 ,, 罗毅, 李晓开, 李学伟四川农业大学动物科技学院,动物遗传育种研究所,成都 611130
,, 罗毅, 李晓开, 李学伟四川农业大学动物科技学院,动物遗传育种研究所,成都 611130Research progress in sRNAs and functional proteins in epididymosomes
Juan Xiao, Xun Wang ,, Yi Luo, Xiaokai Li, Xuewei LiCollege of Animal Science and Technology, Institute of Animal Genetics and Breeding, Sichuan Agricultural University, Chengdu 611130, China
,, Yi Luo, Xiaokai Li, Xuewei LiCollege of Animal Science and Technology, Institute of Animal Genetics and Breeding, Sichuan Agricultural University, Chengdu 611130, China通讯作者:
第一联系人:
编委: 苗龙
收稿日期:2017-09-15修回日期:2018-01-29网络出版日期:2018-03-20
| 基金资助: |
Editorial board:
Received:2017-09-15Revised:2018-01-29Online:2018-03-20
| Fund supported: |

摘要
关键词:
Abstract
Keywords:
PDF (509KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
肖娟, 王讯, 罗毅, 李晓开, 李学伟. 附睾小体功能蛋白及sRNA研究进展. 遗传[J], 2018, 40(3): 197-206 doi:10.16288/j.yczz.17-148
Juan Xiao, Xun Wang, Yi Luo, Xiaokai Li, Xuewei Li.
近年来,由于精液质量下降而引起的男性不育现象正逐年增加,其中精子质量下降是主要因素之一[1]。哺乳动物睾丸曲细精管产生的精子需要在附睾中经过一系列复杂的结构与功能变化才能成熟,经附睾转运后精子膜蛋白新组分呈现出低分子量的特征,这与附睾液中蛋白组分相似,这一变化很可能源自精子与附睾液的相互作用[2]。由于和附睾腔内液体(包括蛋白质[3]和附睾小体[4])相互作用,精子的功能发生了改变。附睾上皮细胞通过顶浆分泌的方式产生的外泌体(exosome)被定义为附睾小体(epididymosomes)[5,6],属于精液外泌体(semen exosomes, SE)中的一种[7]。虽然精液外泌体发现已有30余年,但有关附睾小体的研究却相对较少,现有证据表明附睾小体在精子成熟等过程中发挥重要的调控作 用[4,8,9]。本文基于附睾小体的功能蛋白质及两类主要的sRNAs能参与调节精子成熟及受精等过程,对近年来附睾小体及其生物学功能的最新研究进展进行了综述,通过深入了解附睾小体对精子的作用,有助于揭示雄性动物繁殖能力低下及临床男性不育的发病机制,为提高雄性动物繁育能力及治疗男性不育提供新的思路。
1 附睾小体的发现和产生
哺乳动物精液主要是由精子和生殖道中的多种细胞分泌物组成,而富含高浓度胆固醇、鞘磷脂及结构蛋白等成分的附睾小体也存在于这种复杂流体中[4,7]。1985年,Yanagimachi等[5]首次在中国仓鼠(Chinese hamster)附睾管腔内隔室中发现附睾上皮细胞能产生直径为20~100 nm的纳米囊泡。因其是附睾上皮细胞分泌且与exosome类似, 因此被称为附睾小体[6]。后续的研究表明,除仓鼠外,在猴(Cercopithecidae)[10]、牛(Bos taurus)[11,12,13]、鼠(Mus musculus)[14,15,16]和猫(Felinae)[17]等不同物种的附睾液中也存在附睾小体。顶浆分泌是1923年首次被提 出[18],其分泌过程是分泌细胞首先形成突起囊泡,随后逐渐变大的突起囊泡与细胞分离,最终囊泡破裂将其内容物释放出来。附睾小体是通过顶浆分泌的方式产生,附睾小体的形成主要包括以下几个过程(图1):首先附睾上皮主细胞形成了顶端突起囊泡,突起以出芽的方式生长,从微绒毛之间穿出形成顶端囊泡,然后直径逐渐变大的突起囊泡被释放到附睾管腔液中,最后囊泡膜破裂释放其包裹的能与精子结合的纳米囊泡,即附睾小体[8]。2 附睾小体主要成分及其生物学功能
