的药物靶点和深入研究的课题。
图2
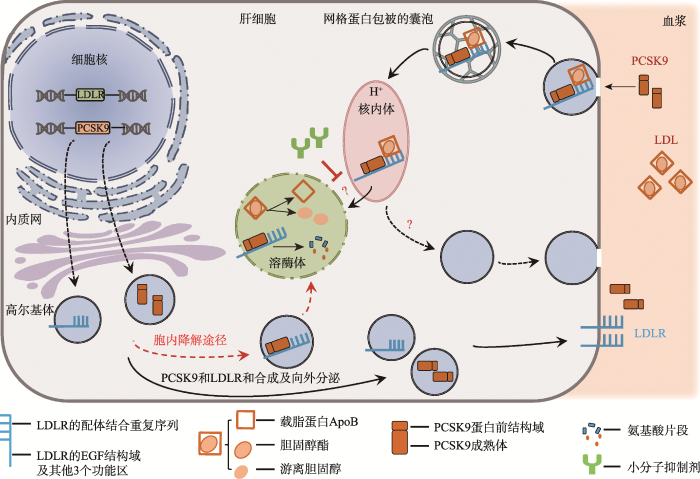 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2PCSK9介导LDLR降解的两种途径
PCSK9降解LDLR的两种途径:胞内途径(红色虚线箭头)和胞外途径(黑色实线箭头)。胞内途径—PCSK9在分泌前与细胞内的LDLR相遇,直接进入溶酶体降解;胞外途径—分泌到胞外的PCSK9与LDLR通过网格蛋白介导的内吞方式进入细胞内,最终在溶酶体降解。
Fig. 2Two pathways of PCSK9-mediated LDLR degradation
2.2.1 PCSK9介导LDLR降解的胞内途径
2004年,Park等[36]首次发现,在Arh 敲除小鼠中过表达PCSK9后,肝脏的LDLR水平依然降低,人家据此推断PCSK9降解LDLR存在一种不依赖于ARH的细胞内途径,后来Homer等[37]在南非人群中发现两种PCSK9突变体——S127R和D129G,这两种突变体的自催化和向胞外分泌过程被强烈抑制,却依旧能降低LDLR的表达和活性[37],进一步证实了“胞内途径”的猜想。2009年,Poirier等[38]发现:通过敲低网格蛋白轻链(clathrin light chain, CLCs)阻断从高尔基体网络到溶酶体的囊泡运输,HepG2细胞内的LDLR水平以PCSK9剂量依赖的方式迅速上升,而外源性PCSK9增强LDLR降解的能力不受影响。由此确定了内源水平上PCSK9诱导的LDLR降解的细胞内路径的存在。分泌前的成熟的PCSK9在从内质网进入高尔基体网络后,与包裹着LDLR的囊泡结合,并直接介导LDLR直接进入溶酶体降解[38]。在肝脏中,PCSK9主要通过细胞外途径促进LDLR的降解,推断可能是当胞外途径被人为阻断或PCSK9过表达时,这种代偿性的不依赖于网格蛋白的胞内途径就被激活[7]。关于PCSK9降解LDLR的胞内途径的具体分子机制尚需要更深一步的研究,但这一发现为治疗高胆固醇血症和冠心病提供了一个新的指导方向。
2.2.2 PCSK9介导LDLR降解的胞外途径
一系列联体共生动物实验清楚地表明,PCSK9降解LDLR的生理过程主要是通过胞外途径[39,40]。
分泌到肝细胞外的PCSK9蛋白与LDLR结合,其中PCSK9的催化结构域和LDLR的EGF-A区域是二者结合的关键结构。PCSK9的催化结构域以钙依赖的方式特异性结合到位于细胞膜上的LDLR的EGF-A的N端区域,由此形成PCSK9/LDLR复合体[41,42,43,44]。ARH与LDLR的胞浆结构域相互作用,从而介导PCSK9/LDLR复合体通过网格蛋白介导的胞吞途径一起内吞进入核内体。由于核内体中pH值降低,带正电荷的PCSK9的C端结构域与带负电荷的LDLR的配体结合结构域上的LRs发生静电相互作用,LDLR的配体结合重复域中的Asp残基(Asp172和Asp203)参与了互作[43],进一步增强了PCSK9与LDLR的亲和力[45,46],由此提升了复合物的稳定性,抑制LDLR恢复闭合构象,使其不能释放配体也无法离开内体返回细胞表面。接下来PCSK9/LDLR复合物进入溶酶体,PCSK9和LDLR均被降解[47]。
一些PCSK9突变体如D374Y和S127R以及LDLR的突变体H306Y会导致家族性高胆固醇血症[17],突变体通过增强PCSK9/LDLR复合物内的分子相互作用来破坏LDLR,进而影响LDL-C。如PCSK9 D374Y突变体通过与LDLR的EGFA结构域之间形成氢键,从而使得其与LDLR结合的亲和性相比于野生型PCSK9提高了6~30倍[48],从而促进LDLR降解,增加循环的LDL-C水平[42~44,49,50],因此,这些突变体携带者也会比其他FH患者更早地受到心血管疾病的影响。
PCSK9介导LDLR降解的过程中是否有其他分子参与调节,是一直以来研究的热点,人们对此也有诸多猜想和发现。一方面立足于PCSK9降解LDLR的生理过程具有的组织特异性,在肝脏中其效果最为明显,在肾上腺和成人脑部分区域中PCSK9不能(或几乎不能)调节LDLR水平[51,52]。由于衔接蛋白ARH是肝细胞中PCSK9/LDLR复合物内化所必需的,在其他类型的细胞中则不是必需的[40,53,54],所以由此人们推测:PCSK9降解LDLR需要组织特异性的分子伴侣,且分子伴侣可能与PCSK9的C端结构域相互作用来参与调控[30,55]。最新的一项研究发现了一种参与PCSK9结合并降解LDLR过程的重要因子—肝素硫酸酯蛋白聚糖(heparin sulfate proteoglycans, HSPG)。HSPG是一种大量存在于多种细胞表面及细胞外基质的蛋白聚糖,在脂蛋白代谢尤其是结合配体的内吞过程中起着重要的生理作用[56]。不同于其他组织和细胞,肝细胞上的HSPG的乙酰肝素链化学性质特殊,其中含有较丰富的N-位硫酸化的氨基葡萄糖(GlcN)和2-O-位硫酸化的艾杜糖醛酸(IDoA),并且三硫酸双糖单位在全链中所占的比例较高。PCSK9对由GlcN和IDoA组成的三硫酸乙酰肝素二糖重复结构表现出高度的选择性和亲和性。由此,肝细胞表面上HSPG可大量招募血液中的PCSK9蛋白并通过PCSK9前结构域的6个暴露的氨基酸位点与之紧密结合,这种结合对于体内和体外PCSK9/LDLR复合体的形成及降解至关重要[57]。因此,针对HSPG结合位点的单克隆抗体和肝素类似物等是有效的PCSK9抑制剂,可作为潜在的CAD治疗手段。
2019年,Huang等[58]发现了PCSK9和LDLR结合及降解过程中的一个新的参与者——黄体酮和脂质受体3(progestin and AdipoQ receptor 3, PAQR3)。PAQR3主要定位于高尔基体,主要通过抑制Raf激酶和PI3K/AKT介导的促生长信号通路发挥抑癌作用[58,59]。研究发现当PAQR3过表达时可剂量依赖性地增强LDLR与PCSK9的相互作用。相反,当肝脏PAQR3被敲除时内源性PCSK9与LDLR的相互作用减少。活体实验也证明了PAQR3的缺失导致肝脏中LDLR/PCSK9复合物的形成减少,LDLR水平升高,导致血浆LDL-C和胆固醇水平下降。通过免疫共沉淀进一步发现PAQR3可在早期核内体中与LDLR的β螺旋结构域结合,又能与PCSK9的前结构域互作,并且PAQR3可通过这种中间体的互作来增强LDLR/PCSK9复合体的相互作用,从而调节LDLR的降解。
关于PCSK9降解LDLR的分子机制的另一猜想是蛋白质的翻译后修饰。PCSK9蛋白转录后会经
历一系列修饰,包括糖基化[60]、硫化[32]和磷酸化修饰[61]。Ben Djoudi Ouadda等[62]首次发现:Fam20C/ Fam20A复合体作为PCSK9的主要的分泌激酶,可在PCSK9蛋白的第47、666、668和688位丝氨酸位点对其进行磷酸化修饰,且这种磷酸化修饰在体内和体外都被证实不仅增强了PCSK9从高尔基体向细胞表面的转运及分泌,还使得PCSK9与LDLR结合亲和力均提升了约2倍,由此最大化诱导PCSK9降解LDLR[62]。此外有研究发现:向表达LDLR的细胞加入PCSK9共培养时LDLR出现了泛素化,同时发现当LDLR分子C端上的两个泛素化位点发生突变时,LDLR不能被PCSK9诱导降解。因此推测,PCSK9介导的LDLR的降解需要LDLR的泛素化[55]。
2.3 PCSK9蛋白的其他功能
PCSK9在多种器官和细胞中的表达意味着它除了控制LDL代谢之外可能还发挥多种生物学功能。多项研究发现PCSK9在中枢神经系统发育、动脉粥样硬化发病、免疫应答和肿瘤发生等多种生物学功能中发挥作用[63]。PCSK9最显著的功能是通过降解肝细胞表面的LDLR来调节血脂代谢[64]。除此之外,有研究发现PCSK9可能通过上调凝集素型氧化低密度脂蛋白受体1 (lectin-like oxidized low density lipoprotein receptor-1, LOX-1)的表达介导巨噬细胞摄取氧化型低密度脂蛋白,从而直接促进动脉粥样硬化的形成[65],这表明PCSK9也可能以非胆固醇依赖的方式直接发挥促动脉粥样硬化作用。
除了与LDLR结合外,PCSK9还与其他受体相互作用并控制其降解,如载脂蛋白E受体(apolipoprotein receptor 2)[66]、LDLR相关蛋白1 (lipoprotein receptor-related protein 1, LRP1)[67],VLDLR (very low density lipoprotein receptors)[68]、CD36[69]、CD81[70]和上皮细胞Na+通道[71]。
PCSK9最早被发现参与皮层神经元的分化,因此被称为神经凋亡调节的转化酶1 (NARC-1)[60]。研究发现:斑马鱼(Danio rerio) Narc-1/Pcsk9的特异性敲除导致小脑神经元的普遍紊乱和后脑中脑边界的丢失,受精后96 h胚胎死亡,说明NARC-1/PCSK9在中枢神经系统发育中起着至关重要的作用[72]。PCSK9与机体感染也有一定关联,其在脓毒症中有调节炎症的作用[73],PCSK9过表达可加重早期脓毒症的多器官病理和促炎状态[74]。抑制PCSK9的表达还能有效抑制神经元、胶质瘤细胞的凋亡[75,76]。已有证据表明,PCSK9可能通过调节LDLR的表达来调节亲脂性病原体相关分子模式的识别参与对某些病原体的免疫应答[73]。
PCSK9信号通路是分子生物学面临的重大挑战,除了脂质代谢外,PCSK9其他的生理功能仍知之甚少,需要进一步地深入探索和发现。
3 PCSK9与人类疾病
PCSK9多种生物学功能决定了其可能与多种疾病的发生发展有关。截至目前研究较为清晰的是PCSK9在高胆固醇血症和脓毒症中的调节作用,其余多种相关的生理病理机制尚有待探索。3.1 人群中PCSK9基因多态性和血脂异常
PCSK9基因的某些突变可以改变蛋白质的氨基酸结构,从而改变其酶活、功能和对LDLR的亲和力。根据PCSK9基因突变对血浆胆固醇水平的影响,将其分为功能获得型突变(gain-of-function, GOF)和功能缺失型突变(loss-of-function, LOF)。功能获得型突变的PCSK9与高胆固醇血症密切相关,会引起动脉粥样硬化[12];2003年,在两个患常染色体高胆固醇血症的法国家庭中发现两种PCSK9-GOF突变——S127R和F216L。此后,引起高胆固醇血症的其他PCSK9 GOF突变如D374Y、E32K、D374H、R469W和D129G被陆续发现[10,32,42,63,77]。功能缺失型突变的PCSK9能减少LDLR的内吞,使LDLR不容易被清除,从而降低血液胆固醇[28,78]。在2005年,Cohen等[34]描述了两种PCSK9 LOF突变——Y142X and C679X,这两种突变携带者在非洲裔美国人群中出现频率更高,LDL-C水平只有(100 ± 45) mg/dL,与正常人的(138 ± 42) mg/dL相比减少了28%,患冠心病的风险可下降88%[11,28]。此后又陆续在南非和新西兰人群中以及欧洲白种人分别发现了两种PCSK9 LOF突变——R237W和R46L[37,61]。基于这些发现,笔者猜想:与胆固醇血症有关的PCSK9突变型与地域及饮食习惯可能有一定规律性和关联性。3.2 PCSK9与其他疾病
有证据表明,PCSK9可能由炎症刺激诱导,本身可能是炎症介质[79],其在脓毒症的调节作用最为突出。脓毒症特点是炎症和凝血的系统激活,严重脓毒症伴有至少一个器官功能障碍,在美国每年有0.3%的人受感染,死亡率为30%[80]。遗传数据表明,在人类中PCSK9 LOF (R46L)多态性与脓毒症患者的生存率升高和降低细胞因子水平相关,而PCSK9 GOF (E670G)多态性则具有相反的效果,并且给健康志愿者静脉注射内毒素后R46L携带者中细胞因子水平明显更低[81]。研究表明内毒素脂多糖(lipopolysaccharide, LPS)与脂蛋白结合,通过LDLR以及包括VLDLR在内的受体内化于肝细胞清除[82]。这说明PCSK9通过降低肝细胞上的LDLR水平,从而在感染脓毒症时降低肝脏对LPS的LDLR依赖性摄取,加剧局部或全身炎症[73]。还有研究发现,PCSK9可通过MAPK通路促进氧化型低密度脂蛋白(oxidized low density lipoprotein, ox-LDL)诱导血管内皮细胞凋亡,由此减弱斑块氧化应激和炎症,提示PCSK9可能通过非LDLR途径直接参与动脉粥样硬化的调控[83]。有研究发现PCSK9的LOF基因变异与乳腺癌发病率降低相关,而PCSK9的GOF基因变异与乳腺癌风险增加相关,PCSK9的表达可抑制肝癌、前列腺癌和黑色素瘤等肿瘤的发生发展[84,85,86],表明PCSK9可能对肿瘤生长有一定影响[87]。除此之外,前人提出PCSK9对血糖代谢和肾功能的调节作用的猜想,但遗传学研究和观察性研究的结果与临床数据往往相悖,并且分子机制未明,所以相关猜想仍存在争议[71,88~93]。PCSK9在某些神经退行性疾病中发挥的作用尚未在人体得到最终验证[63,94]。
总体而言,PCSK9与这些疾病的观察和猜想尽管是初步的且分子机制未明,但也提供了关于PCSK9抑制剂在调节血脂之外的安全性的重要信息。
4 PCSK9抑制剂
PCSK9抑制剂通过抑制PCSK9的表达或PCSK9降解LDLR的结合,起到提升肝细胞膜上LDLR水平,减少血液中LDL-C积累的作用。根据作用机制的不同,PCSK9抑制剂可分为单克隆抗体、RNA干扰(small interfering RNA, siRNA)和多肽抑制物等。4.1 单克隆抗体
目前市场上已有两种FDA批准的药用的人类单克隆抗体:Alirocumab和Evolocumab[95]。单克隆抗体通过中和PCSK9来抑制PCSK9与LDLR的相互作用,导致LDLR数量增加,最终增强LDL的吸收。在高胆固醇血症和非家族性胆固醇升高的血脂异常患者中,当单独使用Alirocumab或Evolocumab或者将单克隆抗体与其他降脂药物联合使用时,均可显著降低高达60%的LDL[96,97,98]。PCSK9单抗可减轻不良反应,降低心血管事件和肝功能异常的发生率,逆转动脉粥样硬化斑块,但静脉给药及抗体制备所导致的成本过高使得其难以推广使用。4.2 RNA干扰药物
目前很有前景的一类的PCSK9抑制药物是通过类脂纳米颗粒(lipidoid nanoparticle)包裹着siRNA来抑制PCSK9的表达[99]。这种方法不仅可以降低循环的PCSK9水平,还可以有效降低细胞内的PCSK9水平。2期临床研究表明,siRNA降脂药物inclisiran 在第1天和第90天注射可使得健康志愿者的LDL-C水平降低52.6%,达到与PCSK9单抗相似的降脂效果[100]。siRNA作为药物的优点在于其选择性相对较高,有利于降低脱靶率,几乎无不良反应发生,到目前为止inlisiran尚未发现副作用,但也需要更多的数据来科学评估其是否可以应用于临床[101,102]。4.3 模拟肽
模拟LDLR的EGF-A结构域的短肽通过与PCSK9竞争性结合LDLR的EGF-A结构域,从而抑制LDLR的降解,增加LDL的吸收。第一个有效抑制PCSK9-LDLR结合的EGF-A类似小分子肽是EGF66,其与PCSK9结合并在HepG2细胞和小鼠中均可抑制PCSK9诱导的LDLR降解[103]。此后又设计出了第一代含16个残基的线性肽MESFPGWNLV (homoR) IGLLR,它可以拮抗PCSK9活性,目前正在改进结构并产生一种有效的口服活性小分子抑制剂[104]。如果能解决肽在血浆中不稳定、口服后难以被吸收等问题,模拟肽将是一种有前途的、经济有效的PCSK9单抗替代药物。4.4 疫苗
基于PCSK9肽的疫苗消除内源性循环的PCSK9是一种新的抑制PCSK9的手段。疫苗的一期临床数据显示:在健康受试者中,诱导免疫是安全的且具有良好耐受性;超过90%的免疫受试者产生PCSK9特异性抗体反应,在首次诱导后70周时LDL-C平均下降13.3%,并在加强免疫后持续至少30周[105]。虽然这种方法看起来更为简单易行,效果也更持久,但关键是仍然无法排除成人肝脏中由于没有PCSK9表达而产生任何严重的意外副作用的危险性,特别是在肝功能受损的情况下。4.5 其他
除了以PCSK9或LDLR作为药物靶点外,人们也尝试通过阻断PCSK9的合成和分泌达到抑制LDLR降解的功效。Sortilin可协助PCSK9转运至高尔基体而促使其从肝细胞分泌。在Sortilin敲低时PCSK9的表达降低,由此减弱PCSK9对LDLR的降解[106]。此外,Sec24a、SRT3025和Q125H等蛋白能抑制PCSK9分泌[107]。硫酸肝素可介导PCSK9和LDLR之间的结合,且这种作用对于LDLR进入溶酶体降解是不可或缺的。小分子肝素类似物可干扰PCSK9与LDLR的关联,增加质膜中LDLR的数量[57]。天然产物槲皮素-3-O-葡萄糖苷(quercetin- 3-b-D-glucoside, Q3G)可减少PCSK9的分泌,同时增加LDLR的表达[108]。这些观察结果为开发治疗高脂血症的新方法提供了新思路。5 结语与展望
近20年的实验和临床研究表明PCSK9 LOF突变与LDL-C水平降低、心血管并发症发生率降低和全因死亡率降低相关[109]。人们也确定了PCSK9通过介导LDLR降解来影响LDL-C的生理过程。PCSK9主要通过胞外途径来影响LDL水平,血浆中循环的PCSK9通过自身催化结构域与肝细胞表面的LDLR的EGF-A域结合,形成PCSK9/LDLR复合体,在ARH和网格蛋白介导下启动内吞。复合体内吞后进入核内体,酸性的环境使得LDLR的构象发生改变,无法与PCSK9解离,由此导致LDLR无法循环到细胞膜表面,而是进入溶酶体降解,进一步降低肝脏对LDL-C的清除,循环LDL-C随之上升[7,8,42~44]。迄今为止,PCSK9介导LDLR降解的通路才刚刚建立“框架”,而其中的作用过程及作用机制仍然未知。比如,胞内途径的作用甚微,其在什么生理状况下才被触发?具体又有哪些分子调控?胞外途径中PCSK9是如何阻止LDLR循环到质膜上?PCSK9/LDLR复合体又是如何被分选到溶酶体中?笔者通过对PCSK9-LDLR复合物的分子作用方式的研究,猜想PCSK9/LDLR复合物内吞进入细胞后到内化至LDLR的溶酶体内降解过程中,可能有一些新的未知基因的参与其中,形成目前未知的通路。另一方面,如若能找到一个小分子可抑制PCSK9/ LDLR复合物向lysosome的转运即可作为有效的PCSK9抑制剂。
除了脂质代谢外,PCSK9的其他生物学功能仍知之甚少。并且,PCSK9的基因多效性的生理作用可能会引发到目前为止进行的试验中尚未描述的各种副作用[63]。PCSK9在糖尿病、慢性肾病以及癌症等多种疾病中的作用和机制存在多种猜想和数据,具体机制也尚无定论。深入研究PCSK9的多效性生理功能和PCSK9决定LDLR命运的分子机制有望为抑制PCSK9的活性提供新的作用靶点,对于高血脂症和冠心病的预防和治疗及开发新的降脂药物具有十分重要的意义。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1016/j.molcel.2017.02.015URLPMID:28344083 [本文引用: 1]

