 ,1
,1Development and innovation of haploid induction technologies in plants
Haiqiang Chen1, Huiyun Liu1, Ke Wang1, Shuangxi Zhang2, Xingguo Ye ,1
,1通讯作者: 叶兴国,博士,研究员,博士生导师,研究方向:小麦细胞工程和基因工程育种。E-mail:yexingguo@caas.cn
责任编辑: 严建兵
收稿日期:2020-02-7修回日期:2020-04-22网络出版日期:2020-05-20
| 基金资助: |
Editor:
Received:2020-02-7Revised:2020-04-22Online:2020-05-20
| Fund supported: |
作者简介 About authors
陈海强,在读硕士研究生,专业方向:小麦分子育种。E-mail:Haiqiang_Chen@163.com。

摘要
单倍体育种是培育作物新品种的主要育种技术之一,提高单倍体诱导频率和简化诱导程序是单倍体育种技术的关键。随着单倍体诱导技术的发展与改进,单倍体育种技术已被广泛应用于许多重要植物的育种研究中,展现出基因纯合快速、育种年限缩短、育种效率提高等优势。单倍体诱导技术与杂交育种、诱变育种、反向育种和分子标记辅助选择育种等技术相结合,在作物品种改良上的作用更加显著。单倍体和双单倍体在遗传群体构建、基因功能鉴定、转基因研究、细胞学研究等方面具有重要应用价值。本文从单倍体诱导技术、单倍体和双单倍体应用等方面综述了植物单倍体诱导技术的发展,尤其是近年来利用基因组编辑技术创制主要作物单倍体诱导系的进展,并分析了目前研究中存在的问题和今后的发展方向,以期促进单倍体诱导技术尤其是利用基因编辑创造诱导系技术在作物育种中的应用。
关键词:
Abstract
Haploid induction is one of the main techniques for breeding new varieties of major crops, and its key steps are improving the haploid induction rate and simplifying the induction procedure. With the development and innovation of plant haploid induction technologies, haploid breeding has been widely used in varietal improvement of important crops, showing the advantages of rapid homozygosity of heterozygous genes, shortening breeding period, and improving breeding efficiency. The combination of haploid breeding with crossing breeding, mutation breeding, reverse breeding, and molecular marker-assisted selection will greatly improve the effectiveness of crop breeding. Haploids and doubled haploids have demonstrated their usefulness in production of genetic populations, characterization of gene functions, and transgenic and cytological studies in plants. In this review, we summarize the progress of haploid induction technologies in view of various haploid induction techniques and applications of haploids and double haploids. In particular, the advances on the haploid induction in several major crops by genome editing were briefly described. Finally, we discuss current issues and future perspectives in this field, so as to promote the application of the haploid induction techniques, especially the techniques of creating haploid inducer lines by genome editing in crop breeding.
Keywords:
PDF (812KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
陈海强, 刘会云, 王轲, 张双喜, 叶兴国. 植物单倍体诱导技术发展与创新. 遗传[J], 2020, 42(5): 466-482 doi:10.16288/j.yczz.20-033
Haiqiang Chen.
植物单倍体植株的出现不但加快了育种进程,而且为遗传学研究提供了背景纯合的植物材料。但植物单倍体诱导技术研究经历了漫长的发展过程。1922年首次在曼陀罗(Datura stramonium)中发现了天然单倍体植株的存在[1],40多年后通过对曼陀罗花粉进行离体培养才获得了单倍体植株[2],继而对水稻(Oryza sativa)小孢子进行离体培养获得了再生植株[3]。后来发现,通过种间杂交染色体消除能够诱导单倍体胚[4],利用诱导系授粉和基因编辑技术可获得发育完整的单倍体籽粒,简化了单倍体诱导程序。到目前为止,单倍体诱导技术已经在200多种植物中获得了成功[5]。同时,染色体加倍技术也逐步完善,提高了单倍体的加倍效率[6],促进了单倍体诱导技术在水稻、小麦(Triticum aestivum)、大麦(Hordeum vulgare)、玉米(Zea mays)等重要作物遗传和育种研究中的应用。通过单倍体诱导的植物育种具有快速纯合基因、缩短育种年限、提高育种效率等优势,可与杂交育种、诱变育种、反向育种、分子标记辅助选择育种等技术结合,在遗传群体构建、基因功能鉴定、转基因植株快速稳定等研究中具有独特作用。因此,单倍体育种已成为与分子标记辅助育种和转基因育种相媲美的育种核心技术之一。
单倍体诱导依据诱导方式分为体外诱导和体内诱导。体外诱导进一步分为雄配子体诱导和雌配子体诱导。雄配子体诱导包括花药培养和小孢子培养,雌配子体诱导包括胚珠培养和胚囊培养。体外诱导受基因型、生理状态、发育阶段、胁迫预处理和培养条件等因素的影响。体内诱导分为远缘杂交诱导、花粉诱导、着丝粒介导、诱导系诱导和CRISPR/Cas9基因编辑诱导。花药培养和小孢子培养在水稻、小麦、大麦等作物中应用比较广泛。虽然诱导系诱导目前还仅限于玉米等少数作物,但随着近几年利用CRISPR/Cas9基因编辑技术创制诱导系的问世,诱导系诱导在水稻和小麦等作物中已经取得了成功。将远缘杂交诱导技术与CRISPR/Cas9基因编辑技术结合,在诱导小麦单倍体植株的同时,实现了对小麦目标基因的编辑,而且不涉及转基因环节[7]。本文将对上述几方面的主要进展进行综述,重点介绍诱导系和基因编辑在植物单倍体诱导中的应用,并对未来发展方向予以展望。
1 体外诱导作物单倍体技术
1.1 雄配子体诱导
雄配子体诱导是植物中诱导产生单倍体的传统方法。综合文献报道,来自茄科、十字花科和禾本科等250多种植物可以通过雄配子体诱导单倍体植株[8,9]。植物雄配子体诱导途径包括花药培养和小孢子培养。花药培养操作简单,适用于多种作物。花药培养存在强烈的基因型依赖性和物种特异性。Machii等[10]对107个小麦基因型进行花药培养,有83个基因型产生了愈伤组织,45个基因型产生了再生植株,其中只有9个基因型产生了绿苗,25个基因型只产生白化苗。然而,豆科植物和木本植物,以及禾本科的玉米等,属于单倍体诱导顽拗型植物,难以通过雄配子体产生单倍体[9]。一般情况下,花药培养诱导的单倍体植株需要经过染色体加倍才能获得正常结实的双单倍体,但一些物种如大麦在小孢子早期细胞分裂过程中通过染色体自然加倍能够获得较高频率的双单倍体[6]。尽管花药培养的应用已经比较普遍,但对水稻、大麦、小麦等花药培养比较容易的作物来说,很多农艺性状优良的顽拗基因型其花药培养仍然不能成功,且对获得诱导单倍体的分子生物学过程知之甚少,需要剖析单倍体诱导的分子机制和花药培养再生植株的遗传基础,从而解决基因型限制问题。花药培养由于花药壁和花丝组织的存在,诱导的单倍体可能存在嵌合体现象,为后续单倍体鉴定和染色体加倍带来了困难。另外,花药培养单个花药的绿苗诱导率非常低,接种量比较大。因此,可以通过小孢子培养避免嵌合体的产生。更重要的是,可以利用细胞生物学、分子生物学和功能基因组学的相关技术研究离体培养小孢子的生理生化过程,以解析通过雄配子体离体诱导单倍体的分子机制[11]。小孢子培养密度高,每毫升培养基中可形成1000个以上胚胎[5],远远多于花药形成的胚胎数目,且避免了体细胞干扰。尽管小孢子培养研究比较晚,但显现出明显优势,已成功应用于小麦、大麦和水稻等重要作物批量获得双单倍体植株。例如,对35个硬粒小麦材料的小孢子在C17培养基上培养,诱导产生了407个再生植株,其中67.0%的植株可以自然加倍产生双单倍体[12]。利用IMI培养基对4个栽培大麦进行小孢子培养,每4×104个小孢子可获得469~534个胚胎,进而发育为32~160个植株;尤其用甘露醇预处理小孢子,染色体自然加倍率提高了8.9%~14.1%[13]。利用不同培养条件对杂交粳稻小孢子进行培养,发现4℃低温处理水稻幼穗12天,其愈伤组织诱导率和绿苗分化率均高于16天的处理;通过比较小孢子不同培养时间(4周、7周和13周)后进行分化培养的效果,发现培养7周后愈伤组织分化效果最好,再生植株自然加倍率为50.0%[14]。