附睾是雄性哺乳动物重要的生殖器官,主要负责精子的传输、浓缩、贮存及成熟,而附睾液中由附睾上皮细胞分泌的附睾小体主要通过转运精子成熟及受精所需的蛋白和RNA促进精子成熟,此外,附睾小体除了富含蛋白质和脂类[7],还含有很多非编码RNA,如tRNA[19]、miRNA[20]等(图2)。附睾分为3段,即附睾头部、附睾体和附睾尾部,这3段均可分泌附睾小体[21]。附睾小体在附睾和精子交流中发挥了重要的作用,能够包裹来源于附睾的sRNA[19,21]和蛋白质[9]并将其转移至精子发挥重要的生理作用。图1
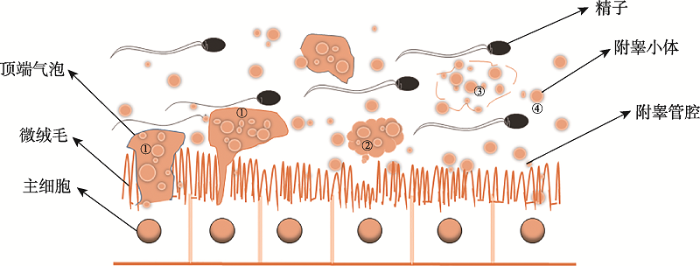 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1附睾小体的生成
①顶端囊泡的产生;②释放到附睾的管腔液中;③囊泡膜破裂释放纳米囊泡;④附睾小体产生。参考文献[5,6,8,18]内容修改绘制。
Fig. 1The formation of the epididymosomes
图2
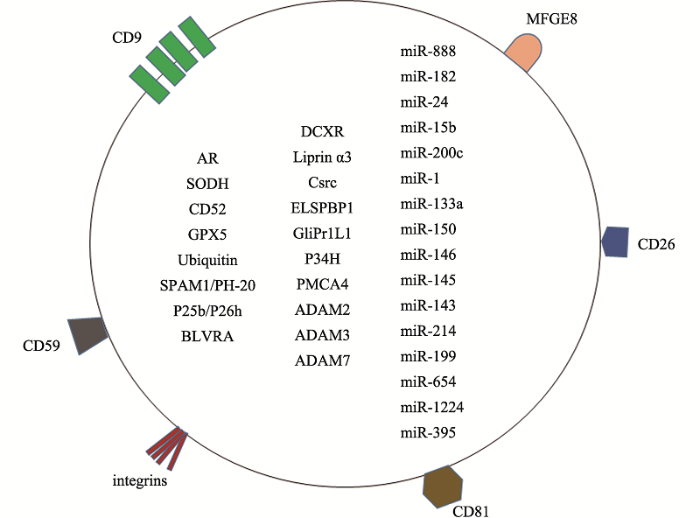 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2附睾小体功能蛋白及部分sRNA
CD9、CD59、integrins、CD81、CD26为附睾小体膜上跨膜连接蛋白,能促进细胞间及细胞内信号转导。MFGE8也是膜上蛋白,其结构域C2区能和暴露在精子细胞表面的磷脂酰丝氨酸残基结合。圆圈内为部分附睾小体内容物。参考文献[8,20,25,26,31,35,39, 41,44,48,50,58,61,62,64,65]内容修改绘制。
Fig. 2The functional proteins and partial list of sRNAs in epididymosomes
除了参与精子成熟及受精[22,23,24],附睾小体中的蛋白[8,25,26]还能参与精子抗氧化或消除缺陷精子等过程[27,28,29]。附睾小体中的miR-15b、 miR-182和miR-24[20]可能影响精子活力和正常形态[30];当炎症存在时,附睾小体miRNA(miR-29a、miR-181a和miR-146)[20,31]还具有抑制附睾上皮细胞增殖,控制T细胞敏感性或抑制促炎通路[32,33,34]。功能研究显示附睾小体内所含的部分miRNA(miR-150[20]和miR- 133b[35])具有影响早期胚胎的发育的功能[36,37],以上结果提示附睾小体内的miRNA随精子进入卵母细胞后还可能参与早期胚胎发育。
2.1 附睾小体蛋白及其功能
Girouard等[38]发现附睾头部和尾部分泌的附睾小体的蛋白种类差异很大,牛附睾头部和附睾尾部的附睾小体中分别含有555和438种蛋白质,其中共有的蛋白质有231种。附睾小体与精子之间存在蛋白转移的现象已经得到广泛认可;研究发现将牛附睾尾部的附睾小体与头部的精子体外培养,附睾小体蛋白会转移到精子上[12]。这些附睾小体蛋白在精子的成熟及运动能力的调节等过程中扮演重要角色(表1),可以影响精子的成熟[39],或有助于精子和透明带结合[23],还能保护精子抵抗氧应激[8]。2.1.1 精子活力及能量代谢相关蛋白
附睾小体中包含醛糖还原酶(aldose reductase, AR)[40]、山梨醇脱氢酶(sorbitol dehydrogenase, SODH)[24,41]和巨噬细胞迁移酶抑制因子(microphage migration inhibitory factor, MIF)[39,40,42,43]等多种蛋白,它们参与精子活力获得及成熟。Kobayashi等[24]和Frenette等 [41]发现AR和SODH都是通过参与多元醇相关通路影响精子的活力及成熟,其中AR是利用电子供体NADPH增加葡萄糖向山梨糖醇的转变,而SODH则通过NAD+作为电子受体将山梨糖醇氧化成果糖,它们通过控制精子能量的来源,进而影响精子的活力。附睾小体的MIF穿过精子质膜被转移到精子鞭毛内从而影响精子的活力[39,42]。此外,小鼠附睾分泌的钙离子-腺苷三磷酸膜蛋白(plasma membrane Ca2+-ATPase 4, PMCA4)[9]作为钙离子外排泵,能影响钙离子信号通路的激活并维持精子内Ca2+的稳态[44,45];精子若缺失PMCA4基因将不能发生超活化和获能反应[45],因此PMCA4对精子运动活力和受精具有极其重要的作用[46]。