Hedgehog (Hh) has been known as the only cholesterol-modified morphogen playing pivotal roles in development and tumorigenesis. A major unsolved question is how Hh signaling regulates the activity of Smoothened (SMO). Here, we performed an unbiased biochemical screen and identified that SMO was covalently modified by cholesterol on the Asp95 (D95) residue through an ester bond. This modification was inhibited by Patched-1 (Ptch1) but enhanced by Hh. The SMO(D95N) mutation, which could not be cholesterol modified, was refractory to Hh-stimulated ciliary localization and failed to activate downstream signaling. Furthermore, homozygous Smo(D99N/D99N) (the equivalent residue in mouse) knockin mice were embryonic lethal with severe cardiac defects, phenocopying the Smo(-/-) mice. Together, the results of our study suggest that Hh signaling transduces to SMO through modulating its cholesterylation and provides a therapeutic opportunity to treat Hh-pathway-related cancers by targeting SMO cholesterylation.
DOI:10.1007/s11427-019-1563-3URLPMID:31686320 [本文引用: 1]

Glucose and fatty acids are the major sources of energy for human body. Cholesterol, the most abundant sterol in mammals, is a key component of cell membranes although it does not generate ATP. The metabolisms of glucose, fatty acids and cholesterol are often intertwined and regulated. For example, glucose can be converted to fatty acids and cholesterol through de novo lipid biosynthesis pathways. Excessive lipids are secreted in lipoproteins or stored in lipid droplets. The metabolites of glucose and lipids are dynamically transported intercellularly and intracellularly, and then converted to other molecules in specific compartments. The disorders of glucose and lipid metabolism result in severe diseases including cardiovascular disease, diabetes and fatty liver. This review summarizes the major metabolic aspects of glucose and lipid, and their regulations in the context of physiology and diseases.
DOI:10.1016/j.bcp.2019.04.003URLPMID:30953636 [本文引用: 1]

Hypercholesterolemia represents a leading cause in the development of atherosclerotic plaques, increasing the risk for ACVS. It actually counts as a major cause of cardiovascular disease etiopathogenesis. The causes of hypercholesterolemia are multifactorial, spanning from genetic constitution, age, sex, to sedentary lifestyle and diets rich in sugars and lipids. Although dietary restriction in saturated fats, increased exercise, and other modification in lifestyle represent a first-line approach to treat very initial stages in hypercholesterolemia, most patients will require the addition of pharmacological agents. Pharmacological approaches include inhibition of cholesterol synthesis, decreased fat absorption from the GI tract, and increased degradation of FA. These strategies present a series of side effects, low therapeutic efficiency in some patients, and reduced tolerability. One of the major goals in treatment for hypercholesterolemia is to decrease the levels of low density lipoproteins (LDL), while maintaining those of high density lipoproteins (HDL). LDL particles contain about 80% of lipids, most of it cholesterol and cholesteryl esters, and 20% of the ApoB-100 protein. LDL carries cholesterol to the tissues, to be incorporated to biological membranes, or to be transformed to steroids. Excess of LDL translates into increased levels of circulating cholesterol particles and accumulation in certain tissues, especially vascular tissue, initiating a fatty streak, which may evolve to an atheroma, causing a series of cardiovascular problems, including impaired circulation, high blood pressure, increased cardiac workload, and coronary artery disease. It is essential to prevent LDL accumulation into the bloodstream to avoid the formation of these fatty streaks and the initiation of a cascade that will lead to the development of atherosclerosis. In healthy individuals. Under physiological conditions, LDL is effectively removed from circulation through receptor-mediated endocytosis. LDL clearance involves binding to its receptor, LDLR, which enables the internalization of the LDL particle and drives its degradation in lysosomes. Once the LDL particle is degraded, the free receptor recycles to the plasma membrane, and captures new LDL particles. Adequate levels of LDLR are essential to remove the excess of cholesterol-laden LDL. Proprotein convertase, subtilysin kexin type 9 (PCSK-9), expressed in liver and intestine, binds to LDLR, and internalized. Once inside the cell, PCSK-9 catalyzes the proteolysis of LDLR, preventing its recycling to the cell surface, and effectively decreasing the number of LDLR, notoriously decreasing the ability to clear LDL from circulation. Levels of PCSK-9 varies with age, gender, and levels of insulin, glucose, and triglycerides. Loss-of-function mutations in PCSK-9 gene invariably translates into lower levels of LDL, and decreased risk of developing coronary artery disease. Conversely, increased activity or expression of this enzyme leads to hypercholesterolemia. Inhibition of PCSK9 has proven to be successful in decreasing LDL levels and risk of the development of hypercholesterolemia with its associated higher risk for ASCVD. Patient with gain-of-function mutations in the PCSK9 undoubtedly benefit from therapies based on PCSK-9 inhibitors. However, millions of patients show statin intolerance, or cannot be efficiently controlled by statins alone- the most prevalent therapy for hypeprcholesterolemia. This commentary will evaluate the possibilities, caveats and future directions in the treatment of hypercholesterolemia, and therapies with combination of drugs.
DOI:10.1016/j.atherosclerosis.2015.08.038URLPMID:26350916 [本文引用: 1]

BACKGROUND: Familial hypercholesterolemia (FH) is undoubtedly associated with premature coronary heart disease, but it is debatable whether FH increases the risk for stroke. OBJECTIVE: To meta-analyze available evidence regarding the incidence of stroke in individuals with heterozygous (He) FH. METHODS: We conducted a systematic review and a meta-analysis of epidemiological studies, including English-language publications until June 2015; four observational studies, with 3374 participants with HeFH, were included in the analysis. Cerebrovascular disease comprised of ischemic stroke or transient ischemic attack. Since studies did not include any control subjects, the corresponding general population of the same reference area and period of time for each HeFH study served as control group. Analyses were performed according to the period of time during which the studies were conducted: prestatin and statin era (before and after 1987 when lovastatin was launched). RESULTS: In the prestatin era, individuals with HeFH exhibited a higher risk for stroke compared with the general population [odds ratio (OR) = 7.658, 95% confidence interval (CI): 6.059-9.678, p < 0.01]. In contrast, FH subjects had a lower odds for stroke following the generalization of statin therapy (OR = 0.251, 95% CI: 0.176-0.358, p < 0.01). CONCLUSIONS: Taking into account the small number of studies and methodological issues, HeFH was associated with a higher risk of cerebrovascular disease compared with the general population in the prestatin era, which was significantly reduced after the introduction of statin therapy.
URLPMID:21943799 [本文引用: 1]
DOI:10.1016/j.jacl.2015.08.002URL [本文引用: 1]
DOI:10.1194/jlr.R026658URL [本文引用: 4]

PCSK9 proprotein convertase subtilisin/kexin type (PCSK9) is a crucial protein in LDL cholesterol (LDL-C) metabolism by virtue of its pivotal role in the degradation of the LDL receptor. In recent years, both in vitro and in vivo studies have greatly supplemented our understanding of the (patho) physiological role of PCSK9 in human biology. In the current review, we summarize studies published or in print before May 2012 concerning the physiological role of PCSK9 in cholesterol metabolism. Moreover, we briefly describe the clinical phenotypes encountered in carriers of mutations in the gene encoding PCSK9. As PCSK9 has emerged as a novel target for LDL-C lowering therapy, methods to inhibit PCSK9 will also be reviewed. Initial data from investigations of PCSK9 inhibition in humans are promising and indicate that PCSK9 inhibition may be a viable new therapeutic option for the treatment of dyslipidemia and associated cardiovascular diseases.-Lambert, G., B. Sjouke, B. Choque, J. J. P. Kastelein, and G. K. Hovingh. The PCSK9 decade. J. Lipid Res. 2012. 53: 2515-2524.
DOI:10.1097/MOL.0000000000000114URLPMID:25110901 [本文引用: 2]

PURPOSE OF REVIEW: Proprotein convertase subtilisin/kexin type-9 (PCSK9) binds to LDL receptor (LDLR) and targets it for lysosomal degradation in cells. Decreased hepatic clearance of plasma LDL-cholesterol is the primary gauge of PCSK9 activity in humans; however, PCSK9's evolutionary role may extend to other lipoprotein classes and processes. This review highlights studies that are providing novel insights into physiological regulation of PCSK9 transcription and plasma PCSK9 activity. RECENT FINDINGS: Recent studies indicate that circulating PCSK9 binds to apolipoprotein B100 on LDL particles, which in turn inhibits PCSK9's ability to bind to cell surface LDLRs. Negative feedback of secreted PCSK9 activity by LDL could serve to increase plasma excursion of triglyceride-rich lipoproteins and monitor lipoprotein remodeling. Recent findings have identified hepatocyte nuclear factor-1alpha as a key transcriptional regulator that cooperates with sterol regulatory element-binding protein-2 to control PCSK9 expression in hepatocytes in response to nutritional and hormonal inputs, as well as acute inflammation. SUMMARY: PCSK9 is an established target for cholesterol-lowering therapies. Further study of PCSK9 regulatory mechanisms may identify additional control points for pharmacological inhibition of PCSK9-mediated LDLR degradation. PCSK9 function could reflect ancient roles in the fasting-feeding cycle and in linking lipoprotein metabolism with innate immunity.
DOI:10.1038/ng1161URLPMID:12730697 [本文引用: 2]

Autosomal dominant hypercholesterolemia (ADH; OMIM144400), a risk factor for coronary heart disease, is characterized by an increase in low-density lipoprotein cholesterol levels that is associated with mutations in the genes LDLR (encoding low-density lipoprotein receptor) or APOB (encoding apolipoprotein B). We mapped a third locus associated with ADH, HCHOLA3 at 1p32, and now report two mutations in the gene PCSK9 (encoding proprotein convertase subtilisin/kexin type 9) that cause ADH. PCSK9 encodes NARC-1 (neural apoptosis regulated convertase), a newly identified human subtilase that is highly expressed in the liver and contributes to cholesterol homeostasis.
DOI:10.1007/s00439-003-1071-9URLPMID:14727179 [本文引用: 2]

Familial hypercholesterolemia results from mutations in the low-density lipoprotein (LDL) receptor or apolipoprotein B genes. We have previously reported the identification of a Utah autosomal-dominant hypercholesterolemia pedigree (kindred 1173) that did not show linkage to either of these loci (Hunt et al. 2000). Expansion of the pedigree and increased marker density within the region of interest have resulted in a multipoint LOD score of 9.6 and enabled us to decrease the size of the linked region to approximately 7.5 Mbp. In addition, we were able to identify additional families sharing the same microsatellite haplotype. While all haplotype carriers in kindred 1173 (K1173) are affected, the haplotype carriers within the newly identified families are unaffected, suggesting that the causal mutation in K1173 had occurred after divergence of these pedigrees from a common ancestor. Mutation screening of genes in the region identified a single nucleotide variant (G-->T) present on the K1173 haplotype that was not present on the same haplotype in the other kindreds. This variant results in a D374Y missense change in the gene PCSK9.
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
DOI:10.1517/14728220802600715URL [本文引用: 1]
DOI:10.1073/pnas.70.10.2804URLPMID:4355366 [本文引用: 1]