1.2 雌配子体诱导
雌核发育指对胚囊或胚珠进行离体培养获得单倍体再生植株。目前,分离和培养卵细胞仍然比较困难。虽然培养具有8个细胞核的胚囊可以诱导雌核发育,但卵细胞、助细胞和反足细胞都有发育为单倍体胚胎的潜力。自首次在大麦中报道雌核发育以来[15],多个物种通过雌核发育获得了单倍体植株,该技术主要应用于甜菜(Beta vulgaris)和洋葱(Allium cepa)等植物。在甜菜育种中,加倍单倍体(double haploids, DH)技术已发挥了一定作用,如将DH亲本用于生产F1杂种[16]。对两个伊朗洋葱品种雌核发育的研究发现,多胺类物质可以改善雌核发育,品种Sefid-e-Kurdistan胚胎再生能力可达6.9%,高于品种Sefid-e-Neishabour的3.3%,其中的73.6%的再生植株是单倍体[17]。利用芸苔素内酯对拟南芥(Arabidopsis thaliana)和芥菜(Brassica juncea)去雄后的柱头后进行处理,获得了单倍体种子[18]。20世纪末,Li等[19]建立了一种简便、高效的水稻未授精子房离体培养技术,并用于水稻育种和遗传研究。Mdarhri-Alaoui等[20]对硬粒小麦未受精子房进行离体培养,研究了绿苗形成过程中形态发生的过程。随后,Sibi等[21]对6个硬粒小麦基因型的未受精子房进行培养,均获得了单倍体和双单倍体植株。另外,通过对26,400个授粉后的玉米子房进行离体培养,诱导产生了24株单倍体植株,其中的2株经秋水仙素处理染色体加倍成功,发现授粉后19.5 h雌核发育频率最高(0.2%)[22]。由于1个胚珠中仅含1个卵细胞,与单个花药中含有数百个小孢子的花药培养在数量上无法相比,导致雌配子体发育的诱导效率远低于雄配子体发育。但是,对于具有孤雄生殖障碍、花粉缺陷或雄配子体诱导产生白化苗的顽拗物种或基因型而言,通过雌配子培养诱导单倍体是有效的途径。总体而言,雌核培养产生的单倍体遗传较为稳定、白化苗率较低[23,24]。1.3 通过配子体诱导单倍体的影响因素
1.3.1 供体植物的基因型和生理状态供体植物种类和基因型决定配子体离体培养诱导单倍体的效率,尤其是雄配子体诱导具有强烈的物种及基因型依赖性,不同物种和基因型对花药或小孢子培养的应答不同。在小麦族中,普通小麦比硬粒小麦对雄配子体培养的基因型特异性更强,普通小麦中冬小麦比春小麦的基因型反应更敏感。而在水稻的雄配子体诱导培养中,粳稻比籼稻的基因型反应更敏感[24]。研究表明,雄配子体诱导培养的顽拗型由花粉中的特异性表达基因调控[25]。例如,大多数小麦基因型中存在抑制Kr基因表达的基因,限制了雄配子体离体培养产生单倍体;以11个小麦品种及其组配的20个F1杂种为材料,发现Alondra、Verry、石4185、新春9号和百农3217的花药容易产生愈伤组织,诱导率25.3%~51.9% [26];对中国大面积推广的24个优良小麦品种连续2年进行花药培养,植株再生率0~41.8%,基因型差异显著,其中,石麦4185和邯6172花药培养愈伤组织诱导率和分化率较高,可作为小麦单倍体育种的亲本材料[27];籼稻花药培养过程中基因型顽固,利用添加10-3 mol/L腐胺的N6培养基对不同籼稻基因型进行花药培养,发现Grogol、Krowal和Sigundil具有良好的花药培养能力[28]。利用FHG培养基对多个冬大麦基因型进行花药培养,发现12个基因型在胚胎诱导、植株再生方面均存在显著差异[29]。另外,对46个玉米基因型在3种培养基(IMSS、N6和YPm)上进行花药培养,表明基因型的影响比培养基的效应更大[30]。
供体植物的生理状态同样显著影响配子体离体培养的效果。主要影响花药内小孢子的数量和活力、花药组织的营养状况及内源生长调节剂的含量,进而影响雄核发育能力和单倍体植株诱导效率。与在温室盆栽条件下生长的供体植株相比,田间自然条件下生长植株雄配子体诱导效率更高。田间自然条件通常是供体植物花药发育的最佳环境,有利于雄核体外培养产生单倍体。一般来说,发育较早的花药和取自主茎穗上的花药相对容易进行雄配子体诱导培养。除水稻外,大多数禾谷类作物主分蘖比侧分蘖花药中的雄核对诱导培养更敏感。
1.3.2 配子体的发育阶段
外植体的发育阶段对单倍体诱导效率有重要影响。在小孢子培养中,发育阶段是影响小孢子全能性的关键因素,只有处于形成花粉粒前的小孢子才能在短时间内快速诱导雄核发育,产生单倍体。对大多数植物而言,小孢子细胞第一次有丝分裂前后,即花粉细胞处于单核晚期或双核早期,是雄核诱导培养最敏感的时期[31]。对模式植物的研究表明,双核期花粉的细胞质中开始积累淀粉,不适合用于雄核诱导培养[32]。在小麦中,单核发育中后期进行雄核诱导培养最有效[33]。在大多数植物中,小孢子发育并不同步,同一花药中可以观察到不同发育阶段的小孢子。因此,选择含有最大比例胚性小孢子的材料进行培养,对提高小孢子的产胚效率至关重要。实际操作中需要对同一花序不同位置的材料进行观察,确定其花药内小孢子发育阶段,从而确定诱导单倍体所用材料的取材标准。
1.3.3 胁迫预处理
通过对外植体培养前后进行物理或化学胁迫预处理可以启动孢子体发生途径,阻止正常的配子体发育过程,诱导产生更多单倍体[34]。尽管胁迫预处理的类型、时间可能因物种和品种而异,但发现多数胁迫预处理方式对小孢子胚胎形成有促进作用[35]。对于不同类型的外植体,预处理方式和时间不同,再生效率也不同。培养起始的胁迫预处理包括高温、低温、碳水化合物、氮饥饿、高渗透压和秋水仙素处理等,对小孢子的重编程起着关键作用。就温度胁迫而言,通常先将花序、花芽或花药置于低温或高温下处理一定时间,然后接种到培养基上进行诱导培养。对小孢子常用的预处理方法有低温、高温、饥饿、甘露醇等[36,37],其中低温预处理应用最多。在大麦小孢子的提取液和预处理液中添加适宜浓度秋水仙素不仅提高胚胎发生率,也提高了再生植株中可育株的比率[38]。据报道,4℃低温预处理5周可提高顽拗型硬粒小麦基因型雄配子体的胚胎诱导率和植株再生率[36]。Prem等[39]报道了一项通过连续 4℃低温处理高效诱导甘蓝型油菜小孢子胚胎形成的技术,单倍体诱导率显著提高。甘露醇预处理也能显著提高硬粒小麦花药培养效率[40]。通过比较4℃低温下利用甘露醇和PEG处理小麦小孢子不同时间对培养效果的影响,发现预处理5周的效果最好[36]。另外,将分离的小麦新鲜小孢子用含有18 mmol/L的2-HNA和9.0%麦芽糖的溶液在25℃、黑暗条件下处理38~52 h,然后与子房共培养,胚状体产率(每个穗子360~4914个胚状体)、绿苗产率(15.0%~95.0%)和单倍体自然加倍率(39.0%~78.0%)都得到提高,并且适用于许多基因型[41]。
1.3.4 培养基和培养条件
植物花药培养或小孢子培养能否成功诱导胚胎的形成,在很大程度上取决于培养基的组成。培养基中碳、氮等大量营养元素和微量营养元素可能决定胚胎发育的开始,植物生长调节剂的种类和浓度,尤其是生长素和细胞分裂素的合理搭配,可能是影响雄配子体胚胎发生的关键因子。研究发现,外植体从配子体向孢子体途径的重编程依赖于碳水化合物的最佳供给和植物生长调节剂的配比及浓度[23]。培养基对植物种类具有特异性,添加或减少培养基中一个或多个组分可以适应特定植物或基因型。目前针对不同植物的花药培养研发了适宜的培养基,如Nitsch培养基适用于烟草[42],MS和N6培养基适用于水稻[43,44],B5培养基适用于大豆[45],C17、W14培养基适用于小麦[46]。通过调整培养基中的大量元素和微量元素,研制出了适于小麦花药培养的癸培养基,花药培养愈伤组织平均诱导率比C17、W14和N6培养基分别提高了30.3%、50.7%和58.0%[47]。在W14D、W14Gd和W14GD等液体培养基中培养小麦花药,出现了经胚胎发生直接成苗现象[26]。同样,针对植物小孢子培养也研发了专用培养基,如适宜于小麦小孢子培养的PB2和NPB99培养基。研究表明,适当增加培养基中硫酸铜的用量可以提高小麦小孢子培养再生植株效率,并且显著减少白化苗率[48];在培养基中添加适量阿拉伯半乳聚糖蛋白(arabinogalactan proteins, AGPs)可以提高小孢子的活性,增加小孢子胚状体诱导数量[49];在4℃条件下用0.4 mol/L甘露醇预处理小麦幼穗,然后在含维生素C的RM5培养基上培养小孢子可产生更多的单倍体植株[50]。除了基本培养基和生长调节剂外,在培养基中添加一些有机添加剂如谷氨酰胺、酪蛋白、脯氨酸、生物素、肌醇、椰子汁和活性炭等,对提高胚胎形成具有促进作用[51]。