Table 1
表1
表1 附睾小体相关蛋白对精子的调控作用
Table 1
| 参与过程 | 相关蛋白名称缩写 | 功能 | 参考文献 |
|---|---|---|---|
| 精子活力及能量代谢 | MIF | 与精子纤维密度相关:参与精子能动性获得 | [39,40,42,43] |
| SODH | 通过NAD作为电子受体将山梨糖醇氧化成果糖,控制精子能量来源 | [24,41] | |
| AR | 利用电子供体NADPH增加葡萄糖向山梨糖醇转变,控制精子能量的来源 | [24,40,41] | |
| PMCA4 | 是小鼠精子的必需蛋白以及主要的钙离子外排泵,调节精子的活力 | [9,44~46] | |
| 精子获能 及受精 | MFGE8 | 与卵子衣壳糖蛋白羧基端的F5/8C部位结合介导精子和卵子粘附,促进精卵结合 | [48~54] |
| SPAM1/PH20/P25b/P26h/DCXR/P34H | 增加精子对卵子周围积云细胞层的渗透力使精子成功附着卵子透明带,促进精卵透明带反应 | [13,23,25,56,59] | |
| Liprin α3 | Liprin α3表达影响精子顶体反应和受精作用 | [57,58] | |
| cSrc | Src激酶家族通过增强胞内蛋白酪氨酸磷酸化作用,转导精子获能的级联信号影响精子获能 | [60] | |
| GLIPR1L1 | 参与透明带反应从而精卵融合过程 | [61] | |
| ADAM2/ADAM3 /ADAM7 | ADAMs家族与卵子膜蛋白整合素β1、α4/α9、α6和CD9相互作用介导了精卵质膜的结合和融合 | [22,43,47] | |
| 保护精子 抵抗氧应激 | ELSPBP1/BLVRA | ELSPBP1/BLVRA复合物能清除ROS,保护活精子抵抗氧化应激 | [63,64] |
| GPX5 | 精子的抗氧化损伤过程。此外,还能避免未成熟精子在ROS作用下过早获能 | [8,28] | |
| 泛素(ubiquitin) | 参与了消除缺陷精子 | [10,29] |
新窗口打开|下载CSV
2.1.2 精子获能及受精相关蛋白
附睾小体中所含的部分蛋白质还能参与精子获能及受精过程,如:金属蛋白酶解离素 (a disintegrin and metalloprotein, ADAMs)[43]家族成员(ADAM2/ ADAM3/ADAM7)属于I型跨膜蛋白。附睾小体中的ADAMs被转移到精子后能与卵子膜蛋白整合素β1、α4/α9、α6和CD9相互作用介导了精卵质膜的结合和融合[22,47]。附睾小体也含有乳脂球表皮生长因子(milk fat globule-EGF factor 8 protein, MFGE8;在小鼠的精子细胞上该蛋白根据其结构不同被命名为:secreted protein containing N-terminal Notch-like type II epidermal growth factor (EGF) repeats and C-terminal discoidin/F5/8C domains, SED1)也具有重要功能[48,49,50]。Ensslin等[48,51]和Shur等[52,53]研究表明MFGE8的结构域C2区能和暴露在精子细胞表面的磷脂酰丝氨酸残基结合,而SED1/MFGE8的结构域C1区则能与卵子衣壳糖蛋白羧基端的F5/8C部位结合介导精子和卵子粘附,使SED1/MFGE8作为二聚体或多聚体促进精卵结合。Hoffhines等[54]研究发现了体内无MFGE8表达的小鼠生育能力低下,且其精子在体外不能与卵细胞结合;证明了SED1/MFGE8的硫酸化在精卵结合中起重要作用。