The homozygous form of the autosomal dominant disorder, familial hypercholesterolemia, is characterized by the presence in children of profound hypercholesterolemia, cutaneous planar xanthomas, and rapidly progressive coronary vascular disease that usually results in death before age 30 years. Cultured skin fibroblasts from three unrelated subjects with this disorder showed 40- to 60-fold higher activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase (EC 1.1.1.34), the rate-controlling enzyme in cholesterol biosynthesis, when compared with fibroblasts of seven control subjects. Enhanced enzyme activity resulted from a complete absence of normal feedback suppression by low-density lipoproteins, which led to a marked overproduction of cholesterol by the mutant cells. The demonstration of apparently identical kinetic properties of the reductase activity of control and mutant cells, coupled with the evidence that this enzyme is normally regulated not by allosteric effectors but by alterations in enzyme synthesis and degradation, suggests that the primary genetic abnormality does not involve the structural gene for the enzyme itself, but a hitherto unidentified gene whose product is necessary for mediation of feedback control by lipoproteins. The fibroblasts of two obligate heterozygotes, the parents of one of the homozygotes, showed a pattern of enzyme regulation intermediate between that of controls and homozygotes.
URLPMID:4359767 [本文引用: 1]
DOI:10.1161/ATVBAHA.108.179564URLPMID:19299327 [本文引用: 3]

In this article, the history of the LDL receptor is recounted by its codiscoverers. Their early work on the LDL receptor explained a genetic cause of heart attacks and led to new ways of thinking about cholesterol metabolism. The LDL receptor discovery also introduced three general concepts to cell biology: receptor-mediated endocytosis, receptor recycling, and feedback regulation of receptors. The latter concept provides the mechanism by which statins selectively lower plasma LDL, reducing heart attacks and prolonging life.
[本文引用: 2]
[本文引用: 2]
DOI:10.1038/88556URL [本文引用: 1]
DOI:10.1016/s1357-4310(95)92412-4URLPMID:17607901 [本文引用: 1]

Familial hypercholesterolaemia is a co-dominant inherited disorder of lipoprotein metabolism, in which defects in the gene for the low-density-lipoprotein (LDL) receptor result in a twofold increase in the plasma concentration of cholesterol and moderate-to-severe premature coronary heart disease. Many mutations in the gene for the LDL receptor that have different effects on the structure and function of this multifunctional protein have been found, but it is not yet clear whether the nature of the mutation determines the severity of the disorder. This question is being answered by comparing patients with well-characterized mutations, and recent work suggests that other genetic or environmental factors may be important in modulating the effect of the defect in LDL-receptor function in patients who are heterozygous for the disorder.
[本文引用: 1]
[本文引用: 1]
DOI:10.1038/ncomms10961URL [本文引用: 1]
DOI:10.1161/CIRCRESAHA.117.312004URLPMID:29545368 [本文引用: 1]

RATIONALE: COMMD (copper metabolism MURR1 domain)-containing proteins are a part of the CCC (COMMD-CCDC22 [coiled-coil domain containing 22]-CCDC93 [coiled-coil domain containing 93]) complex facilitating endosomal trafficking of cell surface receptors. Hepatic COMMD1 inactivation decreases CCDC22 and CCDC93 protein levels, impairs the recycling of the LDLR (low-density lipoprotein receptor), and increases plasma low-density lipoprotein cholesterol levels in mice. However, whether any of the other COMMD members function similarly as COMMD1 and whether perturbation in the CCC complex promotes atherogenesis remain unclear. OBJECTIVE: The main aim of this study is to unravel the contribution of evolutionarily conserved COMMD proteins to plasma lipoprotein levels and atherogenesis. METHODS AND RESULTS: Using liver-specific Commd1, Commd6, or Commd9 knockout mice, we investigated the relation between the COMMD proteins in the regulation of plasma cholesterol levels. Combining biochemical and quantitative targeted proteomic approaches, we found that hepatic COMMD1, COMMD6, or COMMD9 deficiency resulted in massive reduction in the protein levels of all 10 COMMDs. This decrease in COMMD protein levels coincided with destabilizing of the core (CCDC22, CCDC93, and chromosome 16 open reading frame 62 [C16orf62]) of the CCC complex, reduced cell surface levels of LDLR and LRP1 (LDLR-related protein 1), followed by increased plasma low-density lipoprotein cholesterol levels. To assess the direct contribution of the CCC core in the regulation of plasma cholesterol levels, Ccdc22 was deleted in mouse livers via CRISPR/Cas9-mediated somatic gene editing. CCDC22 deficiency also destabilized the complete CCC complex and resulted in elevated plasma low-density lipoprotein cholesterol levels. Finally, we found that hepatic disruption of the CCC complex exacerbates dyslipidemia and atherosclerosis in ApoE3*Leiden mice. CONCLUSIONS: Collectively, these findings demonstrate a strong interrelationship between COMMD proteins and the core of the CCC complex in endosomal LDLR trafficking. Hepatic disruption of either of these CCC components causes hypercholesterolemia and exacerbates atherosclerosis. Our results indicate that not only COMMD1 but all other COMMDs and CCC components may be potential targets for modulating plasma lipid levels in humans.
DOI:10.1016/j.cca.2019.09.022URLPMID:31770510 [本文引用: 1]

The SREBP2/LDLR pathway is sensitive to cholesterol content in the endoplasmic reticulum (ER), while membrane low-density lipoprotein receptor (LDLR) is influenced by sterol response element binding protein 2 (SREBP2), pro-protein convertase subtilisin/kexin type 9 (PCSK9) and inducible degrader of LDLR (IDOL). LDL-C, one of the risk factors in cardiovascular disease, is cleared through endocytosis recycling of LDLR. Therefore, we propose that a balance between LDLR endocytosis recycling and PCSK9-mediated and IDOL-mediated lysosomal LDLR degradation is responsible for cholesterol homeostasis in the ER. For statins that decrease serum LDL-C levels via cholesterol synthesis inhibition, the mechanism by which the statins increase the membrane LDLR may be regulated by cholesterol homeostasis in the ER.
DOI:10.1186/s12929-016-0256-1URL [本文引用: 1]
DOI:10.1038/ncpcardio0836URLPMID:17380167 [本文引用: 1]

Familial hypercholesterolemia (FH) is characterized by raised serum LDL cholesterol levels, which result in excess deposition of cholesterol in tissues, leading to accelerated atherosclerosis and increased risk of premature coronary heart disease. FH results from defects in the hepatic uptake and degradation of LDL via the LDL-receptor pathway, commonly caused by a loss-of-function mutation in the LDL-receptor gene (LDLR) or by a mutation in the gene encoding apolipoprotein B (APOB). FH is primarily an autosomal dominant disorder with a gene-dosage effect. An autosomal recessive form of FH caused by loss-of-function mutations in LDLRAP1, which encodes a protein required for clathrin-mediated internalization of the LDL receptor by liver cells, has also been documented. The most recent addition to the database of genes in which defects cause FH is one encoding a member of the proprotein convertase family, PCSK9. Rare dominant gain-of-function mutations in PCSK9 cosegregate with hypercholesterolemia, and one mutation is associated with a particularly severe FH phenotype. Expression of PCSK9 normally downregulates the LDL-receptor pathway by indirectly causing degradation of LDL-receptor protein, and loss-of-function mutations in PCSK9 result in low plasma LDL levels. Thus, PCSK9 is an attractive target for new drugs aimed at lowering serum LDL cholesterol, which should have additive lipid-lowering effects to the statins currently used.
DOI:10.1042/CS20180190URL [本文引用: 1]
DOI:10.1016/j.atherosclerosis.2008.06.010URLPMID:18649882 [本文引用: 1]

The LDL receptor (LDLr) inhibitor Proprotein Convertase Subtilisin Kexin type 9 (PCSK9) has emerged as a genetically validated target for lowering plasma LDL cholesterol levels. In 2007, PCSK9 was found to act as a chaperone that binds the LDLr, thereby targeting it for lysosomal degradation. The enzymatic activity of PCSK9 is not involved in that process, but rather permits proper intramolecular processing of PCSK9. This was demonstrated by both site directed mutagenesis and independent reports of the PCSK9 crystal structure. These reports also elucidated the mode of action of several naturally occurring mutants of PCSK9 associated with hyper- or hypocholesterolemia. The present review summarizes studies published or in print before May 2008 investigating the functional significance of PCSK9 and its promising aspects as a prognostic tool and a drug target.
DOI:10.1056/NEJMoa054013URLPMID:16554528 [本文引用: 3]

BACKGROUND: A low plasma level of low-density lipoprotein (LDL) cholesterol is associated with reduced risk of coronary heart disease (CHD), but the effect of lifelong reductions in plasma LDL cholesterol is not known. We examined the effect of DNA-sequence variations that reduce plasma levels of LDL cholesterol on the incidence of coronary events in a large population. METHODS: We compared the incidence of CHD (myocardial infarction, fatal CHD, or coronary revascularization) over a 15-year interval in the Atherosclerosis Risk in Communities study according to the presence or absence of sequence variants in the proprotein convertase subtilisin/kexin type 9 serine protease gene (PCSK9) that are associated with reduced plasma levels of LDL cholesterol. RESULTS: Of the 3363 black subjects examined, 2.6 percent had nonsense mutations in PCSK9; these mutations were associated with a 28 percent reduction in mean LDL cholesterol and an 88 percent reduction in the risk of CHD (P=0.008 for the reduction; hazard ratio, 0.11; 95 percent confidence interval, 0.02 to 0.81; P=0.03). Of the 9524 white subjects examined, 3.2 percent had a sequence variation in PCSK9 that was associated with a 15 percent reduction in LDL cholesterol and a 47 percent reduction in the risk of CHD (hazard ratio, 0.50; 95 percent confidence interval, 0.32 to 0.79; P=0.003). CONCLUSIONS: These data indicate that moderate lifelong reduction in the plasma level of LDL cholesterol is associated with a substantial reduction in the incidence of coronary events, even in populations with a high prevalence of non-lipid-related cardiovascular risk factors.
DOI:10.3390/ijms20020245URL [本文引用: 1]
DOI:10.1073/pnas.0703402104URLPMID:17804797 [本文引用: 2]

Mutations in proprotein convertase subtilisin/kexin type 9 (PCSK9) are strongly associated with levels of low-density lipoprotein cholesterol in the blood plasma and, thereby, occurrence or resistance to atherosclerosis and coronary heart disease. Despite this importance, relatively little is known about the biology of PCSK9. Here, the crystal structure of a full-length construct of PCSK9 solved to 1.9-A resolution is presented. The structure contains a fully folded C-terminal cysteine-rich domain (CRD), showing a distinct structural similarity to the resistin homotrimer, a small cytokine associated with obesity and diabetes. This structural relationship between the CRD of PCSK9 and the resistin family is not observed in primary sequence comparisons and strongly suggests a distant evolutionary link between the two molecules. This three-dimensional homology provides insight into the function of PCSK9 at the molecular level and will help to dissect the link between PCSK9 and CHD.
DOI:10.1038/nsmb1235URLPMID:17435765 [本文引用: 1]

Proprotein convertase subtilisin kexin type 9 (PCSK9) lowers the abundance of surface low-density lipoprotein (LDL) receptor through an undefined mechanism. The structure of human PCSK9 shows the subtilisin-like catalytic site blocked by the prodomain in a noncovalent complex and inaccessible to exogenous ligands, and that the C-terminal domain has a novel fold. Biosensor studies show that PCSK9 binds the extracellular domain of LDL receptor with K(d) = 170 nM at the neutral pH of plasma, but with a K(d) as low as 1 nM at the acidic pH of endosomes. The D374Y gain-of-function mutant, associated with hypercholesterolemia and early-onset cardiovascular disease, binds the receptor 25 times more tightly than wild-type PCSK9 at neutral pH and remains exclusively in a high-affinity complex at the acidic pH. PCSK9 may diminish LDL receptors by a mechanism that requires direct binding but not necessarily receptor proteolysis.
DOI:10.1074/jbc.M409699200URLPMID:15358785 [本文引用: 3]

The discovery of autosomal dominant hypercholesterolemic patients with mutations in the PCSK9 gene, encoding the proprotein convertase NARC-1, resulting in the missense mutations suggested a role in low density lipoprotein (LDL) metabolism. We show that the endoplasmic reticulum-localized proNARC-1 to NARC-1 zymogen conversion is Ca2+-independent and that within the zymogen autocatalytic processing site SSVFAQ [downward arrow]SIP Val at P4 and Pro at P3' are critical. The S127R and D374Y mutations result in approximately 50-60% and > or =98% decrease in zymogen processing, respectively. In contrast, the double [D374Y + N157K], F216L, and R218S natural mutants resulted in normal zymogen processing. The cell surface LDL receptor (LDLR) levels are reduced by 35% in lymphoblasts of S127R patients. The LDLR levels are also reduced in stable HepG2 cells overexpressing NARC-1 or its natural mutant S127R, and this reduction is abrogated in the presence of 5 mm ammonium chloride, suggesting that overexpression of NARC-1 increases the turnover rate of the LDLR. Adenoviral expression of wild type human NARC-1 in mice resulted in a maximal approximately 9-fold increase in circulating LDL cholesterol, while in LDLR-/- mice a delayed approximately 2-fold increase in LDL cholesterol was observed. In conclusion, NARC-1 seems to affect both the level of LDLR and that of circulating apoB-containing lipoproteins in an LDLR-dependent and -independent fashion.
DOI:10.1016/j.abb.2003.09.011URLPMID:14622975 [本文引用: 1]

The NARC 1 gene encodes a novel proteinase K family proteinase. The domain structure of rat Narc 1 resembles that of the subtilisin-like proprotein convertases (SPCs), except that rNarc 1 lacks the canonical P-domain of SPCs, retaining only the RGD motif as part of what might be a cryptically functioning P-domain. Narc 1 undergoes autocatalytic intramolecular processing at the site LVFAQ/, resulting in the cleavage of its prosegment and the generation of an active proteinase with a broad alkaline pH optimum and no apparent calcium requirement for activity. Both primary and secondary structural determinants influence Narc 1 substrate recognition. Our functional characterization of Narc 1 reinforces the inference drawn from the analysis of its predicted structure that this enzyme is most closely related to representatives of the proteinase K family, but that it is also sufficiently different to warrant its possible classification in a separate sub-family.
DOI:10.1038/ng1509URLPMID:15654334 [本文引用: 2]

The low-density lipoprotein receptor (LDLR) prevents hypercholesterolemia and atherosclerosis by removing low-density lipoprotein (LDL) from circulation. Mutations in the genes encoding either LDLR or its ligand (APOB) cause severe hypercholesterolemia. Missense mutations in PCSK9, encoding a serine protease in the secretory pathway, also cause hypercholesterolemia. These mutations are probably gain-of-function mutations, as overexpression of PCSK9 in the liver of mice produces hypercholesterolemia by reducing LDLR number. To test whether loss-of-function mutations in PCSK9 have the opposite effect, we sequenced the coding region of PCSK9 in 128 subjects (50% African American) with low plasma levels of LDL and found two nonsense mutations (Y142X and C679X). These mutations were common in African Americans (combined frequency, 2%) but rare in European Americans (<0.1%) and were associated with a 40% reduction in plasma levels of LDL cholesterol. These data indicate that common sequence variations have large effects on plasma cholesterol levels in selected populations.
DOI:10.1194/jlr.M028563URL [本文引用: 1]

Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a secreted protein that promotes degradation of cell surface LDL receptors (LDLRs) in selected cell types. Here we used genetic and pharmacological inhibitors to define the pathways involved in PCSK9-mediated LDLR degradation. Inactivating mutations in autosomal recessive hypercholesterolemia (ARH), an endocytic adaptor, blocked PCSK9-mediated LDLR degradation in lymphocytes but not in fibroblasts. Thus, ARH is not specifically required for PCSK9-mediated LDLR degradation. Knockdown of clathrin heavy chain with siRNAs prevented LDLR degradation. In contrast, prevention of ubiquitination of the LDLR cyto-plasmic tail, inhibition of proteasomal activity, or disruption of proteins required for lysosomal targeting via macroautophagy (autophagy related 5 and 7) or the endosomal sorting complex required for trafficking (ESCRT) pathway (hepatocyte growth factor-regulated Tyr-kinase substrate and tumor suppressor gene 101) failed to block PCSK9-mediated LDLR degradation. These findings are consistent with a model in which the LDLR-PCSK9 complex is internalized via clathrin-mediated endocytosis and then routed to lysosomes via a mechanism that does not require ubiquitination and is distinct from the autophagy and proteosomal degradation pathways. Finally, the PCSK9-LDLR complex appears not to be transported by the canonical ESCRT pathway.-Wang, Y., Y. Huang, H. H. Hobbs, and J. C. Cohen. Molecular characterization of proprotein convertase subtilisin/kexin type 9-mediated degradation of the LDLR. J. Lipid Res. 2012. 53: 1932-1943.
DOI:10.1074/jbc.M410077200URLPMID:15385538 [本文引用: 1]

Lipid homeostasis is transcriptionally regulated by three DNA-binding proteins, designated sterol regulatory element-binding protein (SREBP)-1a, -1c, and -2. Oligonucleotide arrays hybridized with RNA made from livers of transgenic SREBP-1a, transgenic SREBP-2, and SREBP cleavage-activating protein knockout mice recently identified 33 genes regulated by SREBPs in liver, four of which had no known connection to lipid metabolism. One of the four genes was PCSK9, which encodes proprotein convertase subtilisin/kexin type 9a, a protein that belongs to the proteinase K subfamily of subtilases. Mutations in PCSK9 are associated with an autosomal dominant form of hypercholesterolemia. Here, we demonstrate that hepatic overexpression of either wild-type or mutant PCSK9 in mice results in hypercholesterolemia. The hypercholesterolemia is due to a post-transcriptional event causing a reduction in low density lipoprotein (LDL) receptor protein prior to the internalization and recycling of the receptor. Overexpression of PCSK9 in primary hepatocytes and in mice lacking the LDL receptor does not alter apolipoprotein B secretion. These data are consistent with PCSK9 affecting plasma LDL cholesterol levels by altering LDL receptor protein levels via a post-transcriptional mechanism.
DOI:10.1016/j.atherosclerosis.2007.07.022URLPMID:17765244 [本文引用: 3]

We analysed the Proprotein Convertase Subtilisin Kexin type 9 (PCSK9) exons and intronic junctions of 71 patients with familial hypercholesterolemia (FH) in whom LDL receptor (LDLR) or apolipoprotein B100 mutations were excluded. The previously reported S127R and R237W mutations were found in South African families, whereas new missense mutations D129G and A168E were found in families from New Zealand. Only, the S127R and D129G mutations modify a highly conserved residue and segregate with the FH phenotype. We overexpressed those mutants in hepatoma cells and found that both S127R and D129G have reduced autocatalytic activity compared with wild-type PCSK9, whereas the A168E mutant is processed normally. The S127R and D129G mutants were not secreted from cells, unlike the A168E mutant and wild-type PCSK9. By immunoblot, we showed that the expression of the LDLR was reduced by 40% in cells overexpressing wild-type or A168E PCSK9 and further reduced by 30% when the S127R or D129G mutants were used. Paralleling the LDLR levels, LDL cellular binding decreased by 25% upon wild-type PCSK9 or A168E overexpression, and by 45% with both S127R and D129G mutants. Our study therefore indicates that PCSK9 mediated inhibition of the LDLR does not require PCSK9 autocatalytic cleavage or secretion, suggesting that PCSK9 may also function intracellularly.
DOI:10.1074/jbc.M109.037085URLPMID:19635789 [本文引用: 2]

Elevated levels of plasma low density lipoprotein (LDL)-cholesterol, leading to familial hypercholesterolemia, are enhanced by mutations in at least three major genes, the LDL receptor (LDLR), its ligand apolipoprotein B, and the proprotein convertase PCSK9. Single point mutations in PCSK9 are associated with either hyper- or hypocholesterolemia. Accordingly, PCSK9 is an attractive target for treatment of dyslipidemia. PCSK9 binds the epidermal growth factor domain A (EGF-A) of the LDLR and directs it to endosomes/lysosomes for destruction. Although the mechanism by which PCSK9 regulates LDLR degradation is not fully resolved, it seems to involve both intracellular and extracellular pathways. Here, we show that clathrin light chain small interfering RNAs that block intracellular trafficking from the trans-Golgi network to lysosomes rapidly increased LDLR levels within HepG2 cells in a PCSK9-dependent fashion without affecting the ability of exogenous PCSK9 to enhance LDLR degradation. In contrast, blocking the extracellular LDLR endocytosis/degradation pathway by a 4-, 6-, or 24-h incubation of cells with Dynasore or an EGF-AB peptide or by knockdown of endogenous autosomal recessive hypercholesterolemia did not significantly affect LDLR levels. The present data from HepG2 cells and mouse primary hepatocytes favor a model whereby depending on the dose and/or incubation period, endogenous PCSK9 enhances the degradation of the LDLR both extra- and intracellularly. Therefore, targeting either pathway, or both, would be an effective method to reduce PCSK9 activity in the treatment of hypercholesterolemia and coronary heart disease.
DOI:10.1172/JCI29383URLPMID:17080197 [本文引用: 1]

Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a member of the proteinase K subfamily of subtilases that reduces the number of LDL receptors (LDLRs) in liver through an undefined posttranscriptional mechanism. We show that purified PCSK9 added to the medium of HepG2 cells reduces the number of cell-surface LDLRs in a dose- and time-dependent manner. This activity was approximately 10-fold greater for a gain-of-function mutant, PCSK9(D374Y), that causes hypercholesterolemia. Binding and uptake of PCSK9 were largely dependent on the presence of LDLRs. Coimmunoprecipitation and ligand blotting studies indicated that PCSK9 and LDLR directly associate; both proteins colocalized to late endocytic compartments. Purified PCSK9 had no effect on cell-surface LDLRs in hepatocytes lacking autosomal recessive hypercholesterolemia (ARH), an adaptor protein required for endocytosis of the receptor. Transgenic mice overexpressing human PCSK9 in liver secreted large amounts of the protein into plasma, which increased plasma LDL cholesterol concentrations to levels similar to those of LDLR-knockout mice. To determine whether PCSK9 was active in plasma, transgenic PCSK9 mice were parabiosed with wild-type littermates. After parabiosis, secreted PCSK9 was transferred to the circulation of wild-type mice and reduced the number of hepatic LDLRs to nearly undetectable levels. We conclude that secreted PCSK9 associates with the LDLR and reduces hepatic LDLR protein levels.
DOI:10.1194/jlr.M800027-JLR200URLPMID:18354138 [本文引用: 2]

Proprotein convertase subtilisin/kexin type 9 (PCSK9) is a secreted protein that regulates the expression of LDL receptor (LDLR) protein. Gain-of-function mutations in PCSK9 cause hypercholesterolemia, and loss-of-function mutations result in lower plasma LDL-cholesterol. Here, we investigate the kinetics and metabolism of circulating PCSK9 relative to tissue levels of LDLRs. The administration of recombinant human PCSK9 (32 microg) to mice by a single injection reduced hepatic LDLRs by approximately 90% within 60 min, and the receptor levels returned to normal within 6 h. The half-life of the PCSK9 was estimated to be approximately 5 min. Continuous infusion of PCSK9 (32 microg/h) into wild-type mice caused a approximately 90% reduction in hepatic LDLRs within 2 h and no associated change in the level of LDLR in the adrenals. Parallel studies were performed using a catalytically inactive form of PCSK9, PCSK9(S386A), and similar results were obtained. Infusion of PCSK9(D374Y), a gain-of-function mutation, resulted in accelerated clearance of the mutant PCSK9 and a greater reduction in hepatic LDLRs. Combined, these data suggest that exogenously administrated PCSK9 in plasma preferentially reduces LDLR protein levels in liver at concentrations found in human plasma and that PCSK9's action on the LDLR is not dependent on catalytic activity in vivo.
DOI:10.1016/j.drudis.2017.01.006URLPMID:28111330 [本文引用: 1]

Diabetes mellitus (DM) is associated with an increased risk of cardiovascular disease (CVD). Inhibitors of proprotein convertase subtilisin/kexin type 9 (PCSK9) have emerged as effective low-density lipoprotein cholesterol-lowering compounds. Although the results of available epidemiological, preclinical, and clinical studies suggest a positive association of plasma PCSK9 levels with glycemic parameters and risk of type 2 DM (T2DM), genetic findings have shown contradictory results. Overall, the impact of PCSK9 inhibitors on glycemic control parameters in patients with DM remains unclear. Here, we assess the available evidence for the association of PCSK9 status with the incidence and control of DM in preclinical and clinical studies, and identify molecular mechanisms regulating PCSK9 expression in the diabetic state.
URLPMID:19001363 [本文引用: 4]
DOI:10.1038/embor.2011.205URL [本文引用: 2]

The protein PCSK9 (proprotein convertase subtilisin/kexin type 9) is a key regulator of low-density lipoprotein receptor (LDLR) levels and cardiovascular health. We have determined the crystal structure of LDLR bound to PCSK9 at neutral pH. The structure shows LDLR in a new extended conformation. The PCSK9 C-terminal domain is solvent exposed, enabling cofactor binding, whereas the catalytic domain and prodomain interact with LDLR epidermal growth factor(A) and beta-propeller domains, respectively. Thus, PCSK9 seems to hold LDLR in an extended conformation and to interfere with conformational rearrangements required for LDLR recycling.
DOI:10.1074/jbc.M701634200URLPMID:17493938 [本文引用: 3]

Mutations within PCSK9 (proprotein convertase subtilisin/kexin type 9) are associated with dominant forms of familial hyper- and hypocholesterolemia. Although PCSK9 controls low density lipoprotein (LDL) receptor (LDLR) levels post-transcriptionally, several questions concerning its mode of action remain unanswered. We show that purified PCSK9 protein added to the medium of human endothelial kidney 293, HepG2, and Chinese hamster ovary cell lines decreases cellular LDL uptake in a dose-dependent manner. Using this cell-based assay of PCSK9 activity, we found that the relative potencies of several PCSK9 missense mutants (S127R and D374Y, associated with hypercholesterolemia, and R46L, associated with hypocholesterolemia) correlate with LDL cholesterol levels in humans carrying such mutations. Notably, we found that in vitro wild-type PCSK9 binds LDLR with an approximately 150-fold higher affinity at an acidic endosomal pH (K(D) = 4.19 nm) compared with a neutral pH (K(D) = 628 nm). We also demonstrate that wild-type PCSK9 and mutants S127R and R46L are internalized by cells to similar levels, whereas D374Y is more efficiently internalized, consistent with their affinities for LDLR at neutral pH. Finally, we show that LDL diminishes PCSK9 binding to LDLR in vitro and partially inhibits the effects of secreted PCSK9 on LDLR degradation in cell culture. Together, the results of our biochemical and cell-based experiments suggest a model in which secreted PCSK9 binds to LDLR and directs the trafficking of LDLR to the lysosomes for degradation.
DOI:10.1016/j.atherosclerosis.2014.08.038URL [本文引用: 1]

Objective: This article reviews the mechanism by which the low density lipoprotein receptor (LDLR) that has bound proprotein convertase subtilisin/kexin type 9 (PCSK9), is rerouted to intracellular degradation instead of being recycled.
Methods: A search of relevant published literature has been conducted.
Results: PCSK9 binds to the LDLR at the cell surface. It is the catalytic domain of PCSK9 that binds to the epidermal growth factor repeat A of the LDLR. The LDLR: PCSK9 complex is internalized through clathrin-mediated endocytosis. Due to an additional electrostatic interaction at acidic pH between the C-terminal domain of PCSK9 and the ligand-binding domain of the LDLR, PCSK9 remains bound to the LDLR in the sorting endosome. As a consequence, the LDLR fails to adopt a closed conformation and is degraded instead of being recycled. The mechanism for the failure of the LDLR to recycle appears to involve ectodomain cleavage of the extended LDLR by a cysteine cathepsin in the sorting endosome. The cleaved LDLR ectodomain will be confined to the vesicular part of the sorting endosome for degradation in the endosomal/lysosomal tract.
Conclusion: Ectodomain cleavage of an LDLR with bound PCSK9 in the sorting endosome disrupts the normal recycling of the LDLR. (C) 2014 Elsevier Ireland Ltd.
DOI:10.1007/s00395-015-0464-yURLPMID:25589055 [本文引用: 1]

A high proportion of primary percutaneous coronary interventions performed in the setting of acute myocardial infarction, concur with inadequate myocardial perfusion at the microvascular level. This phenomenon, known as
DOI:10.1016/j.tibs.2006.12.008URLPMID:17215125 [本文引用: 1]

Proprotein convertase subtilisin-like kexin type 9 (PCSK9) is a newly discovered serine protease that destroys low density lipoprotein (LDL) receptors in liver and thereby controls the level of LDL in plasma. Mutations that increase PCSK9 activity cause hypercholesterolemia and coronary heart disease (CHD); mutations that inactivate PCSK9 have the opposite effect, lowering LDL levels and reducing CHD. Although the mechanism of PCSK9 action is not yet clear, the protease provides a new therapeutic target to lower plasma levels of LDL and prevent CHD.
DOI:10.1074/jbc.M110.113035URLPMID:20172854 [本文引用: 1]

PCSK9 binds to the low density lipoprotein receptor (LDLR) and leads to LDLR degradation and inhibition of plasma LDL cholesterol clearance. Consequently, the role of PCSK9 in modulating circulating LDL makes it a promising therapeutic target for treating hypercholesterolemia and coronary heart disease. Although the C-terminal domain of PCSK9 is not involved in LDLR binding, the location of several naturally occurring mutations within this region suggests that it has an important role for PCSK9 function. Using a phage display library, we identified an anti-PCSK9 Fab (fragment antigen binding), 1G08, with subnanomolar affinity for PCSK9. In an assay measuring LDL uptake in HEK293 and HepG2 cells, 1G08 Fab reduced 50% the PCSK9-dependent inhibitory effects on LDL uptake. Importantly, we found that 1G08 did not affect the PCSK9-LDLR interaction but inhibited the internalization of PCSK9 in these cells. Furthermore, proteolysis and site-directed mutagenesis studies demonstrated that 1G08 Fab binds a region of beta-strands encompassing Arg-549, Arg-580, Arg-582, Glu-607, Lys-609, and Glu-612 in the PCSK9 C-terminal domain. Consistent with these results, 1G08 fails to bind PCSK9DeltaC, a truncated form of PCSK9 lacking the C-terminal domain. Additional studies revealed that lack of the C-terminal domain compromised the ability of PCSK9 to internalize into cells, and to inhibit LDL uptake. Together, the present study demonstrate that the PCSK9 C-terminal domain contribute to its inhibition of LDLR function mainly through its role in the cellular uptake of PCSK9 and LDLR complex. 1G08 Fab represents a useful new tool for delineating the mechanism of PCSK9 uptake and LDLR degradation.
DOI:10.1186/1471-2121-8-9URL [本文引用: 1]
DOI:10.1016/j.celrep.2015.11.006URLPMID:26628375 [本文引用: 1]