培养温度、光照条件、花药或小孢子的密度等培养条件对雄配子体的胚胎形成有显著影响。在水稻花药培养中,温度被证明是诱导单倍体的一个关键因子[52],然而,有关培养温度调控花药培养效率的研究还比较少。光是影响花药培养效果的另一个环境因素,对花粉脱分化为愈伤组织具有一定抑制作用[53]。所以,花药或小孢子培养的初始阶段一般在无光照下进行,后期转移至光照条件下分化植株。对某些植物来说,光照和黑暗交替培养有利于胚胎再生[54]。
2 体内诱导作物单倍体技术
2.1 远缘杂交诱导
远缘杂交诱导包括属间杂交和种间杂交,通过染色体选择性消除产生母本单倍体(图1A)。1970年首次发现,利用球茎大麦(Hordeum bulbosum)做父本与大麦杂交,获得了大麦单倍体植株[4]。随后发现,利用球茎大麦做父本与小麦品系中国春远缘杂交,也获得了小麦单倍体植株[55]。然而,由于多数小麦品种中存在抑制远缘杂交的可交配性显性基因Kr1和Kr2,该技术仅局限于少数小麦基因型。1986年发现,小麦做母本与玉米远缘杂交,在合子发育过程中由于玉米染色体与小麦染色体有丝分裂进程不同步,玉米染色体被消除,诱导了小麦单倍体幼胚[56]。进一步研究表明,小麦与玉米杂交受精频率不受小麦Kr基因的影响,即小麦品种具有普遍适用性。由于玉米花粉萌发对Kr1和Kr2基因作用不敏感,小麦-玉米杂交诱导小麦单倍体没有小麦基因型的特异性,与其他方法相比更具实用价值。通过对13个小麦杂种F1代与玉米杂交,研究了不同小麦生长环境、生长素处理、培养基和壮苗处理对单倍体及双单倍体产生频率的影响,发现生长在大田环境的小麦去雄后割穗离体培养,然后与玉米杂交,平均得胚率23.9%,诱导效率是返青后从大田移栽到温室盆栽植株的3倍以上;利用2种不同培养基进行胚胎拯救,B5培养基上单倍体幼胚萌发率为82.0%,1/2MS培养基上幼胚萌发率为76.6%,没有显著差异[57]。本实验室分别利用玉米诱导系CI011-1和自交系郑58与矮败小麦DS987杂交,发现诱导系和自交系对诱导小麦单倍胚的效率没有显著差异[58]。玉米花粉不但可以诱导小麦产生单倍体,也能够诱导黑麦和燕麦等禾谷类作物产生单倍体[59,60]。虽然 球茎大麦、大刍草(Purus frumentum)、高粱(Sorghum bicolor)和珍珠粟(Pennisetum americarum)等植物的花粉也可以诱导小麦单倍体植株,但目前诱导最成功、应用最广泛的还是利用玉米花粉。实际工作中,可以通过分期播种的方式调节小麦和玉米花期相遇,创造适合小麦单倍体发育的环境条件,提高成胚率。另外,利用小麦作为母本与白茅(Imperata cylindrica)杂交,通过染色体消减可以诱导小麦单倍体幼胚,在诱导小黑麦单倍体植株中也显示了一定潜力[61,62]。白茅是一种多年生植物,在自然条件下与小麦和小黑麦的开花期吻合,不需要经常播种,在世界上几乎所有种植小麦的地区都可以正常生长,花粉量较大,杂交容易成功。此外,白茅几乎可以与所有小麦和小黑麦或其衍生材料杂交,不存在基因型的特异性。
2.2 花粉诱导
花粉诱导法是通过在授粉前采用物理(辐射和高温)或化学(甲苯胺蓝)处理,甚至通过延迟授粉时间等方法,致使花粉中遗传物质损伤进而诱导单倍体的产生。这些经过处理的花粉不能与胚珠内卵细胞正常受精,但能刺激卵细胞形成单倍体胚胎(图1B)。该技术已成功应用于小麦、玉米、烟草(Nicotiana tabacum)等重要作物的单倍体诱导[8]。例如,利用X射线照射处理一粒小麦(Triticum monococcum)减数分裂时期的植株,获得了单倍体籽粒,诱导率0.5%[63];对玉米花粉进行3个不同温度(40℃、45℃和50℃)热击处理,发现50℃热击处理下单倍体诱导率达到1.5%,而未经热击处理的单倍体诱导率仅为0.6%[64]。通过对一粒小麦延迟授粉时间,单倍体植株诱导频率提高,在延迟授粉9天处理下单倍体诱导率达到37.5%[65]。花粉诱导法最关键的环节在于对花粉的处理,其中,使用辐射处理的剂量需要适中,剂量偏低花粉生殖核部分受损但能与卵细胞结合产生杂交种,剂量偏高胚胎形成率降低但多为单倍体[66],延迟授粉时间要根据不同物种开花规律掌握。图1
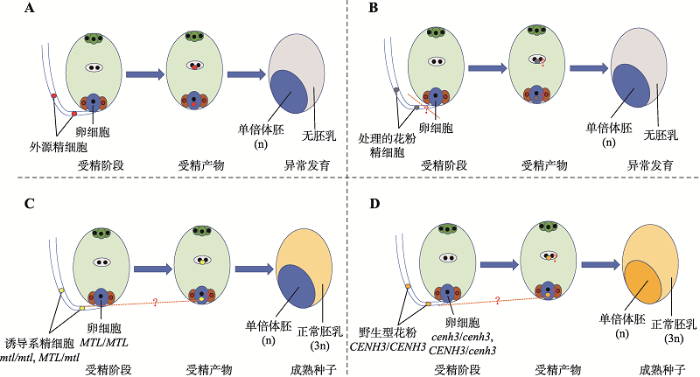 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1体内诱导植物单倍体4种方法示意图
A:远缘杂交诱导;B:花粉诱导;C:诱导系诱导(MTL);D:诱导系诱导(CENH3)。
Fig. 1A diagram of the four methods for in vivo induction of haploid plants
2.3 诱导系直接诱导和遗传修饰间接诱导
2.3.1 诱导系诱导诱导系诱导是目前玉米产生单倍体的主要方式(图1C)。1959年Coe等[67]首次报道,玉米单倍体诱导系Stock 6作为父本可诱导母本产生2.0%~3.0%的单倍体种子。随着WS14、MHI、CAUHOI、RWS、PHI、UH400、BHI306和CAU5等优良玉米单倍体诱导系的培育,玉米单倍体植株诱导率可达8.0%~ 16.0%[68,69,70,71]。中国农业大学陈绍江教授实验室利用分子标记辅助选择方法培育了3个高油诱导系,油份含量8.5%左右,单倍体诱导率约为8.0%[72];在此基础上,创制出诱导率达到8.0%以上的高频诱导系6个,其中两个诱导系的诱导率超过15.0%。研究表明,玉米诱导系中诱导玉米单倍体主要由关键基因MTL/ZmPLA1/NLD控制[73,74,75]。通过精细定位、基因组测序、遗传互补和基因编辑等方法,发现玉米中的单倍体诱导是由于一种精子细胞质特异性磷脂酶(matrilineal, MTL)编码基因的移码突变导致[74]。将玉米诱导系Stock 6的衍生系CAU5与模式玉米基因型B73的参考基因组序列进行比对,发现ZmPLA1第4外显子中存在4 bp (CGAG)的插入,该移码突变导致MTL蛋白20个氨基酸的改变和29个氨基酸的缺失[73]。
2.3.2 CRISPR/Cas9编辑MTL蛋白编码关键基因诱导
通过在ZmPLA1第1外显子中设计gRNA靶位点,构建含ZmPLA1基因靶点和Cas9序列的表达载体,利用农杆菌介导法转化玉米自交系的幼胚,在转基因植株中检测到10株发生基因编辑,然后分别选取在gRNA靶位点发生1 bp插入、11 bp缺失和1 bp缺失的3个T1株系自交,并作为父本与ZD958和JK968杂交,发现单倍体诱导率为2.0%[73]。利用CRISPR/Cas9基因编辑技术对玉米ZmMTL/ZmPLA1基因自然突变发生位点上游5 bp位置进行靶向突变,突变率达87.1%;同时,采用胚特异型启动子ZmESP和胚乳特异性启动子HvASP,构建胚特异表达绿色荧光蛋白(eGFP)和胚乳特异表达红色荧光蛋白(DsRED)的双荧光单倍体筛选与标记鉴定系统,获得的诱导系能够高效诱导单倍体,诱导率可达11.0%,平均诱导率7.5%[76]。
禾谷类作物中MTL高度保守,因此可以利用CRISPR/Cas9编辑技术创制多种作物单倍体诱导系,建立诱导单倍体技术体系。针对水稻中ZmMATL基因的同源基因OsMATL,分别在其第1个外显子的氨基酸末端和第4外显子距离玉米ZmMTL/ZmPLA1基因自然突变发生位点7 bp位置设计两个靶点gRNA进行CRISPR/Cas9基因编辑,突变率达80.0%,自交后代中单倍体平均诱导率为6.0%[77]。另一项研究中,利用CRISPR/Cas9基因编辑技术沉默水稻品种春优84中玉米单倍体诱导基因MTL的同源基因,在248株转基因后代中筛选到具有杂交稻背景的9株单倍体植株和2株双单倍体植株,证明通过基因编辑可以诱导水稻产生单倍体[78]。最新研究表明,采用CRISPR/Cas9基因编辑技术敲除小麦中玉米MTL基因的两个同源等位基因TaPLA-A和TaPLA-D,成功诱导了小麦单倍体籽粒,诱导率为2.0%~3.0%[79]。本实验室针对小麦优化了CRISPR/Cas技术体系,进一步对小麦TaMTL基因进行编辑,发现编辑植株繁茂性和结实率等性状显著降低,通过对编辑植株进行分子细胞遗传学和表型鉴定,单倍体籽粒诱导率高达18.9%,并且首次在禾谷类作物中发现了无胚型和胚及胚乳双无型籽粒[80]。
2.3.3 沉默着丝粒特异性组蛋白CENH3编码基因诱导
编码着丝粒特异性组蛋白的基因CENH3是近年来利用诱导系诱导单倍体的另一个研究热点(图1D)。Ravi等[81]发现,拟南芥cenh3无效突变体具有致死性,将经过遗传修饰的着丝粒特异性组蛋白编码基因CENH3导入该拟南芥突变体,可恢复植株的野生表型,转基因植株与野生型植株杂交可产生只含野生型基因组的单倍体,其原因是CENH3介导了母本基因组的完全消除。利用RNAi技术沉默内源CENH3基因,然后将GFP-CENH3-tailswap表达载体导入沉默内源CENH3基因的材料,CENH3活性恢复,自交后结实率正常。然而,当利用转基因株系与非转基因株系杂交时,从140个子代中鉴定出许多非整倍体和1个单倍体。这些结果表明,CENH3着丝粒介导和RNAi技术可用于多倍体作物的单倍体诱导[82]。利用EMS (ethymethyl sulfonate)诱变和TILLING (targeting induced local lesions in genomes)技术结合,在拟南芥中已鉴定到CENH3的多个保守氨基酸位点(如L130F、P82S、G83E、A132T、A136T和A86V等),cenh3-1突变体中任一氨基酸的互补后与野生型杂交,可诱导单倍体的产生,后代单倍体比例为0.6%~12.2%[83,84]。EMS诱变结合TILLING分析技术操作简便、通量高,可以对CENH3每个氨基酸进行点突变,而且该技术不涉及转基因[8]。最近,从拟南芥EMS诱变后代中鉴定了31个单个氨基酸替换的CENH3等位基因,将其导入cenh3敲除突变体,可以补偿内源CENH3的敲除效应,转基因植株接受野生型花粉后能够诱导单倍体的产生。并且,利用CRISPR/Cas9编辑技术获得了保守的CENH3组蛋白折叠结构域中αN螺旋的移码突变类型,该类型为优良的单倍体诱导系,用野生型花粉授粉时诱导系生长和发育正常[85]。由于CENH3广泛存在于真核生物中,且具有高度进化保守性,人们尝试在其他植物中利用CENH3组蛋白介导的方法诱导产生单倍体。通过对玉米CENH3进行遗传修饰,成功获得了单倍体,虽然单倍体诱导率非常低,但为其他重要作物建立单倍体诱导技术提供了借 鉴[86]。