此外,附睾小体中精子粘附分子1(sperm adhesion molecule 1,SPAM1)/PH-20[23,26,55]、P25b/P26h[13] [在人类被称为二羰基/L-木酮糖还原酶(dicarbonyl/ L-xylulose reductase,DCXR)或P34H[56]]和酪氨酸磷酸酶受体相互作用蛋白α3 (liprin α3)[57,58]等都是与精子获能及受精相关的蛋白。SPAM1/PH20是一种透明质酸酶,其主要生物功能包括在透明质酸酶活性作用下协助精子穿过放射冠,在顶体反应后参与透明带的次级结合[23,55,59]。Joshi等[57]发现Liprin α3的表达影响精子的顶体反应和受精作用,但其具体作用机制还有待研究。具有酪氨酸蛋白激酶(protein tyrosine kinase, PTK)活性的cSrc激酶家族通过转导精子获能的级联信号可影响精子获能[60]。此外,附睾小体中还含有胶质瘤致病相关蛋白1 (glioma pathogenesis-related protein 1, GLIPR1L1)[61],Gibbs等[62]的研究显示GLIPR1L1蛋白在精子与透明带结合过程中发挥一定作用。
2.1.3 精子抗氧化相关蛋白
除上述附睾小体蛋白发挥的生物学功能外,部分附睾小体蛋白还表现出和精子死亡存在高度密切关系。牛附睾小体中ELSPBP1[63](epididymal sperm binding protein1)等蛋白水平与牛射精前附睾中死亡的精子数量相关。ELSPBP1/BLVRA(biliverdin reductase A, BLVRA)复合物的形成能清除活性氧簇(reactive oxygen species, ROS),从而保护活精子抵抗氧化应激[64]。此外,小鼠的谷胱甘肽过氧化物酶5(glutathione peroxidase 5, GPX5)是一种由附睾头部上皮细胞分泌的附睾小体蛋白,是GPXs家族中的一员。该家族蛋白在附睾精子的抗氧化损伤过程中起关键作用,是有效的抗氧化剂清除剂,可保护精子抵抗氧化损伤,从而保证其完整性[8,28]。泛素(ubiquitin)是另一种附睾小体蛋白,该蛋白在精子经过附睾的过程中转移到精子上,参与了消除缺陷精子的过程[10,25],此外,泛素-蛋白酶体途径(Ubiquitin- proteasome pathway, UPP)在精子变态过程中细胞器降解及精子细胞中多余蛋白质的去除中发挥重要调控作用,在精子变态过程中UPP调控异常会导致精子畸形及精子活力降低,并引发少精子症、不育及睾丸肿瘤等生殖系统疾病[29]。
近年来,关于附睾小体蛋白的研究逐渐增多,以上证据均表明它们与精子的发生、成熟及受精等关系密切,然而其具体作用机制仍有待进一步确定。
2.2 附睾小体中两种主要sRNA及其功能
sRNA(small RNAs)包括多种非编码RNA,但目前附睾小体中miRNA (microRNA)和tsRNA (tRNA- derived small RNAs)的研究较多。miRNA是一类高度保守的非编码调控单链sRNA,参与各种生物功能的调节途径,包括发育、病毒防御、细胞增殖和凋亡等,此外miRNA还参与生殖功能相关途径[65,66]。而tsRNA是由tRNA及前体tRNA通过一系列酶作用断裂后形成的短链RNA,普遍存在于哺乳动物精子和附睾小体中,目前研究表明tsRNA同样具有调控基因转录、细胞的增殖凋亡和应激反应等功能,很可能通过精子传递行使其生物学功能[19,67]。附睾小体具有选择性包裹sRNA的能力,Belleannée等[20]研究表明小鼠附睾不同部位分泌的附睾小体所含的miRNA丰度存在差异,例如miR-654、miR-1224和miR-395在附睾尾分泌的附睾小体中高度富集,而miR-145、 miR-143、 miR-214和 miR-199则在头部分泌的附睾小体中含量较高,小鼠的附睾小体能和精子发生融合并将其中的sRNA转移到精子中,从而发挥其生物学功能(表2)。2.2.1 精子活力相关miRNA
附睾小体中含有miR-888[20,31],它可调控SPAG6和SPAG1基因从而维持精子鞭毛的蠕动和成熟的精子结构[66,68]。附睾小体中还含有miR-182、miR-24及miR-15b等miRNA[20],靶基因预测显示miR-15b可靶向调节IDH3A(Isocitrate dehydrogenase 3 (NAD) alpha)基因的表达控制三羧酸循环中能量代谢;而miR-182和miR-24可靶向调节糖原合成酶激酶3α(Glycogen synthase kinase 3 alpha, GSK3A)基因的表达,而GSK3A的磷酸化可影响精子的运动能力;此外,miR-24还可靶向调节肌动蛋白(Actin)结合蛋白Fascin1(fascin actin-bundling protein 1, FSCN1)基因的表达, FSCN1基因编码的肌动蛋白结合蛋白与细胞运动相关[30]。因此附睾小体中miRNA和精子质量关系密切,这些miRNA可能是今后研究男性不育等疾病及雄性动物繁殖力低下的重要靶点。