Clearance of circulating low-density lipoprotein cholesterol (LDLc) by hepatic LDL receptors (LDLR) is central for vascular health. Secreted by hepatocytes, PCSK9 induces the degradation of LDLR, resulting in higher plasma LDLc levels. Still, it remains unknown why LDLR and PCSK9 co-exist within the secretory pathway of hepatocytes without leading to complete degradation of LDLR. Herein, we identified the ER-resident GRP94, and more precisely its client-binding C-terminal domain, as a PCSK9-LDLR inhibitory binding protein. Depletion of GRP94 did not affect calcium homeostasis, induce ER stress, nor did it alter PCSK9 processing or its secretion but greatly increased its capacity to induce LDLR degradation. Accordingly, we found that hepatocyte-specific Grp94-deficient mice have higher plasma LDLc levels correlated with approximately 80% reduction in hepatic LDLR protein levels. Thus, we provide evidence that, in physiological conditions, binding of PCSK9 to GRP94 protects LDLR from degradation likely by preventing early binding of PCSK9 to LDLR within the ER.
DOI:10.1194/jlr.M014118URL [本文引用: 1]

Proprotein convertase subtilisin/kexin type 9 (PCSK9) plays a major role in cholesterol homeostasis through enhanced degradation of the LDL receptor (LDLR) in liver. As novel inhibitors/silencers of PCSK9 are now being tested in clinical trials to treat hypercholesterolemia, it is crucial to define the physiological consequences of the lack of PCSK9 in various organs. LDLR regulation by PCSK9 has not been extensively described during mouse brain development and injury. Herein, we show that PCSK9 and LDLR are co-expressed in mouse brain during development and at adulthood. Although the protein levels of LDLR and apolipoprotein E (apoE) in the adult brain of Pcsk9(-/-) mice are similar to those of wild-type (WT) mice, LDLR levels increased and were accompanied by a reduction of apoE levels during development. This suggests that the upregulation of LDLR protein levels in Pcsk9(-/-) mice enhances apoE degradation. Upon ischemic stroke, PCSK9 was expressed in the dentate gyrus between 24 h and 72 h following brain reperfusion. Although mouse behavior and lesion volume were similar, LDLR protein levels dropped similar to 2-fold less in the Pcsk9(-/-) -lesioned hippocampus, without affecting apoE levels and neurogenesis. Thus, PCSK9 downregulates LDLR levels during brain development and following transient ischemic stroke in adult mice.-Rousselet, E., J. Marcinkiewicz, J. Kriz, A. Zhou, M. E. Hatten, A. Prat, and N. G. Seidah. PCSK9 reduces the protein levels of the LDL receptor in mouse brain during development and after ischemic stroke. J. Lipid Res. 2011. 52: 1383-1391.
DOI:10.1097/MOL.0b013e3281338531URLPMID:17495605 [本文引用: 1]

PURPOSE OF REVIEW: Proprotein convertase subtilisin kexin type 9 (PCSK9) has emerged as a potential target for lowering plasma LDL cholesterol levels. This review summarizes recent studies published in print or online before January 2007 which have investigated the functional significance of this intriguing protease. RECENT FINDINGS: Increasing interest in PCSK9 has given rise to landmark epidemiological studies, the generation of animal models, the discovery of new human mutations, as well as numerous in-vitro studies. These studies have helped to unravel the molecular functions of PCSK9. SUMMARY: Mutations of PCSK9 are associated either with hypercholesterolemia or with hypocholesterolemia. In the latter case, the incidence of coronary heart disease is reduced, thereby demonstrating that low LDL cholesterol levels from birth are highly beneficial. PCSK9 promotes the degradation of the LDL receptor in hepatocytes apparently both intracellularly and by being a secreted protein that can bind the LDL receptor and be internalized. By virtue of its role as a major inhibitor of the LDL receptor, PCSK9 is a promising therapeutic target. Specific PCSK9 pharmacological inhibitors may prove to be useful in amplifying the well documented benefits of statins.
DOI:10.1194/jlr.R800091-JLR200URL [本文引用: 1]
DOI:10.1016/j.str.2007.04.004URL [本文引用: 1]

Summary
Proprotein convertase subtilisin kexin type 9 (PCSK9) has been shown to be involved in the regulation of extracellular levels of the low-density lipoprotien receptor (LDLR). Although PCSK9 is a subtilase, it has not been shown to degrade the LDLR, and its LDLR-lowering mechanism remains uncertain. Here we report the crystal structure of human PCSK9 at 2.3 Å resolution. PCSK9 has subtilisin-like pro- and catalytic domains, and the stable interaction between these domains prevents access to PCSK9's catalytic site. The C-terminal domain of PCSK9 has a novel protein fold and may mediate protein-protein interactions. The structure of PCSK9 provides insight into its biochemical characteristics and biological function.DOI:10.1016/j.bbrc.2011.10.110URLPMID:22074827 [本文引用: 2]

The proprotein convertases subtilisin kexin 9 (PCSK9) binds to the epidermal growth factor domain A (EGF-A) of low-density lipoprotein receptor (LDLR) and leads to its destruction. However, the intracellular processes leading to LDLR degradation have not been fully delineated. In this report, we show that PCSK9 treatment can lead to ubiquitination of LDLR, which was enhanced in the presence of proteasome inhibitor MG132. Furthermore, LDLR protein carrying mutations in the C-terminal ubiquitination sites was resistant to PCSK9-mediated degradation. Our data suggest that the ubiquitination system is involved in PCSK9-induced LDLR degradation.
DOI:10.1016/S1877-1173(10)93010-XURLPMID:20807647 [本文引用: 1]

Hypertriglyceridemia, characterized by the accumulation of triglyceride-rich lipoproteins in the blood, affects 10-20% of the population in western countries and increases the risk of atherosclerosis, coronary artery disease, and pancreatitis. The etiology of hypertriglyceridemia is complex, and much interest exists in identifying and characterizing the biological and environmental factors that affect the synthesis and turnover of plasma triglycerides. Genetic studies in mice have recently identified that heparan sulfate proteoglycans are a class of receptors that mediate the clearance of triglyceride-rich lipoproteins in the liver. Heparan sulfate proteoglycans are expressed by endothelial cells that line the hepatic sinusoids and the underlying hepatocytes, and are present in the perisinusoidal space (space of Disse). This chapter discusses the dependence of lipoprotein binding on heparan sulfate structure and the identification of hepatocyte syndecan-1 as the primary proteoglycan that mediates triglyceride-rich lipoprotein clearance.
URLPMID:28894089 [本文引用: 2]
DOI:10.1016/j.metabol.2019.02.005URLPMID:30831144 [本文引用: 2]

OBJECTIVE: Low-density lipoprotein cholesterol (LDL-C) is the hallmark of atherosclerotic cardiovascular diseases. The hepatic LDL receptor (LDLR) plays an important role in clearance of circulating LDL-C. PCSK9 facilitates degradation of LDLR in the lysosome and antagonizing PCSK9 has been successfully used in the clinic to reduce blood LDL-C level. Here we identify a new player that modulates LDLR interaction with PCSK9, thus controlling LDLR degradation and cholesterol homeostasis. METHODS: The blood LDL-C and cholesterol levels were analyzed in mice with hepatic deletion of Paqr3 gene. The half-life of LDLR was analyzed in HepG2 cells. The interaction of PAQR3 with LDLR and PCSK9 was analyzed by co-immunoprecipitation and immunofluorescent staining. RESULTS: The blood LDL-C and total cholesterol levels in the mice with hepatic deletion of Paqr3 gene were significantly lower than the control mice after feeding with high-fat diet (p<0.001 and p<0.05 respectively). The steady-state level of LDLR protein is elevated by Paqr3 knockdown/deletion and reduced by PAQR3 overexpression. The half-life of LDLR protein is increased by Paqr3 knockdown and accelerated by PAQR3 overexpression. PAQR3 interacts with the beta-sheet domain of LDLR and the P-domain of PCSK9 respectively. In addition, PAQR3 can be localized in early endosomes and colocalized with LDLR, PCSK9 and LDL. Mechanistically, PAQR3 enhances the interaction between LDLR and PCSK9. CONCLUSION: Our study reveals that PAQR3 plays a pivotal role in controlling hepatic LDLR degradation and blood LDL-C level via modulating LDLR-PCSK9 interaction.
DOI:10.1016/j.canlet.2017.07.015URLPMID:28743532 [本文引用: 1]

Activation of class I Phosphoinositide 3-kinases (PI3Ks) by mutation or overexpression closely correlates with the development of various human cancers. Class I PI3Ks are heterodimers composed of p110 catalytic subunits and regulatory subunits represented by p85. PAQR3 has been found to inhibit p110alpha activity by blocking its interaction with p85. In this study, we identified the N-terminal 6-55 amino acid residues of PAQR3 being sufficient for its interaction with p110alpha. A synthetic peptide, P6-55, that contains the N-terminus of PAQR3 could disrupt the interactions of p110alpha with both PAQR3 and p85. The activity of PI3K was also inhibited by P6-55, accompanied by significant inhibition of cancer cell proliferation. In a xenograft mouse model, P6-55 was able to reduce tumor growth in vivo. Furthermore, P6-55 was capable of inhibiting the elevated basal PI3K activity of H1047R, a hotspot mutation found in many types of human cancers. The cell proliferation and migration of cancer cells bearing H1047R mutation were also reduced by P6-55. In conclusion, our study provides a proof of concept that blocking the interaction of p110alpha with p85 by a peptide can serve as a new strategy to inhibit the oncogenic activity of PI3K in cancer therapy.
URLPMID:12552133 [本文引用: 2]
DOI:10.1111/j.1742-4658.2008.06495.xURLPMID:18498363 [本文引用: 2]

Proprotein convertase subtilisin/kexin 9 (PCSK9) is a secreted glycoprotein that regulates the degradation of the low-density lipoprotein receptor. Single nucleotide polymorphisms in its gene associate with both hypercholesterolemia and hypocholesterolemia, and studies have shown a significant reduction in the risk of coronary heart disease for 'loss-of-function' PCSK9 carriers. Previously, we reported that proPCSK9 undergoes autocatalytic processing of its prodomain in the endoplasmic reticulum and that its inhibitory prosegment remains associated with it following secretion. Herein, we used a combination of mass spectrometry and radiolabeling to report that PCSK9 is phosphorylated at two sites: Ser47 in its propeptide and Ser688 in its C-terminal domain. Site-directed mutagenesis suggested that a Golgi casein kinase-like kinase is responsible for PCSK9 phosphorylation, based on the consensus site, SXE/S(p). PCSK9 phosphorylation was cell-type specific and occurs physiologically because human plasma PCSK9 is phosphorylated. Interestingly, we show that the naturally occurring 'loss-of-function' variant PCSK9(R46L) exhibits significantly decreased propeptide phosphorylation in the Huh7 liver cell line by 34% (P < 0.0001). PCSK9(R46L) and the engineered, unphosphorylated variant PCSK9(E49A) are cleaved following Ser47, suggesting that phosphorylation protects the propeptide against proteolysis. Phosphorylation may therefore play an important regulatory role in PCSK9 function. These findings will be important for the future design of PCSK9 inhibitors.
DOI:10.1161/ATVBAHA.119.313247URLPMID:31553664 [本文引用: 2]

OBJECTIVE: PCSK9 (proprotein convertase subtilisin-kexin 9) enhances the degradation of the LDLR (low-density lipoprotein receptor) in endosomes/lysosomes. This study aimed to determine the sites of PCSK9 phosphorylation at Ser-residues and the consequences of such posttranslational modification on the secretion and activity of PCSK9 on the LDLR. Approach and Results: Fam20C (family with sequence similarity 20, member C) phosphorylates serines in secretory proteins containing the motif S-X-E/phospho-Ser, including the cholesterol-regulating PCSK9. In situ hybridization of Fam20C mRNA during development and in adult mice revealed a wide tissue distribution, including liver, but not small intestine. Here, we show that Fam20C phosphorylates PCSK9 at Serines 47, 666, 668, and 688. In hepatocytes, phosphorylation enhances PCSK9 secretion and maximizes its induced degradation of the LDLR via the extracellular and intracellular pathways. Replacing any of the 4 Ser by the phosphomimetic Glu or Asp enhanced PCSK9 activity only when the other sites are phosphorylated, whereas Ala substitutions reduced it, as evidenced by Western blotting, Elisa, and LDLR-immunolabeling. This newly uncovered PCSK9/LDLR regulation mechanism refines our understanding of the implication of global PCSK9 phosphorylation in the modulation of LDL-cholesterol and rationalizes the consequence of natural mutations, for example, S668R and E670G. Finally, the relationship of Ser-phosphorylation to the implication of PCSK9 in regulating LDL-cholesterol in the neurological Fragile X-syndrome disorder was investigated. CONCLUSIONS: Ser-phosphorylation of PCSK9 maximizes both its secretion and activity on the LDLR. Mass spectrometric approaches to measure such modifications were developed and applied to quantify the levels of bioactive PCSK9 in human plasma under normal and pathological conditions.
URLPMID:32639091 [本文引用: 4]

Overly active acyl-coenzyme A: cholesterol acyltransferases (ACATs) are known to contribute to the development of atherosclerosis, cancer cell proliferation and de novo lipogenesis. However, the role of ACAT in systemic lipid metabolism and its consequence of aging is unknown. Using avasimibe, a clinically proven ACAT inhibitor, and mboa-1 mutant strain, a homologous to mammalian ACAT, herein, we found that Ava treatment and mboa-1 mutant exhibited a decreased fat accumulation during feeding and increased lipolysis with extended lifespan of C. elegans during fasting. Our study highlights the essential role of ACAT inhibitor and mboa-1 in fat mobilization and the survival of C. elegans in fasting through the modulation of the genes involved in lipolysis and insulin/IGF-1 signaling.
URLPMID:15118091 [本文引用: 1]
DOI:10.1093/cvr/cvv178URL [本文引用: 1]
DOI:10.1007/s00018-012-0977-6URL [本文引用: 1]

The secreted protease proprotein convertase subtilisin/kexin type 9 (PCSK9) binds to low-density lipid (LDL) receptor family members LDLR, very low density lipoprotein receptor (VLDLR) and apolipoprotein receptor 2 (ApoER2), and promotes their degradation in intracellular acidic compartments. In the liver, LDLR is a major controller of blood LDL levels, whereas VLDLR and ApoER2 in the brain mediate Reelin signaling, a critical pathway for proper development of the nervous system. Expression level of PCSK9 in the brain is highest in the cerebellum during perinatal development, but is also increased in the adult brain after ischemia. The mechanism of PCSK9 function and its involvement in neuronal apoptosis is poorly understood. We show here that RNAi-mediated knockdown of PCSK9 significantly reduced the death of potassium-deprived cerebellar granule neurons (CGN), as shown by reduced levels of nuclear phosphorylated c-Jun and activated caspase-3, as well as condensed apoptotic nuclei. ApoER2 protein levels were increased in PCSK9 RNAi cells. Knockdown of ApoER2 but not of VLDLR was sufficient to reverse the protection provided by PCSK9 RNAi, suggesting that proapoptotic signaling of PCSK9 is mediated by altered ApoER2 function. Pharmacological inhibition of signaling pathways associated with lipoprotein receptors suggested that PCSK9 regulates neuronal apoptosis independently of NMDA receptor function but in concert with ERK and JNK signaling pathways. PCSK9 RNAi also reduced staurosporine-induced CGN apoptosis and axonal degeneration in the nerve growth factor-deprived dorsal root ganglion neurons. We conclude that PCSK9 potentiates neuronal apoptosis via modulation of ApoER2 levels and related anti-apoptotic signaling pathways.
DOI:10.1371/journal.pone.0064145URLPMID:23675525 [本文引用: 1]