2.3.4 诱导系诱导单倍体的机理
目前,玉米单倍体诱导系的作用机制还不清楚,主要有2种假说,即单受精孤雌生殖和合子后基因组消除[87,88]。研究表明,玉米单倍体诱导过程中发生了双受精,染色体消失是玉米单倍体诱导的基础[71,87,88]。为了明确玉米单倍体诱导的机理,Zhao等[88]检测了利用两个新玉米诱导系CAUB (含有B染色体)和CAUYFP (含有CENH3-YFP)诱导的单倍体植株,在少数单倍体中检测到B染色体和44 Mb左右的诱导系染色体片段,发现了染色体选择性消失的部分证据,进一步证实单倍体的形成发生了双受精,存在诱导系染色体渗入现象。进一步利用HI-Edit方法获得只含母本全套染色体的单倍体编辑植株,表明处在瞬时合子状态下,雌雄配子融合,卵细胞受精,为合子后基因组消除假说提供了直接证据[7]。综上所述,玉米单倍体诱导系诱导单倍体的原理是其染色体在合子胚中选择性消失,但不排除单受精促进单倍体诱导的可能性,染色体消失的原因和具体过程尚不清楚。着丝粒介导的染色体消除的机制可能是由于修饰的着丝粒姐妹染色单体在合子发生有丝分裂时,纺锤丝未能牵引CENH3突变的姐妹染色单体分离,从而使突变体亲本的染色体消失,形成了单倍体[89]。MTL和CENH3是植物单倍体诱导的关键基因,可以利用MTL和CENH3介导的诱导系诱导单倍体的产生,然后进行细胞学和遗传学等研究,以阐明单倍体诱导的确切机制[73,74,88,90]。
3 单倍体诱导技术的应用
3.1 新品种培育
通常采用传统育种方法获得作物纯合稳定品系需要连续7代自交和选择,且无法实现所有性状的绝对纯合,而双单倍体技术只需1~2代即可获得遗传上100.0%纯合的品系。高效的双单倍体技术能节约育种时间和成本,克服常规育种的许多局限,加速育种进程[5]。油菜(Brassica napus)品种Maris Haplona是第一批利用单倍体技术育成的作物品种,其次是大麦品种Mingo[91,92]。随着单倍体诱导技术和染色体加倍方法不断完善,双单倍体技术被全球众多实验室和生物技术公司用于多种作物的改良,包括大麦、小麦、油菜、水稻、玉米和烟草等[8]。欧洲目前种植的大麦品种中约50.0%是通过DH技术选育出来的[5]。法国、加拿大等育成了一批高产优质的小麦新品种[93],并在欧洲和中东地区得到推广,同时建立了整套单倍体育种规模化技术体系,每年可获得数以万计的小麦单倍体植株。20世纪80年代以来,中国科学家利用花培技术培育了中花8号、中花9号、中花10号、中花14、中花15和汕优163等水稻新品种,以及京花1号、花培5号、花培8号、奎花2号、宁春50号等小麦新品种。利用小麦-玉米杂交技术培育了高产、节水、抗条锈病和白粉病的小麦新品种中麦533,在区域试验和生产试验中比对照增产5.2%~9.7%[94]。3.2 DH群体构建和遗传分析
育种家通常采用分子标记辅助选择(molecular marker assisted selection, MAS)和全基因组选择(genome wide selection, GWS)提高育种效率。利用目标基因的特异性分子标记定向选择目标植株,丢弃大量非目标基因组合的植株,最小化田间试验和减少世代数量,发挥MAS的最大效用[24,95]。MAS结合DH技术为提高遗传增益和缩短育种时间提供了新的策略,并已成功加速禾谷类作物的育种进程。通过MAS与DH技术的有机结合,创制了一系列含有抗锈病基因的小麦DH品系[96]。回交转育是改良具有明显缺陷优良品种的一种有效方法。在回交过程中通过分子标记辅助选择,将1个或有限数量的基因以易位方式转移至的优良品种中,实现优良基因的积聚[97]。但回交完成后,需要自交固定目的基因。对于单基因易位转移,所需纯合基因型个体的预期概率是1/4。而对于多基因的渐渗,预期基因型的频率呈1/4n指数下降(其中,n是分离目的基因的个数)。然而,单倍体目标基因型以1/2n的频率产生[97,98,99]。因此,利用DH技术显著减少了选择目标基因型所需的群体大小,结合分子标记辅助选择提高了选择目标基因的效率和精确度。分子标记辅助选择育种方法受遗传标记数量及分布的影响,多数条件下仅能检测出少量遗传变异,随着多种植物全基因组测序的完成,大量单核苷酸多态性(single nucleotide polymorphism, SNP)标记被开发出来,利用DH系进行GWS育种能大大提高育种效率[24,66]。与MAS相比,GWS基于表型和基因型数据,采用全基因组标记预测对照群体基因组预估育种值(genomic estimated breeding values, GEBVs);同时,在一个育种周期内,利用对照群体中估算的标记效应用于没有表型分析的GEBVs预测,该GEBVs可以提早或在没有出现表型特征之前预 测,使育种者做出遗传增益的早期选择,缩短选择周期[100,101]。通过对两个玉米自交系杂交产生的DH和F2群体进行的GWS和MAS分析表明,GWS效果优于MAS;在使用GWS情况下,DH群体效果优于F2群体[102]。
反向育种包括利用一定方法获得杂交种原亲本品系,将原亲本品系重新固定杂种优势以获得更多的杂交种,增加育种中可筛选的杂交种数量。利用杂交育种技术时,期望通过促进减数分裂时期同源染色体的交叉和交换,实现基因重组,打破有利基因与不利基因间的连锁,聚集有利基因[103]。反向育种正好与此相反,期望完全阻止同源染色体的交叉和交换,使得同源染色体在减数分裂中期Ⅰ和后期Ⅰ如同单价体一样随机分离[103]。Wijnker等[104]成功实现拟南芥反向育种,验证了着丝粒介导的单倍体诱导技术的可行性,为其他植物提供了新思路。DH技术与反向育种结合能解密杂交品种的亲本基因型,从杂交种中获得纯合的原亲本品系,重建原始杂种,固定杂种优势[66]。
3.3 遗传图谱构建和基因组研究
由于DH群体完全纯合,遗传稳定,背景一致,是遗传作图的理想材料。通过构建拟南芥亲本的DH系和重组自交系,比较两个群体在数量性状基因座(quantitative trait locus, QTL)定位上的差异,发现二者的重组率和亲本等位基因频率相似,并利用新构建的DH群体定位到开花时间和叶柄长度的QTL,表明DH群体可以代替重组自交系成为拟南芥遗传研究的便捷材料[105]。DH群体遗传作图已成功应用于多种重要作物,尤其在大麦、水稻、油菜和小麦等研究中应用普遍[106]。DH群体已被广泛用于QTL作图,如玉米虫漆酶,大麦抗赤霉病,水稻砷富集、产量相关性状、株高和蒸煮品质,以及小麦株高、抽穗期、面粉颜色、磷利用效率、缺锌和抗黑穗病等QTL研究[8]。尤其在水稻中,DH群体已被用于产量、品质等农艺性状的QTL定位。DH群体在分离群体分组分析(bulked segregation analysis, BSA)中建立标记与性状关联方面也是非常理想的材料选择。BSA分析主要用于在双亲群体分离后代中选择具有极端表型的个体混样,通过比较不同极端混池之间的多态性并结合表型,对受环境影响较小的质量性状基因或主效基因进行初步定位。构建遗传分离世代的DH群体,从选定的DH群体中提取DNA,然后通过分子标记逐步进行基因分型。BSA分析结合DH技术可以建立与病虫害和品质等性状关联的标记辅助选择体系[107]。DH群体在基因组学研究中也有重要作用,可以在DH群体中利用表达序列标签(expressed sequence tag, EST)和QTL相对位置寻找候选基因,利用BAC (bacterial artificial chromosome)文库确定候选基因在染色体上的物理位置。DH群体在整合遗传和物理图谱中扮演至关重要的角色,提高了靶向候选基因的精确性。单倍体和双单倍体也可用于基因组测序。双单倍体相比杂交种,基因位点纯合,遗传基础简单,且可以长期保存,重复利用,在全基因组测序、组装和拼接中具有较大优势。目前多种植物的双单倍体已被用于参考基因组测序。诱变提供了另一种将基因与表型联系起来的方法。TILLING突变体库已成功应用于几个物种的正向和反向遗传学研究,利用突变体库优选DH系可以避免由于原始材料遗传变异导致的假阳性。DH技术也可用于突变体后代的快速分离和纯合。诱变处理的材料通常是种子,但也可以有目的性选择小孢子进行处理。通过诱变处理配子细胞,然后诱导胚胎形成和DH系产生,可直接构建纯合突变体系[108]。这样避免了嵌合体或杂合子的掩蔽效应,隐性性状和显性性状在单倍体细胞、组织和植株水平上能够正常表达和被识别。在许多植物中,单倍体诱变处理结合改进的DH技术成为一种可行的途径。
3.4 基因工程改良