2.2.2 胚胎生长及发育相关miRNA
附睾小体能和精子发生融合并将其中的miRNA转移到精子内,在受精过程中,这些miRNA被带入卵母细胞中进而可能对胚胎发育产生重要影响。如图2所示,附睾小体内含有miR-200c、miR-150、miR-1、miR-133a等多种miRNA[20,21],功能富集分析显示这些miRNA对于胚胎发育具有重要功能。Huang等[69]研究表明miR-200c可靶向抑制GATA4 (GATA binding protein 4) 基因的表达,且敲除人胚胎干细胞(Human embryonic stem cells,hESC)中的miR-200c将上调GATA4的表达进而诱导hESC的凋亡。Wystub等[37]报道了miR-1-1/133a-2和miR-1-2/ 133a-1簇可通过调节平滑肌基因表达使胚胎心肌细胞从不成熟状态转变为更成熟的表型,miR-1/133簇的缺失诱导平滑肌特异性基因的上调从而导致胚胎心脏多重转录变化,最终导致心脏发育异常并引起胚胎致死,因此miR-1/133a对于早期的心脏发育非常重要。Lin等[36]表明受精卵中miR-150的表达水平和c-Myb基因呈负相关,而c-Myb基因的缺失将导致机体后期出现严重的表型缺陷,这暗示miR- 150/c-Myb的相互作用对胚胎发育至关重要。Reilly等[35]通过靶基因预测、功能富集分析gene ontology(GO)和kyoto encyclopedia of genes and genomes (KEGG)通路分析,发现附睾小体中大多数miRNA的靶基因是和细胞生长、增殖、发育、死亡等相关,其中还有6%~7%和胚胎发育相关。
Table 2
表2
表2 附睾小体相关miRNA的生物学功能
Table 2
| 参与过程 | 相关miRNA | 功能 | 参考文献 |
|---|---|---|---|
| 精子活力 相关 | miR-888 | 调节SPAG6 和SPAG1而维持精子鞭毛蠕动和成熟精子结构 | [20,31,66,68] |
| miR-15b | 调节靶基因IDH3A表达控制三羧酸循环中能量代谢。 | [20,30] | |
| miR-182/miR-24 | 调节GSK3A的表达从而使精子获能 | [20,30] | |
| 胚胎生长及 发育 | miR-200c | 靶向抑制GATA4表达,GATA4高表达会诱导hESC凋亡 | [20,69] |
| miR-150 | 抑制原癌基因c-Myb表达,而该基因的缺失将导致机体后期出现严重的表型缺陷 | [20,36] | |
| miR-1-1/miR-1-2 | 调节平滑肌基因表达使胚胎心肌细胞成熟 | [20,37] | |
| miR133a-1/miR-133a-2 | 调节平滑肌基因表达促使胚胎心肌细胞成熟 | [20,37] | |
| 雄性生殖 免疫、炎症 及细胞增殖 | miR-181a | 调节B细胞分化和T细胞受体信号参与炎症反应 | [20,34] |
| miR-146 | 下调各种促炎性细胞因子来抑制炎症及先天免疫反应 | [20,33] | |
| miR-1224 | 下调TNF-α的表达,在免疫反应中发挥重要作用 | [20,71] | |
| miR-29a | 通过抑制细胞NASP表达,抑制附睾上皮细胞增殖 | [20,32] |
新窗口打开|下载CSV
2.2.3 雄性生殖免疫、炎症及细胞增殖相关miRNA
雄性生殖道感染及其所引起的炎症反应均会影响精液的质量,从而导致雄性生育能力降低[70]。附睾小体中还存在和炎症相关的miRNA,如miR-181a和miR-1224[20]。Li等[34]研究表明miR-181a通过调节B细胞分化和T细胞受体信号参与炎症反应。Cheng等[33]发现miR-146可通过下调各种促炎性细胞因子来抑制炎症及先天免疫反应,但其具体机制需进一步研究。当炎症存在时,miR-1224[71]通过下调肿瘤坏死因子α(tumor necrosis factor-α, TNF-α)的表达从而在免疫反应中发挥重要作用。附睾小体中还含有miR-29a[20],其表达上调可靶向抑制核自身抗原精子蛋白(nuclear autoantigenic sperm protein, NASP)基因的表达从而抑制附睾上皮细胞增殖[32]。这些附睾小体包裹的miRNA可作为一种非倾入性的生物标记来评估附睾自身生理和病理学变化[31]。