Elevated LDL-cholesterol (LDLc) levels are a major risk factor for cardiovascular disease and atherosclerosis. LDLc is cleared from circulation by the LDL receptor (LDLR). Proprotein convertase subtilisin/kexin 9 (PCSK9) enhances the degradation of the LDLR in endosomes/lysosomes, resulting in increased circulating LDLc. PCSK9 can also mediate the degradation of LDLR lacking its cytosolic tail, suggesting the presence of as yet undefined lysosomal-targeting factor(s). Herein, we confirm this, and also eliminate a role for the transmembrane-domain of the LDLR in mediating its PCSK9-induced internalization and degradation. Recent findings from our laboratory also suggest a role for PCSK9 in enhancing tumor metastasis. We show herein that while the LDLR is insensitive to PCSK9 in murine B16F1 melanoma cells, PCSK9 is able to induce degradation of the low density lipoprotein receptor-related protein 1 (LRP-1), suggesting distinct targeting mechanisms for these receptors. Furthermore, PCSK9 is still capable of acting upon the LDLR in CHO 13-5-1 cells lacking LRP-1. Conversely, PCSK9 also acts on LRP-1 in the absence of the LDLR in CHO-A7 cells, where re-introduction of the LDLR leads to reduced PCSK9-mediated degradation of LRP-1. Thus, while PCSK9 is capable of inducing degradation of LRP-1, the latter is not an essential factor for LDLR regulation, but the LDLR effectively competes with LRP-1 for PCSK9 activity. Identification of PCSK9 targets should allow a better understanding of the consequences of PCSK9 inhibition for lowering LDLc and tumor metastasis.
DOI:10.1016/j.bbrc.2008.07.106URL [本文引用: 1]
DOI:10.1161/ATVBAHA.115.306032URLPMID:26494228 [本文引用: 1]

OBJECTIVE: Proprotein convertase subtilisin/kexin type 9 (PCSK9) promotes the degradation of the low-density lipoprotein receptor thereby elevating plasma low-density lipoprotein cholesterol levels and the risk of coronary heart disease. Thus, the use of PCSK9 inhibitors holds great promise to prevent heart disease. Previous work found that PCSK9 is involved in triglyceride metabolism, independently of its action on low-density lipoprotein receptor, and that other yet unidentified receptors could mediate this effect. Therefore, we assessed whether PCSK9 enhances the degradation of CD36, a major receptor involved in transport of long-chain fatty acids and triglyceride storage. APPROACH AND RESULTS: Overexpressed or recombinant PCSK9 induced CD36 degradation in cell lines and primary adipocytes and reduced the uptake of the palmitate analog Bodipy FL C16 and oxidized low-density lipoprotein in 3T3-L1 adipocytes and hepatic HepG2 cells, respectively. Surface plasmon resonance, coimmunoprecipitation, confocal immunofluorescence microscopy, and protein degradation pathway inhibitors revealed that PCSK9 directly interacts with CD36 and targets the receptor to lysosomes through a mechanism involving the proteasome. Importantly, the level of CD36 protein was increased by >3-fold upon small interfering RNA knockdown of endogenous PCSK9 in hepatic cells and similarly increased in the liver and visceral adipose tissue of Pcsk9(-/-) mice. In Pcsk9(-/-) mice, increased hepatic CD36 was correlated with an amplified uptake of fatty acid and accumulation of triglycerides and lipid droplets. CONCLUSIONS: Our results demonstrate an important role of PCSK9 in modulating the function of CD36 and triglyceride metabolism. PCSK9-mediated CD36 degradation may serve to limit fatty acid uptake and triglyceride accumulation in tissues, such as the liver.
DOI:10.1002/hep.22911URLPMID:19489072 [本文引用: 1]

UNLABELLED: Human PCSK9 is known to enhance the degradation of membrane-bound receptors such as the hepatocyte low-density lipoprotein receptor (LDLR), ApoER2, and very low-density lipoprotein receptor. Because the LDLR is suspected to be involved in hepatitis C virus (HCV) entry, we also tested whether PCSK9 can affect the levels of CD81, a major HCV receptor. Interestingly, stable expression of PCSK9 or a more active membrane-bound form of the protein (PCSK9-ACE2) resulted in a marked reduction in CD81 and LDLR expression. Therefore, we analyzed the antiviral effect of PCSK9 in vitro using the HCV genotype 2a (JFH1) virus. The results clearly demonstrated that cells expressing PCSK9 or PCSK9-ACE2, but not the ACE2 control protein, were resistant to HCV infection. Furthermore, addition of purified soluble PCSK9 to cell culture supernatant impeded HCV infection in a dose-dependent manner. As expected, HuH7 cells expressing PCSK9-ACE2 were also resistant to infection by HCV pseudoparticles. In addition, we showed that CD81 cell surface expression is modulated by PCSK9 in an LDLR-independent manner. Finally, in the liver of single Pcsk9 and double (Pcsk9 + Ldlr) knockout mice, both LDLR and/or CD81 protein expression levels were significantly reduced, but not those of transferrin and scavenger receptor class B type 1. CONCLUSION: Our results demonstrate an antiviral effect of the circulating liver PCSK9 on HCV in cells and show that PCSK9 down-regulates the level of mouse liver CD81 expression in vivo. Therefore, we propose that the plasma level and/or activity of PCSK9 may modulate HCV infectivity in humans.
DOI:10.1074/jbc.M112.363382URLPMID:22493497 [本文引用: 2]

The epithelial Na(+) channel (ENaC) is critical for Na(+) homeostasis and blood pressure control. Defects in its regulation cause inherited forms of hypertension and hypotension. Previous work found that ENaC gating is regulated by proteases through cleavage of the extracellular domains of the alpha and gamma subunits. Here we tested the hypothesis that ENaC is regulated by proprotein convertase subtilisin/kexin type 9 (PCSK9), a protease that modulates the risk of cardiovascular disease. PCSK9 reduced ENaC current in Xenopus oocytes and in epithelia. This occurred through a decrease in ENaC protein at the cell surface and in the total cellular pool, an effect that did not require the catalytic activity of PCSK9. PCSK9 interacted with all three ENaC subunits and decreased their trafficking to the cell surface by increasing proteasomal degradation. In contrast to its previously reported effects on the LDL receptor, PCSK9 did not alter ENaC endocytosis or degradation of the pool of ENaC at the cell surface. These results support a role for PCSK9 in the regulation of ENaC trafficking in the biosynthetic pathway, likely by increasing endoplasmic reticulum-associated degradation. By reducing ENaC channel number, PCSK9 could modulate epithelial Na(+) absorption, a major contributor to blood pressure control.
DOI:10.1111/j.1471-4159.2006.03928.xURLPMID:16893422 [本文引用: 1]

Neural apoptosis-regulated convertase-1/proprotein convertase subtilisin-kexin like-9 (NARC-1/PCSK9) is a proprotein convertase recently described to play a major role in cholesterol homeostasis through enhanced degradation of the low-density lipoprotein receptor (LDLR) and possibly in neural development. Herein, we investigated the potential involvement of this proteinase in the development of the CNS using mouse embryonal pluripotent P19 cells and the zebrafish as models. Time course quantitative RT-PCR analyses were performed following retinoic acid (RA)-induced neuroectodermal differentiation of P19 cells. Accordingly, the mRNA levels of NARC-1/PCSK9 peaked at day 2 of differentiation and fell off thereafter. In contrast, the expression of the proprotein convertases subtilisin kexin isozyme 1/site 1 protease and Furin was unaffected by RA, whereas that of PC5/6 and PC2 increased within and/or after the first 4 days of the differentiation period respectively. This pattern was not affected by the cholesterogenic transcription factor sterol regulatory element-binding protein-2, which normally up-regulates NARC-1/PCSK9 mRNA levels in liver. Furthermore, in P19 cells, RA treatment did not affect the protein level of the endogenous LDLR. This agrees with the unique expression pattern of NARC-1/PCSK9 in the rodent CNS, including the cerebellum, where the LDLR is not significantly expressed. Whole-mount in situ hybridization revealed that the pattern of expression of zebrafish NARC-1/PCSK9 is similar to that of mouse both in the CNS and periphery. Specific knockdown of zebrafish NARC-1/PCSK9 mRNA resulted in a general disorganization of cerebellar neurons and loss of hindbrain-midbrain boundaries, leading to embryonic death at approximately 96 h after fertilization. These data support a novel role for NARC-1/PCSK9 in CNS development, distinct from that in cholesterogenic organs such as liver.
DOI:10.1002/jcp.25767URLPMID:28063230 [本文引用: 3]

Sepsis, a complex and dynamic syndrome resulting from microbial invasion and immune system dysregulation, is associated with an increased mortality, reaching up to 35% worldwide. Cholesterol metabolism is often disturbed during sepsis, with low plasma cholesterol levels being associated with poor prognosis. Proprotein convertase subtilisin/kexin type 9 (PCSK9) promotes degradation of the low-density lipoprotein receptor (LDLR), thus regulating intracellular and plasma cholesterol levels. PCSK9 is often upregulated during sepsis and might have a detrimental effect on immune host response and survival. Accordingly, PCSK9 reduces lipopolysaccharide uptake and clearance by human hepatocytes. Moreover, PCSK9 upregulation exacerbates organ dysfunction and tissue inflammation during sepsis, whereas a protective effect of PCSK9 deficiency has been documented in septic patients. Although a possible detrimental impact of PCSK9 on survival has been described, some beneficial effects of PCSK9 on immune response may be hypothesized. First, PCSK9 is associated with increased plasma cholesterol levels, which might be protective during sepsis. Second, PCSK9, by stimulating LDLR degradation and inhibiting reverse cholesterol transport (RCT), might promote preferential cholesterol accumulation in macrophages and other immune cells; these events might improve lipid raft composition and augment toll-like receptor function thus supporting inflammatory response. Hence, a more clear definition of the role of PCSK9 in septic states might provide additional insight in the understanding of the sepsis-associated immune dysregulation and enhance therapeutic outcomes.
DOI:10.1097/SHK.0000000000000682URLPMID:27405064 [本文引用: 1]

INTRODUCTION: Proprotein convertase subtilisin/kexin type 9 (PCSK9) targets lipoprotein receptors for degradation, thereby reducing hepatic lipid clearance. PCSK9 inhibition reduces mortality in septic mice, presumably through increased hepatic clearance of pathogen lipids due to increased lipoprotein receptor concentrations. However, PCSK9 overexpression in vivo has not been studied in sepsis. Therefore, this study aimed to evaluate the effects of differential PCSK9 expression on systemic infection, inflammation, and coagulation in sepsis. METHODS: Wild-type, PCSK9 knockout (KO), and transgenic (Tg) mice that overexpress PCSK9 were subjected to sham surgery or cecal ligation and puncture (CLP). Bacterial loads were measured in lungs, peritoneal cavity fluid, and blood. Organ pathology was assessed in lungs, liver, and kidneys. Lung myeloperoxidase activity, and plasma concentrations of alanine aminotransferase (ALT), creatinine, cell-free DNA (cfDNA), protein C, thrombin-antithrombin (TAT) complexes, interleukin (IL)-6, and IL-10 were also measured 6 h postoperatively. Morbidity was assessed for 16 h following CLP. RESULTS: Overexpression of PCSK9 in mice increased liver and kidney pathology, plasma IL-6, ALT, and TAT concentrations during sepsis, whereas PCSK9 KO mice exhibited reduced bacterial loads, lung and liver pathology, myeloperoxidase activity, plasma IL-10, and cfDNA during CLP-induced sepsis. All septic mice had reduced plasma levels of protein C, but the protein C ratio relative to normal was significantly decreased in PCSK9 Tg mice. Dyspnea, cyanosis, and overall grimace scores were greatest in septic mice overexpressing PCSK9, whereas PCSK9 KO mice retained core body temperature during sepsis. CONCLUSION: These findings demonstrate that PCSK9 deficiency confers protection against systemic bacterial dissemination, organ pathology, and tissue inflammation, particularly in the lungs and liver, while PCSK9 overexpression exacerbates multi-organ pathology as well as the hypercoagulable and pro-inflammatory states in early sepsis.
[本文引用: 1]
DOI:10.3892/br.2013.213URLPMID:24649090 [本文引用: 1]

Neuronal apoptosis is crucial in neurodegenerative diseases. However, a lower apoptotic rate of nerve cells is detected in the brain compared to that in other organs in neurodegenerative patients or in animal models, suggesting that neuronal apoptosis induced by any type of risk factors is intricately regulated. Human and animal studies demonstrated that a high concentration of oxidized LDL (ox-LDL) in the brain, which is associated with hyperlipidemia, is one of the key apoptosis inducers in neurodegenerative diseases. However, the mechanism underlying the ox-LDL-mediated regulation of neuronal apoptosis has not been fully elucidated. Recently, we investigated proprotein convertase subtilisin/kexin type 9 (PCSK9), a striking gene involved in lipid metabolism that exhibits a positive correlation with macrophage and endothelial cell apoptosis induced by ox-LDL. Moreover, PCSK9 may degrade beta-site amyloid precursor protein-cleaving enzyme 1 (BACE1), the key enzyme cleaving amyloid precursor protein (APP) to generate amyloid beta peptide (Abeta). Abeta is another key apoptosis inducer in neurodegenerative diseases. Our findings indicated that PCSK9 may be upregulated by the high levels of ox-LDL in the brain associated with hyperlipidemia and promote neuronal apoptosis through the NF-kappaB-B-cell lymphoma 2 (Bcl-2)/Bax-caspase 9-caspase 3 signaling pathways. Moreover, increased PCSK9 levels may inhibit the APP/Abeta metabolic pathway and reduce Abeta generation by degrading BACE1, thereby decreasing Abeta-induced neuronal apoptosis. The dual regulation mechanism of PCSK9 on apoptosis maintains neuronal apoptosis induced by risk factors at low levels.
DOI:10.1097/MOL.0000000000000386URLPMID:28157721 [本文引用: 1]

PURPOSE OF REVIEW: There are many reports of human variants in proprotein convertase subtilisin-kexin type 9 (PCSK9) that are either gain-of-function (GOF) or loss-of-function (LOF), with downstream effects on LDL cholesterol and cardiovascular disease (CVD) risk. However, data on particular mechanisms have only been minimally curated. RECENT FINDINGS: GOF variants are individually ultrarare, affect all domains of the protein, act to reduce LDL receptor expression through several mechanisms, are a minor cause of familial hypercholesterolemia, have been reported mainly within families, have variable LDL cholesterol-raising effects, and are associated with increased CVD risk mainly through observational studies in families and small cohorts. In contrast, LOF variants can be either ultrarare mutations or relatively more common polymorphisms seen in populations, affect all domains of the protein, act to increase LDL receptor expression through several mechanisms, have variable LDL cholesterol-lowering effects, and have been associated with decreased CVD risk mainly through Mendelian randomization studies in epidemiologic populations. SUMMARY: There is considerable complexity underlying the clinical concept of both LOF and GOF variants of PCSK9. But despite the underlying mechanistic heterogeneity, altered PCSK9 secretion or function is ultimately correlated with plasma LDL cholesterol level, which is also the driver of CVD outcomes.
DOI:10.1093/hmg/ddl077URLPMID:16571601 [本文引用: 1]

The proprotein convertase subtilisin/kexin type 9 (PCSK9) gene is involved in the post-transcriptional regulation of the low-density lipoprotein (LDL) receptors (LDLR). Mutations in the PCSK9 gene have been associated with both hypocholesterolemia and hypercholesterolemia through 'loss-of-function' and 'gain-of-function' mechanisms, respectively. We have studied the effect of the four loss-of-function mutations R46L, G106R, N157K and R237W and the two gain-of-function mutations S127R and D374Y on the autocatalytic activity of PCSK9, as well as on the amount of the cell surface LDLR and internalization of LDL in transiently transfected HepG2 cells. The two groups of mutations did not differ with respect to autocatalytic activity of PCSK9, but they did differ with respect to the amount of cell surface LDLR and internalization of LDL. The four loss-of-function mutations had a 16% increased level of cell surface LDLR and a 35% increased level of internalization of LDL as compared with WT-PCSK9. The two gain-of-function mutations had a 23% decreased level of cell surface LDLR and a 38% decreased level of internalization of LDL as compared with WT-PCSK9. Our studies have also shown that transfer of media from transiently transfected HepG2 cells to untransfected HepG2 cells, reduces the amount of cell surface LDLR and internalization of LDL in the untransfected cells within 20 min of media transfer. Thus, PCSK9 or a factor acted upon by PCSK9, is secreted from the transfected cells and degrades LDLR both in transfected and untransfected cells.
DOI:10.1093/ehjcvp/pvz022URLPMID:31236571 [本文引用: 1]