随着多种植物全基因组序列的释放,越来越多的基因功能被注释。然而,利用多种正向遗传和反向遗传学方法验证基因功能还比较困难。二倍体或多倍体隐性突变基因或转基因不能在M1或T0代植株中表现出来,需要自交多代获得纯合基因型,同时验证多个基因的功能则需要自交更多代,花费的时间和成本成倍增加,成为植物基因功能验证的重要障碍和瓶颈之一[66]。单倍体和双单倍体均可用于目标基因转化,产生遗传稳定的转基因植株,从而用于目标基因定位和功能鉴定等研究[5]。利用3种不同的转化方法(基因枪法、电激转化法和农杆菌介导法)对小麦小孢子或小孢子胚胎进行遗传转化,获得了纯合的转基因植株[109]。利用基因枪介导法对大麦小孢子进行转化,获得纯合转基因植株[110]。将大麦HVA1基因转入小麦花药来源的单倍体胚胎,获得耐旱单倍体植株,对其进行加倍获得纯合双单倍体转基因植株[111]。利用农杆菌介导法转化水稻花药愈伤组织,经自然加倍和秋水仙素诱导加倍,获得含Xa21基因双单倍体转基因植株[112]。单倍体技术结合基因工程技术在获得纯合转基因植株的同时,可以去除标记基因,获得无筛选标记转基因植株[113]。单倍体技术结合基因编辑技术可以在一个生育期内获得含有目标性状的DH系,继而进行基因功能分析,该技术可用于商业化作物品种的遗传修饰[7,114]。总之,诱导T0代转基因植株产生单倍体,加倍后对双单倍体植株进行分子检测,只要检测到转入基因的存在,即可筛选到纯合转基因植株,缩短了通过自交获得纯合转基因植株的时间,加快对转基因植株进行功能鉴定的速度。4 植物单倍体诱导研究的思考与展望
4.1 提高植物单倍体诱导频率的策略
半个世纪以来,单倍体诱导技术在水稻、小麦和玉米等重要农作物遗传改良中广泛应用,虽然取得了许多成果,但单倍体诱导频率依然偏低。目前,在单倍体诱导研究和应用中,水稻主要采用花药培养和小孢子培养技术,小麦主要采用花药培养、小麦-玉米杂交(染色体消除)和小孢子培养技术,玉米主要采用诱导系诱导技术。相比之下,花药培养存在强烈的基因型特异性,基因型间差异显著,同时受母体生理状态、培养基和培养条件的影响。因此,筛选和利用花药培养力高的基因型作为亲本之一,在适宜的栽培环境中保证母体植株生长健壮,改进培养基和培养条件,以提高花药培养技术诱导单倍体植株的效率。小孢子培养在诱导单倍体植株的数量上具有优势,基因型的特异性小,但操作技术比较复杂,容易污染,简化培养程序、采用适宜的预处理方式、减少污染等可以提高小孢子培养技术诱导单倍体植株的效率。染色体消除技术的基因型依赖性也比较小,但授粉后在温和、高湿的生长环境中才能获得较高的得胚率(可达50.0%以上),并且需要离体培养进行胚胎拯救,采用甜玉米花粉和授粉后在人工气候室中水培小麦植株可以提高染色体消除技术诱导小麦单倍体植株的效率。尽管诱导系诱导玉米单倍体的效率最高可达20.0%~30.0%,但诱导单倍体的同时伴有不良农艺性状的出现,严重时甚至死亡,即诱导系的遗传背景一定程度上限制了这些诱导系的应用,需要利用高诱导率的诱导系与优良自交系杂交,创制农艺性状好、诱导频率高的诱导系。另外,现已明确了不同玉米诱导系诱导单倍体胚胎发生主要由两个关键基因MTL和DMP控制[73~75,115],可以通过杂交育种途径培育聚合这两个基因的新型诱导系,提高利用该技术诱导玉米单倍体植株的效率。4.2 通过编辑或诱变MTL基因获得单倍体诱导系
近年来,植物单倍体发生的遗传机理逐步成为了研究热点。CENH3介导的基因组消除诱导单倍体是单倍体技术的一项重大突破,该方法已成功用于玉米和芥菜等作物新品种培育,可望应用于更多作物单倍体育种工作。在种间杂交诱导单倍体中,认为染色体消除与单亲本着丝粒失活有关[116]。在种内杂交中,发现精子特异性磷脂酶(MTL)是玉米单倍化产生的主要因子[73,74,75],并克隆了玉米MTL基因。通过CRISPR/Cas9技术编辑玉米、水稻和小麦中的MTL基因,相继获得了玉米、水稻和小麦单倍体植株[73,76,77,79]。该基因在禾谷类作物中具有保守性,利用CRISPR/Cas9技术编辑更多禾谷类作物中的MTL基因,可以建立这些作物单倍体诱导体系。同时,可以克隆双子叶植物尤其豆科植物中的MTL基因,进一步通过CRISPR/Cas9技术编辑这些植物中的MTL基因,获得单倍体植株,解决豆科植物单倍体育种的瓶颈问题。另外,由于基因编辑技术仍然依赖于转基因环节,利用CRISPR/Cas9编辑MTL基因获得的单倍体诱导系在实际应用中受到限制,可以利用辐射诱变的方法处理植物种子或花粉,在后代群体中筛选MTL基因发生沉默的突变体,用于商业化目标的作物单倍体育种工作。最近,先正达公司将远缘杂交诱导单倍体与CRISPR/Cas9编辑技术结合,获得了TaGT1-4A、TaGT1-4B和TaGT1-4D基因编辑,且不涉及转基因环节和无外源DNA序列整合的小麦单倍体种子,显示了良好的应用前景[7]。同样道理,可以将小麦近缘植物中MTL基因编辑靶点gRNA和Cas9转入玉米或大麦中,利用近缘植物与玉米或大麦杂交过程中,玉米或大麦花粉携带的MTL基因的gRNA和Cas9在近缘植物子房中瞬间表达,进而编辑雌配子中的MTL基因,获得MTL基因编辑的单倍体,通过染色体加倍获得单倍体诱导系,用于这些作物的单倍体育种。最近,利用诱导系CAUHOI和CAU5组配的群体,在qhir8位点定位到了另外一个单倍体诱导基因ZmDMP,将其定位在成熟花粉的细胞膜上,发现ZmDMP基因单碱基突变体单倍体诱导率提高2~3倍,完全敲除ZmDMP基因单倍体诱导率提高5~6倍,丰富了诱导植物单倍体诱导的基因资源。尤其,同时编辑ZmMTL和ZmDMP基因显著提高了单倍体诱导率[115]。因此,利用基因编辑技术敲除与植物胚胎发生的相关基因,能够简单、快速获得作物单倍体,具有广阔应用前景。4.3 单倍体诱导技术结合基因编辑技术创造突变体
基因编辑技术结合单倍体诱导技术不但可以编辑重要基因,而且能够获得纯合编辑植株。根据小麦GRASSY TILLER1同源基因TaGT1-4A、TaGT1-4B和 TaGT1-4D设计gRNA靶位点,构建CRISPR/Cas9表达载体,转入到玉米自交系NP2222中,然后利用转基因玉米花粉给小麦授粉,获得了小麦GRASSY TILLER1基因编辑的单倍体植株和加倍单倍体植株[7]。利用基因编辑技术同时敲除杂交水稻品种春优84中4个内源基因PAIR1、REC8、OSD1和MTL,获得了可以发生无融合生殖的Fix (Fixation of hybrids)材料;在145个Fix后代植株中有136个四倍体,9个二倍体;通过测序鉴定,二倍体子代基因型与亲本完全一致,均为稻春优84遗传背景,且这些F2代植株的表型也与野生型高度相似;证明通过基因编辑技术同时编辑这4个基因,可将无融合生殖特性引入到杂交稻中,实现杂合基因型的固定[78]。将靶定ZmVLHP和ZmGW2基因的CRISPR/ Cas9载体分别转化玉米自交系NP2222,然后与玉米诱导系RWKS杂交,在F2代分离群体中筛选到mtl纯合并且含Cas9基因的诱导系植株,再分别与玉米自交系GP721、GP650、154F04、ID5829 和412F杂交,获得编辑ZmVLHP和ZmGW2的单倍体植株,该方法被称为HI-Edit[7]。另外,将含ZmLG1基因靶点的CRISPR/Cas9载体转入玉米自交系ZC01,转基因植株与诱导系CAU5杂交,创制了携带CRISPR/Cas9载体(用于编辑ZmLG1基因)的诱导系;然后将该诱导系用作父本与B73杂交,获得245株单倍体植株,其中10株中ZmLG1基因发生了编辑,编辑效率约为4.1%,该方法被称为IMGE (haploid inducer- mediated genome editing)[117]。HI-Edit和IMGE方法是将单倍体诱导与CRISPR/Cas9结合的典范,在两个世代内即可创造经基因编辑改良的双单倍体系。4.4 通过诱导系或MTL基因编辑技术诱导单倍体的鉴定
单倍体在植株水平可以利用细胞学、细胞遗传学、基因组学和形态学等方法进行鉴定,包括保卫细胞长度、染色体数目、DNA总量、基因拷贝数和植株高度等[58,79]。通过花药培养、子房培养和远缘杂交等技术诱导产生的植株几乎全部为单倍体,可以全部进行染色体加倍处理获得二倍体植株。但通过CRISPR/Cas9技术编辑MTL基因或诱导系授粉产生的单倍体胚,其频率较低,需要在籽粒发育阶段辨别单倍体和二倍体,以便进行染色体加倍。花青素基因R1-nj可以作为胚和胚乳特异性标记,用于鉴定玉米诱导系诱导的单倍体。含有纯合R1-nj标记基因的玉米诱导系与单交种杂交,诱导产生的单倍体胚无颜色,胚乳紫色,可以快速鉴别单倍体种子[118]。然而,R1-nj基因表达受供体基因型和环境的影响。另外,胚和胚乳特异性标记的颜色变化范围难以明确分级,如果母本中含有抑制R1-nj表达的基因如C1-L12,利用R1-nj标记无法准确鉴定单倍体,且不能实现批量和自动化鉴定[119]。基于高油直感效应鉴定玉米单倍体系统克服了花青素基因R1-nj的局限性。具体来说,高油诱导系与单交种杂交后诱导产生的单倍体油份含量低于单交种,而杂种油份含量介于高油诱导系与单交种之间,与单倍体存在显著性差异,通过油份含量可以准确鉴定单倍体[118]。玉米籽粒油份含量可以通过快速、准确的自动化核磁共振筛选系统进行,具有较强的可操作性[120]。
最近,利用胚特异型启动子ZmESP和胚乳特异性启动子HvASP分别构建胚特异表达绿色荧光蛋白和胚乳特异表达红色荧光蛋白的双荧光单倍体筛选与标记鉴定系统,可有效、准确鉴定玉米单倍体种子,即:用CRISPR/Cas9编辑MTL基因并携带双荧光蛋白标记的诱导系做父本与其他玉米品种杂交,单倍体胚乳中显红色荧光、胚中没有荧光,而二倍体胚乳显红色荧光、胚中显绿色荧光,为作物单倍体鉴定提供了新思路[76]。但是,遗传标记的添加需要借助转基因技术,操作比较繁琐。流式细胞技术是一种根据细胞核DNA含量可快速、准确判断植物倍性的方法,已广泛用于多种作物的单倍体鉴定[74]。