2.2.4 精子成熟及胚胎发育相关tsRNA
附睾小体中除了蛋白质和miRNA等主要成分能发挥重要作用外,来源于tRNA的小分子tsRNA (tRNA-derived small RNAs)也具有重要的功能。研究表明tsRNA同样具有调控基因转录、细胞的增殖凋亡和应激反应等功能,并能通过精子传递行使其生物学功能[19,72]。Sharma等[19]研究表明附睾尾部分泌的附睾小体富含tsRNA,且这些tsRNA与成熟的精子中的极其相似。通过显微注射法将部分tsRNA注入正常胚胎中,结果表明tsRNA能引起胚泡阶段多种基因表达量的改变且大部分基因聚集在代谢调控通路中[72]。Chen等[72]研究发现tRNA(transfer RNA)的修饰会影响tsRNA的功能,而tsRNA自身的修饰同样会影响遗传代谢疾病,通过级联效应调控下游大量基因表达的重编程从而引起早期胚胎的转录本变化。目前,精子自身是否具有剪切tRNA的能力尚不清楚,但来源于附睾小体的tsRNA的确是精子重要的表观遗传因子并影响子代的代谢。因此,通过研究附睾小体中的tsRNA对精子及早期胚胎的具体调控机制将对后代的健康和疾病预防产生重大的影响。与此同时,关于附睾小体中的piRNA、rRNA和lncRNA等其他生物学成分的研究较少,其具体调控机制还需要进一步研究。
3 附睾小体其他生物学功能
除参与精子成熟及受精外,精液exosome还具有抗HIV 1病毒的功能以及作为疾病诊断的生物标记。Madison等[73]从健康个体得到的纯化精液exosome可通过阻断病毒RNA后续逆转录来抑制HIV-1病毒在不同类型细胞中的复制。人类精液exosome能够限制艾滋病毒在LP-BM5感染小鼠模型体内传播[74],但这种抗HIV 1病毒的功能是否是由精液exosome中附睾小体实现的还有待进一步研究。附睾小体中的RNA(let-7d和let-7e)[20]能反映精液中不同细胞来源的RNA表达情况,可作为雄性生殖系统的生物标志物,阐明某些疾病的病因及发病机制,如某些特发性雄性动物不育或繁殖力低下[30,31]。输精管结扎逆转手术是目前可靠的男性避孕方法,通过检查输精管切除术的上游和下游的相关miRNA和蛋白质(DCXR/P34H)变化可以鉴定结扎或恢复结扎手术是否成功[31],研究进一步表明结扎手术后前后相关miRNA出现显著改变,在这些miRNA中表达上调的miR-421[75]及下调的miR-941[76]分别与炎症反应、细胞增殖及细胞分化相关。所以了解精液中附睾小体是如何改变细胞程序性免疫应答对于开发下一代疫苗和预防性治疗性传播等疾病非常重要。4 结语与展望
精子质量下降引起的特发性少精子症、弱精子症及无精子症等正逐年上升,已成为影响男性生育及雄性动物繁育能力的最大杀手。近年来研究发现附睾小体中sRNAs和蛋白质等生物学成分与精子的质量密切相关,它们分别作用于精子成熟相关的转录因子以及各种促炎性细胞因子从而调控精子成熟和抑制雄性生殖系统感染,以保证机体具备良好的受精能力。附睾小体中miRNA表达量变化对精子及早期胚胎的影响提示人们是否可以通过注射相关miRNA达到治疗疾病的目的,而tsRNA对精子及早期胚胎的调控也暗示了附睾小体中可能还有更多调控因子可影响精子的质量。因此,通过对附睾小体内容物及其与精子相互作用的深入研究、从而构建完善的附睾小体对精子调控网络,不但有助于进一步了解附睾内精子的成熟及附睾小体与精子相互作用的分子机制,同时又将成为临床上附睾问题造成的男性不育的诊断与治疗开辟新途径,这将成为未来提高雄性动物繁育能力及治疗男性不育的新方向。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URLPMID:11794171 [本文引用: 1]