Proprotein convertase subtilisin/kexin Type 9 (PCSK9) is now identified as an important and major player in hypercholesterolaemia and atherosclerosis pathophysiology. PCSK9, through promoting lysosomal degradation of hepatic low-density lipoprotein (LDL) receptor, can decrease the clearance of plasma LDLs, leading to hypercholesterolaemia and consequent atherosclerotic plaque formation. Hypercholesterolaemia has been found to promote systemic and vascular inflammation, which can cause atherosclerotic lesion formation and progression and subsequent incidence of cardiovascular disease. Recent studies have shown the involvement of PCSK9 in the inflammatory pathway of atherosclerosis. Although trials with PCSK9 inhibitors have not shown any alteration in plasma C-reactive protein levels, there is accumulating evidence showing lessened inflammatory response in the arterial wall that could attenuate atherosclerotic plaque development beyond the established LDL-lowering effect of PCSK9 inhibition. In this review, we represent mounting evidence indicating that PCSK9 can locally increase vascular inflammation and contribute to atherosclerotic plaque progression in patients with hypercholesterolaemia.
DOI:10.1097/CCM.0b013e31827c09f8URLPMID:23442987 [本文引用: 1]

BACKGROUND: In 1992, the first consensus definition of severe sepsis was published. Subsequent epidemiologic estimates were collected using administrative data, but ongoing discrepancies in the definition of severe sepsis produced large differences in estimates. OBJECTIVES: We seek to describe the variations in incidence and mortality of severe sepsis in the United States using four methods of database abstraction. We hypothesized that different methodologies of capturing cases of severe sepsis would result in disparate estimates of incidence and mortality. DESIGN, SETTING, PARTICIPANTS: Using a nationally representative sample, four previously published methods (Angus et al, Martin et al, Dombrovskiy et al, and Wang et al) were used to gather cases of severe sepsis over a 6-year period (2004-2009). In addition, the use of new International Statistical Classification of Diseases, 9th Edition (ICD-9), sepsis codes was compared with previous methods. MEASUREMENTS: Annual national incidence and in-hospital mortality of severe sepsis. RESULTS: The average annual incidence varied by as much as 3.5-fold depending on method used and ranged from 894,013 (300/100,000 population) to 3,110,630 (1,031/100,000) using the methods of Dombrovskiy et al and Wang et al, respectively. Average annual increase in the incidence of severe sepsis was similar (13.0% to 13.3%) across all methods. In-hospital mortality ranged from 14.7% to 29.9% using abstraction methods of Wang et al and Dombrovskiy et al. Using all methods, there was a decrease in in-hospital mortality across the 6-year period (35.2% to 25.6% [Dombrovskiy et al] and 17.8% to 12.1% [Wang et al]). Use of ICD-9 sepsis codes more than doubled over the 6-year period (158,722 - 489,632 [995.92 severe sepsis], 131,719 - 303,615 [785.52 septic shock]). CONCLUSION: There is substantial variability in incidence and mortality of severe sepsis depending on the method of database abstraction used. A uniform, consistent method is needed for use in national registries to facilitate accurate assessment of clinical interventions and outcome comparisons between hospitals and regions.
DOI:10.1126/scitranslmed.aaa0481URLPMID:30112101 [本文引用: 1]
DOI:10.1371/journal.pone.0155030URLPMID:27171436 [本文引用: 1]

Sepsis is the leading cause of death in critically ill patients. While decreased Proprotein Convertase Subtilisin/Kexin type 9 (PCSK9) function improves clinical outcomes in murine and human sepsis, the mechanisms involved have not been fully elucidated. We tested the hypothesis that lipopolysaccharide (LPS), the major Gram-negative bacteria endotoxin, is cleared from the circulation by hepatocyte Low Density Lipoprotein Receptors (LDLR)-receptors downregulated by PCSK9. We directly visualized LPS uptake and found that LPS is rapidly taken up by hepatocytes into the cell periphery. Over the course of 4 hours LPS is transported towards the cell center. We next found that clearance of injected LPS from the blood was reduced substantially in Ldlr knockout (Ldlr-/-) mice compared to wild type controls and, simultaneously, hepatic uptake of LPS was also reduced in Ldlr-/- mice. Specifically examining the role of hepatocytes, we further found that primary hepatocytes isolated from Ldlr-/- mice had greatly decreased LPS uptake. In the HepG2 immortalized human hepatocyte cell line, LDLR silencing similarly resulted in decreased LPS uptake. PCSK9 treatment reduces LDLR density on hepatocytes and, therefore, was another independent strategy to test our hypothesis. Incubation with PCSK9 reduced LPS uptake by hepatocytes. Taken together, these findings demonstrate that hepatocytes clear LPS from the circulation via the LDLR and PCSK9 regulates LPS clearance from the circulation during sepsis by downregulation of hepatic LDLR.
DOI:10.3892/mmr.2017.6803URLPMID:28656218 [本文引用: 1]

The present study aimed to explore the direct toxicity of proprotein convertase subtilisin/kexin type 9 (PCSK9) to atherosclerosis (AS) and its association with apoptotic endothelial cells. Apolipoprotein E/ mice were randomly divided into two groups, control and experimental. The control group was administered a normal diet and the experimental group was administered a highfat diet. After 20 weeks, the aorta was isolated and dissected. Hematoxylin and eosin staining, and immunohistochemical analysis were performed. Human umbilical vein endothelial cells were incubated with varied concentrations of oxidized lowdensity lipoprotein (oxLDL) for different times. The apoptotic rate was detected by flow cytometry. Western blotting and reverse transcriptionquantitative polymerase chain reaction analysis were conducted to detect the expression of PCSK9, Bcell lymphoma 2 (Bcl2), bcl2like protein 4 (Bax) and caspase-3. Short hairpin (sh) RNAPCSK9 was transfected into endothelial cells using lentiviral transfection. The expression levels of PCSK9, Bax, Bcl2, caspase-3 and the mitogenactivated protein kinase (MAPK) pathway proteins were detected. The highfat group was successfully established as an AS model and PCSK9 was highly expressed in the AS plaque. Treatment with oxLDL induced apoptosis and increased mRNA and protein levels of PCSK9. PCSK9 mRNA and proteins levels were downregulated by shRNAPCSK9. The deficiency of PCSK9 markedly inhibited the expression of proapoptotic proteins and promoted antiapoptotic proteins. In addition, phosphorylation of p38 and cJun Nterminal kinases was altered by shRNAPCSK9. Targeting of PCSK9 by shRNAPCSK9 may repress endothelial cell apoptosis through MAPK signaling in AS, providing a novel direction for understanding the mechanism and treatment of AS.
URLPMID:28442922 [本文引用: 1]
DOI:10.1186/s40170-018-0187-2URL [本文引用: 1]
DOI:10.1593/neo.121252URLPMID:23308045 [本文引用: 1]

High circulating cholesterol is associated with hypercholesterolemia, atherosclerosis, and stroke. However, the relation between cholesterol and tumorigenesis/metastasis is controversial. The proprotein convertase subtilisin/kexin type 9 (PCSK9) regulates low-density lipoprotein cholesterol homeostasis by targeting the low-density lipoprotein receptor (LDLR) for degradation. PCSK9 is mostly expressed in liver, which is one of the most common sites for metastatic disease. To reveal the function of PCSK9 and also evaluate the impact of cholesterol in liver metastasis development, B16F1 melanoma cells were injected into wild-type (WT) and Pcsk9(-/-) mice to induce liver metastasis. On chow diet, Pcsk9(-/-) mice harbored two-fold less liver metastases than WT mice. This decrease is related to low cholesterol levels in Pcsk9(-/-) mice, as the protection was lost after normalizing Pcsk9(-/-) cholesterol levels by a 2-week high cholesterol diet. Furthermore, a prolongation of this diet strongly increased metastasis in both genotypes, suggesting that high cholesterol levels promote metastatic progression. The protective effect of the PCSK9 deficiency is also associated with increased apoptosis in liver stroma and metastases. Tumor necrosis factor.alpha (TNFalpha) mRNA and protein were, respectively, higher in liver stroma and plasma of injected mice, likely increasing the apoptotic TNFalpha signaling. Furthermore, the anti-apoptotic factor B-cell lymphoma 2 was downregulated. TNFalpha regulation is LDLR-independent, as its mRNA level was similarly upregulated in mice lacking both PCSK9 and LDLR. Our findings show that PCSK9 deficiency reduces liver metastasis by its ability to lower cholesterol levels and by possibly enhancing TNFalpha-mediated apoptosis.
DOI:10.1038/s41467-018-06467-9URLPMID:30262900 [本文引用: 1]

Observational studies have reported inconsistent associations between circulating lipids and breast cancer risk. Using results from >400,000 participants in two-sample Mendelian randomization, we show that genetically raised LDL-cholesterol is associated with higher risk of breast cancer (odds ratio, OR, per standard deviation, 1.09, 95% confidence interval, 1.02-1.18, P = 0.020) and estrogen receptor (ER)-positive breast cancer (OR 1.14 [1.05-1.24] P = 0.004). Genetically raised HDL-cholesterol is associated with higher risk of ER-positive breast cancer (OR 1.13 [1.01-1.26] P = 0.037). HDL-cholesterol-raising variants in the gene encoding the target of CETP inhibitors are associated with higher risk of breast cancer (OR 1.07 [1.03-1.11] P = 0.001) and ER-positive breast cancer (OR 1.08 [1.03-1.13] P = 0.001). LDL-cholesterol-lowering variants mimicking PCSK9 inhibitors are associated (P = 0.014) with lower breast cancer risk. We find no effects related to the statin and ezetimibe target genes. The possible risk-promoting effects of raised LDL-cholesterol and CETP-mediated raised HDL-cholesterol have implications for breast cancer prevention and clinical trials.
DOI:10.1210/jc.2009-0141URLPMID:19351729 [本文引用: 1]

CONTEXT: PCSK9 is a secreted protein that influences plasma levels of low-density lipoprotein cholesterol (LDL-C) and susceptibility to coronary heart disease. PCSK9 is present in human plasma, but the factors that contribute to differences in plasma concentrations of PCSK9 and how they impact on the levels of lipoproteins have not been well-characterized. OBJECTIVE: The aim of the study was to measure PCSK9 levels in a large, ethnically diverse population (n = 3138) utilizing a sensitive and specific sandwich ELISA. DESIGN: We conducted an observational study in the Dallas Heart Study, a multiethnic, probability-based sample of Dallas County. RESULTS: Plasma levels of PCSK9 varied over approximately 100-fold range (33-2988 ng/ml; median, 487 ng/ml). Levels were significantly higher in women (517 ng/ml) than in men (450 ng/ml), and in postmenopausal women compared to premenopausal women (P < 0.0001), irrespective of estrogen status. Plasma levels of PCSK9 correlated with plasma levels of LDL-C (r = 0.24) but explained less than 8% of the variation in LDL-C levels (r(2) = 0.073). Other factors that correlated with PCSK9 levels included plasma levels of triglycerides, insulin, and glucose. Individuals with loss-of-function mutations in PCSK9 and reduced plasma levels of LDL-C also had significantly lower plasma levels of PCSK9 after adjusting for age, gender, and LDL-C levels (P < 0.0001). CONCLUSION: Multiple metabolic and genetic factors contribute to variation in plasma levels of PCSK9 in the general population. Although levels of PCSK9 correlate with plasma levels of LDL-C, they account for only a small proportion of the variation in the levels of this lipoprotein.
URLPMID:27701660
DOI:10.1007/s00125-015-3659-8URL
DOI:10.1016/j.jdiacomp.2015.08.003URLPMID:26412029

AIM: Recent in vitro researches have shown that plasma Lp(a) can be reduced using a proprotein convertase subtilisin/kexin type 9 (PCSK9)-inhibitory monoclonal antibody. In our clinical study we tried to investigate the association between plasma Lp(a) and PCSK9 in Type 2 diabetic patients with elevated plasma Lp(a), and to check whether such an association would be related to LDL-receptor (LDL-R) levels. METHODS: Plasma PCSK9 and LDL-R concentrations were measured by sandwich ELISA methods using recombinant human PCSK9 protein and LDL-R protein as standards in a cohort with type 2 diabetic patients (n=50) compared to an age- and sex-matched control group (n=50). Both clinical and biochemical parameters were determined in all patients. RESULTS: Plasma PCSK9 level was significantly elevated in T2DM patients compared to controls (44.61+/-14.44 and 33.22+/-11.79ng/mL, respectively, P<0.0001). However LDL-R levels did not differ between the two groups. Remarkably, plasma PCSK9 levels were positively correlated with Lp(a) levels in whole population (r=+0.227, P=0.03) as well as in T2DM group (r=+0.398, P=0.0061) but not in control group. Multiple linear regression analysis showed that plasma Lp(a) levels were independently associated to those of PCSK9. CONCLUSION: Lp(a) has been proposed as a contributing factor to the accelerated development of macrovascular complications in T2DM. Its synergic effect with PCSK9 may explain the enhanced atherogenicity in T2DM patients.
DOI:10.1159/000365935URL

Background: A high level of circulating PCSK9 binds to the LDL receptor, reduces its cell's surface density and leads to hypercholesterolemia. The aim of this study was to examine the circulating PCSK9 level in patients with kidney disease. Methods: Out of the patients treated in our Departments we selected: (a) 44 patients with CKD stage 3 and 4 (b) 29 patients with CKD stage 5 on maintenance hemodialysis treatment; and (c) 20 patients after successful renal transplantation. Thirty-four subjects, without CKD formed the control group. Serum biochemical parameters' concentrations were assayed by a certified laboratory. Serum PCSK9 concentration was estimated by a commercially available ELISA kit. Results: The mean serum concentration of PCSK9 in patients with kidney disease was higher than in the control group (238.7 +/- 64.5 vs. 536.7 +/- 190.4; p < 0.001). A strong negative correlation between serum PCSK9 concentration and eGFR was found (r = -0.66; p < 0.001), as well as between serum concentrations of PCSK9 and total-(r = 0.482; p < 0.05) or LDL-cholesterol (r = 0.533; p < 0.05), but exclusively in patients not receiving statins. The elevated serum concentration of PCSK9 in patients before hemodialysis session declined afterwards, reaching the values observed in patients after kidney transplantation and in the control group. Conclusion: The circulating PCSK9 concentration is increased in patients with CKD; however, this is not accompanied by hypercholesterolemia. The positive correlations between PCSK9/TCh and PCSK9/LDL-Ch have been found only in patients not treated with statins. The elevated circulating PCSK9 level is corrected by maintenance hemodialysis treatment and normalized by a successful kidney transplantation. (C) 2014 S. Karger AG, Basel
DOI:10.1016/j.jacl.2016.10.005URLPMID:28391915 [本文引用: 1]

BACKGROUND: The association between proprotein convertase subtilisin/kexin type 9 (PCSK9), a critical regulator of low-density lipoprotein (LDL) metabolism, and kidney function is a matter of debate. OBJECTIVE: We aimed to assess the association of circulating PCSK9 concentrations with both glomerular filtration rate (eGFR) and serum lipid parameters in nondiabetic patients with chronic kidney disease (CKD). METHODS: Fasting plasma PCSK9 concentrations were measured by ELISA in 94 nondiabetic nondialysis CKD (ND-CKD) patients not receiving statins, at different stages of CKD. RESULTS: Plasma PCSK9 levels were associated neither to eGFR (P = .770) nor to proteinuria (P = .888) at several stages of CKD. In addition, plasma PCSK9 levels did not vary significantly between the different CKD stages. Plasma PCSK9 concentrations were positively correlated with apolipoprotein B (r = 0.221; P = .03) and triglycerides (r = 0.211; P = .04) but not with total cholesterol, calculated LDL-cholesterol, HDL cholesterol, lipoprotein(a), or CRP. CONCLUSION: In a homogeneous population of nondiabetic subjects without lipid-lowering therapy, plasma PCSK9 concentrations are not associated to eGFR at several stages of CKD. These data suggest that kidney function per se does not impact significantly PCSK9 metabolism.
DOI:10.1124/pr.116.012989URLPMID:27920219 [本文引用: 1]