4.5 安全高效单倍体加倍技术建立和完善
单倍体植株的自然加倍率因物种和所采用的技术而不同。以小麦为例,花药培养获得单倍体植株的自然加倍率为20.0%~30.0%,小孢子培养的自然加倍率为30.0%~40.0%,玉米花粉诱导的单倍体植株完全不能自然加倍,需要用人工方法进行染色体加倍。目前,单倍体植株染色体加倍主要采用秋水仙素处理,但秋水仙素存在毒性较强、危及人体健康、污染环境、用量较大和价格较高等问题,需要寻找安全、低廉和环境友好型加倍试剂。在加倍方法上,利用最多的是在通气泵介导的供氧条件下处理单倍体幼苗分蘖节,然后流水冲洗若干小时。研究表明,甲基胺草磷、炔苯酰草胺和氟乐灵等除草剂对单倍体植株具有加倍效果,利用适宜浓度甲基胺草磷溶液滴心处理三叶期至五叶期源自玉米诱导系诱导的玉米单倍体植株,加倍率20.0%~30.0%。利用适宜浓度秋水仙素溶液涂抹拔节期源于玉米花粉诱导的小麦单倍体植株的叶片,加倍率7.7%。培养基表面添加不同浓度秋水仙素溶液处理来自花药培养诱导的小麦单倍体植株,加倍率26.7%~85.7%;培养基表面添加不同浓度炔苯酰草胺溶液处理来自玉米花粉诱导的小麦单倍体植株,加倍率28.6%~ 100.0%。说明炔苯酰草胺溶液对小麦和玉米单倍体植株均有较好加倍效果,可以在其他作物中尝试;培养基表面添加适宜浓度加倍试剂不但加倍效果明显,而且简单易行。另外,利用植物中存在的自然加倍的遗传机制实现对人工诱导单倍体植株的自然加倍,也是一个极具潜力的研究方向。研究表明,小麦单倍体染色体组自然加倍率与未减数配子形成率呈高度正相关,并在四倍体小麦-节节麦的单倍体群体中检测到1个依赖单倍体、影响未减数配子形成的主效QTL位点QTug.sau-3B,该位点可能位于Ttam.3B基因区域,进一步发现Ttam基因的低表达可能促进小麦单倍体中高频率未减数配子发生[121,122]。所以,利用基因编辑技术沉默小麦中的Ttam基因可能会提高单倍体植株自然加倍频率。尽管单倍体诱导技术已经取得了良好进展,但在利用方面依然存在很多挑战,如单倍体诱导严重受物种和基因型的影响,高粱、大豆等重要经济作物至今仍然无法大量获得单倍体植株。虽然着丝粒介导基因组消失法在理论上可用于诱导所有植物的单倍体,然而目前除了拟南芥外很少有成功的实例,需要进一步研究。另外,利用单倍体器官和组织进行的转基因效率还非常低[64]。对单倍体诱导及胚胎形成的分子遗传机制和单倍体基因组自然加倍的遗传机理等方面的了解还比较少,尚不清楚玉米体内单倍体诱导的机制是染色体消除还是单一受精。通过深入了解单倍体诱导过程,可以改进单倍体诱导系统并将单倍体诱导技术应用到重要作物遗传改良中。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1126/science.55.1433.646URL [本文引用: 1]
[本文引用: 1]
DOI:10.2183/pjab1945.44.554URL [本文引用: 1]
DOI:10.1038/225874a0URL [本文引用: 2]
DOI:10.1016/j.tplants.2007.06.007URL [本文引用: 5]
[本文引用: 2]
DOI:10.1038/s41587-019-0038-xURL [本文引用: 6]
DOI:10.1111/pbi.2010.8.issue-4URL [本文引用: 5]
[本文引用: 2]
DOI:10.1023/A:1006023725640URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/S0168-9452(02)00424-7URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1266/jjg.69.35URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/s11240-005-9017-7URL [本文引用: 1]
[本文引用: 2]
DOI:10.1016/j.biotechadv.2015.07.001URL [本文引用: 4]
[本文引用: 1]
DOI:10.3724/SP.J.1006.2010.01209URL [本文引用: 2]

Wheat anther culture is affected greatly by many factors, such as wheat genotype, culture medium, and the temperature during the growing period of donors. For further improving the regeneration efficiency from wheat anthers and understanding the genetic control of wheat anther culture ability, some wheat genotypes including Verry, Xinchun 9, Chinese Spring, and Ningchun 4, and their F1 hybrids by crossing each other were used for anther cultures in different years, and the callus induction frequencies were evaluated. The results indicated that haploid calli were easily induced from the anthers of Alondra, Verry, Shi 4185, Xinchun 9, and Bainong 3217. The frequency of callus production ranged from 25.3% to 51.9%. Shi 4185 and Xinchun 9 showed higher potentials for wheat haploid breeding because of their good agronomic traits and high anther culture response. Some F1environment conditions for the donor plants of the anthers were long vegetative growing period, moderate temperature during the tillering period, and high temperature during the hybrids derived from Ningchun 4 had high callus production rates, indicating that Ningchun 4 had high combining ability with some crossing parents in anther culture. However, the F1 hybrids from the parents with high anther culture response were not certain to perform high callus frequency, which was more than 10.0% in majority of the crosses. This suggested that the genetic control of wheat anther culture appeared to be complicated and performed as a quantitative trait. The optimal late jointingperiod. Haploid plantlets could be induced directly from wheat anthers in the liquid media with low concentrations of auxin and glucose. It was also found that 2,4-D was better than dicamba in liquid media for callus induction. Besides, the frequency of callus induced from wheat anthers showed increase trend with the promotion of temperature during the growing period of wheat donor plants, which was caused by climate warming.
DOI:10.3724/SP.J.1006.2010.01209URL [本文引用: 2]