Abstract BACKGROUND: Although semen analysis is routinely used to evaluate the male partner in infertile couples, sperm measurements that discriminate between fertile and infertile men are not well defined. METHODS: We evaluated two semen specimens from each of the male partners in 765 infertile couples and 696 fertile couples at nine sites. The female partners in the infertile couples had normal results on fertility evaluation. The sperm concentration and motility were determined at the sites; semen smears were stained at the sites and shipped to a central laboratory for an assessment of morphologic features of sperm with the use of strict criteria. We used classification-and-regression-tree analysis to estimate threshold values for subfertility and fertility with respect to the sperm concentration, motility, and morphology. We also used an analysis of receiver-operating-characteristic curves to assess the relative value of these sperm measurements in discriminating between fertile and infertile men. RESULTS: The subfertile ranges were a sperm concentration of less than 13.5 x 10(6) per milliliter, less than 32 percent of sperm with motility, and less than 9 percent with normal morphologic features. The fertile ranges were a concentration of more than 48.0 x 10(6) per milliliter, greater than 63 percent motility, and greater than 12 percent normal morphologic features. Values between these ranges indicated indeterminate fertility. There was extensive overlap between the fertile and the infertile men within both the subfertile and the fertile ranges for all three measurements. Although each of the sperm measurements helped to distinguish between fertile and infertile men, none was a powerful discriminator. The percentage of sperm with normal morphologic features had the greatest discriminatory power. CONCLUSIONS: Threshold values for sperm concentration, motility, and morphology can be used to classify men as subfertile, of indeterminate fertility, or fertile. None of the measures, however, are diagnostic of infertility.