The secretory proprotein convertase (PC) family comprises nine members, as follows: PC1/3, PC2, furin, PC4, PC5/6, paired basic amino acid cleaving enzyme 4, PC7, subtilisin kexin isozyme 1/site 1 protease (SKI-1/S1P), and PC subtilisin/kexin type 9 (PCSK9). The first seven PCs cleave their substrates at single/paired basic residues and exhibit specific and often essential functions during development and/or in adulthood. The essential SKI-1/S1P cleaves membrane-bound transcription factors at nonbasic residues. In contrast, PCSK9 cleaves itself once, and the secreted inactive protease drags the low-density lipoprotein receptors (LDLR) and very LDLR (VLDLR) to endosomal/lysosomal degradation. Inhibitory PCSK9 monoclonal antibodies are now prescribed to treat hypercholesterolemia. This review focuses on the implication of PCs in cardiovascular functions and diseases, with a major emphasis on PCSK9. We present a phylogeny of the PCs and the analysis of PCSK9 haplotypes in modern and archaic human species. The absence of PCSK9 in mice led to the discovery of a sex- and tissue-specific subcellular distribution of the LDLR and VLDLR. PCSK9 inhibition may have other applications because it reduces inflammation and sepsis in a LDLR-dependent manner. Our present understanding of the cellular mechanism(s) that enables PCSK9 to induce the degradation of receptors is reviewed, as well as the consequences of its key natural mutations. The PCSK9 ongoing clinical trials are reviewed. Finally, how the other PCs may impact cardiovascular disease and the metabolic syndrome, and become relevant targets, is discussed.
[本文引用: 1]
DOI:10.2147/DDDT.S67498URLPMID:26109850 [本文引用: 1]

Despite the proven efficacy of statins, they often fail to achieve low-density lipoprotein (LDL) cholesterol goals, especially in high-risk patients. Moreover, a large number of subjects cannot tolerate statins or full doses of these drugs, in particular patients with familial hypercholesterolemia. Thus, there is a need for additional effective LDL cholesterol-reducing agents. Evolocumab (AMG145) is a monoclonal antibody inhibiting proprotein convertase subtilisin/kexin type 9 that binds to the liver LDL receptor and prevents it from normal recycling by targeting it for degradation. Phase I, II, and III trials revealed that, on subcutaneous injection, either alone or in combination with statins, evolocumab is able to reduce high LDL cholesterol levels from 54% to 80%, apolipoprotein B100 from 31% to 61%, and lipoprotein(a) from 12% to 36%, in a dose-dependent manner. The incidence of side effects seems to be low and mainly limited to nasopharyngitis, injection site pain, arthralgia, and back pain. Evolocumab is an innovative powerful lipid-lowering drug, additive to statins and/or ezetimibe, with a large therapeutic range associated with a low rate of mild adverse events. If the available data are confirmed in long-term trials with strong outcome measures, evolocumab will become an essential tool in the treatment of a large number of high-risk patients, such as those affected by familial hypercholesterolemia, those who are unable to tolerate an efficacious statin dosage, and those at very high cardiovascular risk and unable to achieve their target LDL cholesterol levels with currently available lipid-lowering therapies.
DOI:10.1517/14712598.2016.1148136URL [本文引用: 1]
DOI:10.1007/s13167-017-0106-6URLPMID:29209441 [本文引用: 1]

In the following review, authors described the structure and biochemical pathways of PCSK9, its involvement in LDL metabolism, as well as significances of proprotein convertase subtilisin/kexin type 9 targeted treatment. PCSK9 is a proprotein convertase, which plays a crucial role in LDL receptor metabolism. Transcription and translation of PCSK9 is controlled by different nuclear factors, such as, SREBP and HNF1alpha. This review focuses on interactions between PCSK9 and LDL receptor, VLDLR, ApoER2, CD36, CD81, and others. The role of PCSK9 in the inflammatory process is presented and its influence on cytokine profile (IL-1, IL-6, IL-10, TNF) in atherosclerotic plaque. Cholesterol metabolism converges also with diabetes by mTORC1 pathways. PCSK9 can be altered by oncologic pathways with utilization of kinases, such as Akt, JNK, and JAK/STAT. Finally, the article shows that blocking PCSK9 has proapoptotic capabilities. Administration of monoclonal antibodies against PCSK9 reduced mortality rate and cardiovascular events in randomized trials. On the other hand, immunogenicity of new drugs may play a crucial role in their efficiency. Bococizumab ended its career following SPIRE-1,2 outcome. PCSK9 inhibitors have enormous potential, which had been reflected by introducing them (as a new class of drugs reducing LDL concentration cholesterol) into New Lipid Guidelines from Rome 2016. Discoveries in drugs development are focused on blocking PCSK9 on different levels. For example, silencing messenger RNA (mRNA of PCSK9) is a new alternative against hypercholesterolemia. Peptides mimicking EGF-A domain of the LDL receptor are gaining significance and hopefully they will soon join others. The significance of PCSK9 has just been uncovered and further data is still required to understand their activity.
DOI:10.1073/pnas.0805434105URLPMID:18695239 [本文引用: 1]

Proprotein convertase subtilisin/kexin type 9 (PCSK9) regulates low density lipoprotein receptor (LDLR) protein levels and function. Loss of PCSK9 increases LDLR levels in liver and reduces plasma LDL cholesterol (LDLc), whereas excess PCSK9 activity decreases liver LDLR levels and increases plasma LDLc. Here, we have developed active, cross-species, small interfering RNAs (siRNAs) capable of targeting murine, rat, nonhuman primate (NHP), and human PCSK9. For in vivo studies, PCSK9 and control siRNAs were formulated in a lipidoid nanoparticle (LNP). Liver-specific siRNA silencing of PCSK9 in mice and rats reduced PCSK9 mRNA levels by 50-70%. The reduction in PCSK9 transcript was associated with up to a 60% reduction in plasma cholesterol concentrations. These effects were shown to be mediated by an RNAi mechanism, using 5'-RACE. In transgenic mice expressing human PCSK9, siRNAs silenced the human PCSK9 transcript by >70% and significantly reduced PCSK9 plasma protein levels. In NHP, a single dose of siRNA targeting PCSK9 resulted in a rapid, durable, and reversible lowering of plasma PCSK9, apolipoprotein B, and LDLc, without measurable effects on either HDL cholesterol (HDLc) or triglycerides (TGs). The effects of PCSK9 silencing lasted for 3 weeks after a single bolus i.v. administration. These results validate PCSK9 targeting with RNAi therapeutics as an approach to specifically lower LDLc, paving the way for the development of PCSK9-lowering agents as a future strategy for treatment of hypercholesterolemia.
DOI:10.1056/NEJMoa1615758URL [本文引用: 1]
DOI:10.1038/mt.2011.260URL [本文引用: 1]

Proprotein convertase subtilisin/kexin type 9 (PCSK9) has emerged as a therapeutic target for the reduction of low-density lipoprotein cholesterol (LDL-C). PCSK9 increases the degradation of the LDL receptor, resulting in high LDL-C in individuals with high PCSK9 activity. Here, we show that two locked nucleic acid (LNA) antisense oligonucleotides targeting PCSK9 produce sustained reduction of LDL-C in nonhuman primates after a loading dose (20 mg/kg) and four weekly maintenance doses (5 mg/kg). PCSK9 messenger RNA (mRNA) and serum PCSK9 protein were reduced by 85% which resulted in a 50% reduction in circulating LDL-C. Serum total cholesterol (TC) levels were reduced to the same extent as LDL-C with no reduction in high-density lipoprotein levels, demonstrating a specific pharmacological effect on LDL-C. The reduction in hepatic PCSK9 mRNA correlated with liver LNA oligonucleotide content. This verified that anti-PCSK9 LNA oligonucleotides regulated LDL-C through an antisense mechanism. The compounds were well tolerated with no observed effects on toxicological parameters (liver and kidney histology, alanine aminotransferase, aspartate aminotransferase, urea, and creatinine). The pharmacologic evidence and initial safety profile of the compounds used in this study indicate that LNA antisense oligonucleotides targeting PCSK9 provide a viable therapeutic strategy and are potential complements to statins in managing high LDL-C.
DOI:10.1007/s11789-017-0085-0URL [本文引用: 1]
DOI:10.1074/jbc.M113.514067URLPMID:24225950 [本文引用: 1]

PCSK9 (proprotein convertase subtilisin/kexin type 9) is a negative regulator of the hepatic LDL receptor, and clinical studies with PCSK9-inhibiting antibodies have demonstrated strong LDL-c-lowering effects. Here we screened phage-displayed peptide libraries and identified the 13-amino acid linear peptide Pep2-8 as the smallest PCSK9 inhibitor with a clearly defined mechanism of inhibition that has been described. Pep2-8 bound to PCSK9 with a KD of 0.7 mum but did not bind to other proprotein convertases. It fully restored LDL receptor surface levels and LDL particle uptake in PCSK9-treated HepG2 cells. The crystal structure of Pep2-8 bound to C-terminally truncated PCSK9 at 1.85 A resolution showed that the peptide adopted a strand-turn-helix conformation, which is remarkably similar to its solution structure determined by NMR. Consistent with the functional binding site identified by an Ala scan of PCSK9, the structural Pep2-8 contact region of about 400 A(2) largely overlapped with that contacted by the EGF(A) domain of the LDL receptor, suggesting a competitive inhibition mechanism. Consistent with this, Pep2-8 inhibited LDL receptor and EGF(A) domain binding to PCSK9 with IC50 values of 0.8 and 0.4 mum, respectively. Remarkably, Pep2-8 mimicked secondary structural elements of the EGF(A) domain that interact with PCSK9, notably the beta-strand and a discontinuous short alpha-helix, and it engaged in the same beta-sheet hydrogen bonds as EGF(A) does. Although Pep2-8 itself may not be amenable to therapeutic applications, this study demonstrates the feasibility of developing peptidic inhibitors to functionally relevant sites on PCSK9.
DOI:10.1038/nsmb.3471URLPMID:28981076 [本文引用: 1]
DOI:10.1093/cvr/cvz003URLPMID:30629143 [本文引用: 1]

Since the discovery of the role of proprotein convertase subtilisin kexin 9 (PCSK9) in the regulation of low-density lipoprotein cholesterol (LDL-C) in 2003, a paradigm shift in the treatment of hypercholesterolaemia has occurred. The PCSK9 secreted into the circulation is a major downregulator of the low-density lipoprotein receptor (LDLR) protein, as it chaperones it to endosomes/lysosomes for degradation. Humans with loss-of-function of PCSK9 exhibit exceedingly low levels of LDL-C and are protected from atherosclerosis. As a consequence, innovative strategies to modulate the levels of PCSK9 have been developed. Since 2015 inhibitory monoclonal antibodies (evolocumab and alirocumab) are commercially available. When subcutaneously injected every 2-4 weeks, they trigger a approximately 60% LDL-C lowering and a 15% reduction in the risk of cardiovascular events. Another promising approach consists of a liver-targetable specific PCSK9 siRNA which results in approximately 50-60% LDL-C lowering that lasts up to 6 months (Phases II-III clinical trials). Other strategies under consideration include: (i) antibodies targeting the C-terminal domain of PCSK9, thereby inhibiting the trafficking of PCSK9-LDLR to lysosomes; (ii) small molecules that either prevent PCSK9 binding to the LDLR, its trafficking to lysosomes or its secretion from cells; (iii) complete silencing of PCSK9 by CRISPR-Cas9 strategies; (iv) PCSK9 vaccines that inhibit the activity of circulating PCSK9. Time will tell whether other strategies can be as potent and safe as monoclonal antibodies to lower LDL-C levels.
DOI:10.1016/j.cmet.2013.12.006URL [本文引用: 1]

Circulating PCSK9 destines low-density lipoprotein receptor for degradation in lysosomes, resulting in increased LDL cholesterol. Accordingly, it is an attractive drug target for hypercholesterolemia, and results from clinical trials are promising. While the physiological role of PCSK9 in cholesterol metabolism is well described, its complex mechanism of action remains poorly understood, although it is known to depend on intracellular trafficking. We here identify sortilin, encoded by the hypercholesterolemia-risk gene SORT1, as a high-affinity sorting receptor for PCSK9. Sortilin colocalizes with PCSK9 in the trans-Golgi network and facilitates its secretion from primary hepatocytes. Accordingly, sortilin-deficient mice display decreased levels of circulating PCSK9, while sortilin overexpression in the liver confers increased plasma PCSK9. Furthermore, circulating PCSK9 and sortilin were positively correlated in a human cohort of healthy individuals, suggesting that sortilin is involved in PCSK9 secretion in humans. Taken together, our findings establish sortilin as a critical regulator of PCSK9 activity.
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.fob.2014.08.003URL [本文引用: 1]
DOI:10.1016/S0140-6736(17)32290-0URLPMID:28859947 [本文引用: 1]

BACKGROUND: LDL cholesterol is a well established risk factor for atherosclerotic cardiovascular disease. How much one should or safely can lower this risk factor remains debated. We aimed to explore the relationship between progressively lower LDL-cholesterol concentrations achieved at 4 weeks and clinical efficacy and safety in the FOURIER trial of evolocumab, a monoclonal antibody to proprotein convertase subtilisin-kexin type 9 (PCSK9). METHODS: In this prespecified secondary analysis of 25 982 patients from the randomised FOURIER trial, the relationship between achieved LDL-cholesterol concentration at 4 weeks and subsequent cardiovascular outcomes (primary endpoint was the composite of cardiovascular death, myocardial infarction, stroke, coronary revascularisation, or unstable angina; key secondary endpoint was the composite of cardiovascular death, myocardial infarction, or stroke) and ten prespecified safety events of interest was examined over a median of 2.2 years of follow-up. We used multivariable modelling to adjust for baseline factors associated with achieved LDL cholesterol. This trial is registered with ClinicalTrials.gov, number NCT01764633. FINDINGS: Between Feb 8, 2013, and June 5, 2015, 27 564 patients were randomly assigned a treatment in the FOURIER study. 1025 (4%) patients did not have an LDL cholesterol measured at 4 weeks and 557 (2%) had already had a primary endpoint event or one of the ten prespecified safety events before the week-4 visit. From the remaining 25 982 patients (94% of those randomly assigned) 13 013 were assigned evolocumab and 12 969 were assigned placebo. 2669 (10%) of 25 982 patients achieved LDL-cholesterol concentrations of less than 0.5 mmol/L, 8003 (31%) patients achieved concentrations between 0.5 and less than 1.3 mmol/L, 3444 (13%) patients achieved concentrations between 1.3 and less than 1.8 mmol/L, 7471 (29%) patients achieved concentrations between 1.8 to less than 2.6 mmol/L, and 4395 (17%) patients achieved concentrations of 2.6 mmol/L or higher. There was a highly significant monotonic relationship between low LDL-cholesterol concentrations and lower risk of the primary and secondary efficacy composite endpoints extending to the bottom first percentile (LDL-cholesterol concentrations of less than 0.2 mmol/L; p=0.0012 for the primary endpoint, p=0.0001 for the secondary endpoint). Conversely, no significant association was observed between achieved LDL cholesterol and safety outcomes, either for all serious adverse events or any of the other nine prespecified safety events. INTERPRETATION: There was a monotonic relationship between achieved LDL cholesterol and major cardiovascular outcomes down to LDL-cholesterol concentrations of less than 0.2 mmol/L. Conversely, there were no safety concerns with very low LDL-cholesterol concentrations over a median of 2.2 years. These data support further LDL-cholesterol lowering in patients with cardiovascular disease to well below current recommendations. FUNDING: Amgen.