Wheat anther culture is affected greatly by many factors, such as wheat genotype, culture medium, and the temperature during the growing period of donors. For further improving the regeneration efficiency from wheat anthers and understanding the genetic control of wheat anther culture ability, some wheat genotypes including Verry, Xinchun 9, Chinese Spring, and Ningchun 4, and their F1 hybrids by crossing each other were used for anther cultures in different years, and the callus induction frequencies were evaluated. The results indicated that haploid calli were easily induced from the anthers of Alondra, Verry, Shi 4185, Xinchun 9, and Bainong 3217. The frequency of callus production ranged from 25.3% to 51.9%. Shi 4185 and Xinchun 9 showed higher potentials for wheat haploid breeding because of their good agronomic traits and high anther culture response. Some F1environment conditions for the donor plants of the anthers were long vegetative growing period, moderate temperature during the tillering period, and high temperature during the hybrids derived from Ningchun 4 had high callus production rates, indicating that Ningchun 4 had high combining ability with some crossing parents in anther culture. However, the F1 hybrids from the parents with high anther culture response were not certain to perform high callus frequency, which was more than 10.0% in majority of the crosses. This suggested that the genetic control of wheat anther culture appeared to be complicated and performed as a quantitative trait. The optimal late jointingperiod. Haploid plantlets could be induced directly from wheat anthers in the liquid media with low concentrations of auxin and glucose. It was also found that 2,4-D was better than dicamba in liquid media for callus induction. Besides, the frequency of callus induced from wheat anthers showed increase trend with the promotion of temperature during the growing period of wheat donor plants, which was caused by climate warming.
DOI:10.3724/SP.J.1006.2018.00208URL [本文引用: 1]
DOI:10.3724/SP.J.1006.2018.00208URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/s11738-016-2085-yURL [本文引用: 1]
DOI:10.1023/A:1005748614261URL [本文引用: 1]
DOI:10.1046/j.1365-313X.1997.12040949.xURL [本文引用: 1]
[本文引用: 1]
DOI:10.1007/s10681-006-9238-9URL [本文引用: 1]

Isolated plant microspores, when stressed and cultured in vitro, can be diverted from their normal gametophytic pathway towards sporophytic development, with the formation of haploid embryos and ultimately doubled-haploid plants. This process is called androgenesis or microspore embryogenesis, and is widely used in plant breeding programmes to generate homozygous lines for breeding purposes. Protocols for the induction of microspore embryogenesis and the subsequent regeneration of doubled haploid (DH) plants have been successfully developed for more than 200 species. These practical advances stand in stark contrast to our knowledge of the underlying molecular genetic mechanism controlling this process. The majority of information regarding the genetic and molecular control of the developmental switch from gametophytic to sporophytic development has been garnered from four intensely studied (crop) plants comprising two dicotyledonous species, rapeseed (Brassica napus) and tobacco (Nicotiana tabacum), and two monocotyledonous species, wheat (Triticum aestivum) and barley (Hordeum vulgare). In these species the efficiency of microspore embryogenesis is very high and reproducible, making them suitable models for molecular studies. In the past, molecular studies on microspore embryogenesis have focussed mainly on the identification of genes that are differentially expressed during this developmental transition and/or early in embryo development, and have identified a number of genes whose expression marks or predicts the developmental fate of stressed microspores. More recently, functional genomics approaches have been used to obtain a broad overview of the molecular processes that take place during the establishment of microspore embryogenesis. In this review we summarise accumulated molecular data obtained in rapeseed, tobacco, wheat and barley on embryogenic induction of microspores and define common aspects involved in the androgenic switch.
DOI:10.1111/ppl.2006.127.issue-4URL [本文引用: 1]
[本文引用: 3]
DOI:10.1007/BF02173100URL [本文引用: 1]
DOI:10.1007/s11240-007-9288-2URL [本文引用: 1]
DOI:10.1186/1471-2229-12-127URL [本文引用: 1]
DOI:10.1111/pbr.2007.126.issue-6URL [本文引用: 1]
DOI:10.1007/s00299-001-0393-0URL [本文引用: 1]
DOI:10.1126/science.163.3862.85URL [本文引用: 1]
DOI:10.1111/ppl.1962.15.issue-3URL [本文引用: 1]
[本文引用: 1]
DOI:10.1016/0014-4827(68)90403-5URL [本文引用: 1]
DOI:10.5897/AJBURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/s00299-005-0013-5URL [本文引用: 1]

The objective of this study was to improve induction of embryogenesis in wheat microspore culture in order to obtain a high number of regenerable embryos. The arabinogalactan (AG) Larcoll and the arabinogalactan-protein (AGP) from gum arabic were tested on two spring genotypes to see if they could increase microspore viability and induce embryogenesis in the microspore culture. Adding Larcoll significantly decreased microspore mortality in both genotypes regardless of the presence or absence of ovaries in the culture. Similarly, gum arabic had a strong effect on the number of embryos produced and regenerated green plants. In fact, by using only gum arabic we were able to obtain green plants from wheat microspore cultures without the presence of ovaries. In addition to preventing a high mortality rate of the cells, our results show that the induction of embryogenesis in wheat microspore cultures is strongly affected by the use of both AG or AGP.
DOI:10.2135/cropsci2012.03.0141URL [本文引用: 1]

Microspore culture is used to generate completely homozygous plants in a single generation, thereby reducing cost and time required for the production of doubled haploid (DH) plants for breeding and genetic studies. Many factors are known to influence green plant recovery including composition of regeneration media, donor plant genotype, microspore developmental stage, and pretreatment conditions. The objectives of this study were to: (i) develop an improved regeneration medium for wheat microspore culture and (ii) determine the optimal pretreatment conditions and regeneration media combination for increasing green plant recovery rates. Four wheat cultivars, two pretreatment methods (0.4 M mannitol at 4 C and solution B containing 0.3 M mannitol with inorganic components at room temperature), and five regeneration media were tested. Green plant recovery rates from each treatment combination were analyzed using the Proc Logistic model in SAS. Regeneration medium fortified with ascorbic acid produced the highest number of green plants across the different pretreatment conditions, regeneration media, and cultivars tested. Pretreating wheat (Triticum aestivum L.) spikes with 0.4 M mannitol at 4 C followed by embryoid induction and regeneration in a medium fortified with ascorbic acid resulted in the recovery of the highest number of green plants. This high-efficiency method may prove useful for producing DH populations for wheat improvement efforts.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.3724/SP.J.1006.2013.02247URL [本文引用: 1]
DOI:10.3724/SP.J.1006.2013.02247URL [本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1508/cytologia.5.235URL [本文引用: 1]
[本文引用: 2]
DOI:10.15281/jplantres1887.54.178URL [本文引用: 1]
[本文引用: 4]
[本文引用: 4]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 7]
[本文引用: 5]
[本文引用: 3]
DOI:10.1016/j.molp.2018.06.011URL [本文引用: 3]
[本文引用: 2]
DOI:10.1038/s41587-018-0003-0URL [本文引用: 2]
[本文引用: 3]
DOI:10.1093/jxb/erz529URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1073/pnas.1504333112URL [本文引用: 1]
URL [本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 4]
DOI:10.1111/pbi.2017.15.issue-11URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.17221/CJGPBURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1111/pbr.2012.131.issue-3URL [本文引用: 2]
DOI:10.1038/ncomms6334URL [本文引用: 1]
DOI:10.1016/j.tibtech.2015.07.005URL [本文引用: 1]
DOI:10.1534/genetics.115.178038URL [本文引用: 1]
DOI:10.2135/cropsci2008.08.0512URL [本文引用: 1]
DOI:10.2135/cropsci2008.10.0587URL [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
DOI:10.1038/nprot.2014.049URL [本文引用: 1]

Hybrid crop varieties are traditionally produced by selecting and crossing parental lines to evaluate hybrid performance. Reverse breeding allows doing the opposite: selecting uncharacterized heterozygotes and generating parental lines from them. With these, the selected heterozygotes can be recreated as F-1 hybrids, greatly increasing the number of hybrids that can be screened in breeding programs. Key to reverse breeding is the suppression of meiotic crossovers in a hybrid plant to ensure the transmission of nonrecombinant chromosomes to haploid gametes. These gametes are subsequently regenerated as doubled-haploid (DH) offspring. Each DH carries combinations of its parental chromosomes, and complementing pairs can be crossed to reconstitute the initial hybrid. Achiasmatic meiosis and haploid generation result in uncommon phenotypes among offspring owing to chromosome number variation. We describe how these features can be dealt with during a reverse-breeding experiment, which can be completed in six generations (similar to 1 year).
DOI:10.1073/pnas.1117277109URL [本文引用: 1]
[本文引用: 1]
DOI:10.1007/s11032-009-9280-0URL [本文引用: 1]
DOI:10.1007/s10681-006-9241-1URL [本文引用: 1]

Doubled haploid (DH) systems have many attractive features for inducing and fixing mutations. Doubled haploidy provides the fastest route to homozygosity with the greatest fidelity. The ability to fix mutations via doubled haploidy is a key factor, especially as induced mutations␣are predominantly recessive and cannot normally be detected until the M2 generation at the earliest. The DH systems themselves provide an opportunity to target haploid as well as doubled haploid cells for mutation treatment and capture the mutation in a homozygous, pure line.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1371/journal.pone.0092046URL [本文引用: 1]
[本文引用: 2]
DOI:10.1073/pnas.1103190108URL [本文引用: 1]
DOI:10.1016/j.molp.2019.03.006URL [本文引用: 1]
DOI:10.1007/s00425-009-0943-1URL [本文引用: 2]

The phenomenon of maternal haploid induction in maize was first described many years ago, but the underlying mechanism is still unclear. In this study, the Stock-6-derived, haploid-inducing line CAUHOI with high kernel oil content (KOC), was used as the pollinator to produce maternal haploids from the maize hybrid ZD958 with low KOC. CAUHOI is homozygous for the dominant marker gene R1-nj. Haploids were identified by morphological and cytological investigations. The frequency of haploid induction from this cross was 2.21%. Unexpectedly, many haploid kernels had weakly pigmented purple color on the embryo, and some haploid kernels had high KOC. Simple sequence repeat (SSR) analysis showed that 43.18% of the haploids carried segments from CAUHOI, and a small proportion (average 1.84%) of the genome of CAUHOI was introgressed into haploids. Haploid kernels with high KOC had a higher frequency of segment introgression from CAUHOI (2.92%) than that in haploid kernels with low KOC (1.79%), showing that the marker gene R1-nj and high-oil genes from CAUHOI were expressed during the development of some haploid embryos, and confirmed that the DNA introgression from the inducer parent occurred during maternal haploid induction. Together, these results suggested that the chromosome elimination was probably responsible for haploid induction in maize, and late somatic elimination might occur. Several possible mechanisms underlying haploid formation are discussed.
[本文引用: 1]
[本文引用: 1]
DOI:10.1534/g3.114.013078URL [本文引用: 1]
DOI:10.1007/s10681-009-0081-7URL [本文引用: 1]

Spontaneous chromosome doubling via union of unreduced (2n) gametes has been thought to be the way that common wheat (Triticum aestivum L.) was originated from the hybridization of T. turgidum L. with Ae. tauschii Cosson. Previous works have observed unreduced gametes in F1 hybrids of Ae. tauschii with six of the eight T. turgidum subspecies. It is not clear, however, whether the formation of these unreduced gametes is a norm in the F1 hybrids. In the present study, we tried to answer this question by assessing the occurrence frequency of unreduced gametes in 115 T. turgidum–Ae. tauschii hybrid combinations, involving 76 genotypes of seven T. turgdium subspecies and 24 Ae. tauschii accessions. Our data show that these hybrid combinations differed significantly (P≤ 0.01, F =11.40) in selfed seedset, an indicator for production of unreduced gametes. This study clearly showed that meiotic restitution genes are widely distributed within T. turgidum. However, significant differences were found between as well as within T. turgidum subspecies and in the interaction of the T. turgidum genotypes with those of Ae. taushii. The possible application of the meiotic restitution genes from T. turgidum in production of double haploids is also discussed.