URLPMID:6640035 [本文引用: 1]

To study successive surface changes of maturing ram spermatozoa, we determined the 125I-labeling patterns of testicular spermatozoa and of spermatozoa from 10 consecutive regions of the epididymis. Overall, three phases of cell surface transformations are distinguishable: Phase I occurs in the caput epididymidis and it is characterized by a series of rapid surface changes. The most striking surface transformations occur during transport of spermatozoa from the testis into the proximal caput epididymidis. All major surface components in the zones 78 to 115 kd disappear or are lost from the surface of testicular spermatozoa. Concurrently, several low molecular weight components (17 to 65 kd) appear or become increasingly accessible to 125I. Phase II represents a period of relative quiescence which is confined to the corpus epididymidis. Phase III takes place in the cauda epididymidis where several existing (97, 65 and 41 kd) and new (24 kd) proteins become the predominant features of the sperm cell surface. Electrophoretic analyses of luminal fluid proteins from corresponding regions of the testis and epididymis also show that the most striking changes occur between the rete testis and the proximal caput epididymidis. No rete testis fluid (RTF) components are detectable in luminal fluid of the proximal caput epididymidis. In the epididymis, however, fluid proteins are more persistent than sperm surface components. Several major fluid components (i.e., 95, 76, 21.5, 19.5 and 16 kd) persist throughout the epididymis. Other fluid proteins are of a more transient nature as, for example, a 25 kd molecular weight component (regions E1 through E6) or the 180, 62, 37 and 32 kd components in regions E4 to E10, the 270, 115 and 105 kd proteins in regions E6 to E10 and the 360, 145, 125 and 62 kd molecular weight components in regions E7 to E10. No direct relationships could be established between intrinsic surface components and exogenous fluid proteins from corresponding regions of the testis and epididymis. These results demonstrate a much greater complexity of sequential surface transformation in maturing epididymal spermatozoa than was predictable from our earlier studies of testicular and ejaculated spermatozoa. Apparently, ram spermatozoa must undergo extensive surface renovations in the caput epididymidis before the surface protein pattern typical of mature spermatozoa slowly develops.
URLPMID:20202020202020 [本文引用: 1]

When mammalian spermatozoa exit the testis, they show a highly specialized morphology; however, they are not yet able to carry out their task: to fertilize an oocyte. This property, that includes the acquisition of motility and the ability to recognize and to fuse with the oocyte investments, is gained only after a transit through the epididymis during which the spermatozoa from the testis travel to the vas deferens. The exact molecular mechanisms that turn these cells into fertile gametes still remain mysterious, but surface-modifying events occurring in response to the external media are key steps in this process. Our laboratory has established cartographies of secreted (secretomes) and present proteins (proteomes) in the epididymal fluid of different mammals and have shown the regionalized variations in these fluid proteins along the epididymis. We have found that the main secreted proteins are common in different species and that enzymatic activities, capable of controlling the sperm surface changes, are present in the fluid. Our studies also indicate that the epididymal fluid is more complex than previously thought; it contains both soluble and particulate compartments such as exosome-like vesicles (epididymosomes) and certainly specific glycolipid-protein micelles. Understanding how these different compartments interplay to modify sperm components during their transit will be a necessary step if one wants to control and to ameliorate sperm quality and to obtain valuable fertility markers helpful to establish a male fertility based genetic selection.
URLPMID:20981306 [本文引用: 3]

After spermatogenesis, testicular spermatozoa are not able to fertilize an oocyte, they must undergo sequential maturational processes. Part of these essential processes occurs during the transit of the spermatozoa through the male reproductive tract. Since the sperm become silent in terms of translation and transcription at the testicular level, all the maturational changes that take place on them are dependent on the interaction of spermatozoa with epididymal and accessory gland fluids. During the last decades, reproductive biotechnologies applied to bovine species have advanced significantly. The knowledge of the bull reproductive physiology is really important for the improvement of these techniques and the development of new ones. This paper focuses on the importance of the sperm interaction with the male reproductive fluids to acquire the fertilizing ability, with special attention to the role of the membranous vesicles present in those fluids and the recent mechanisms of protein acquisition during sperm maturation.
URLPMID:3887886 [本文引用: 2]

Abstract Chinese hamster spermatozoa gain their ability to move when they descend from the testis to the distal part of the caput epididymis, but it is not until they enter the corpus epididymis that they become capable of fertilizing eggs. The maturation of the spermatozoa proceeds as they further descend the tract and perhaps continues even in the vas deferens. During transit between the distal caput and proximal cauda epididymides, small membrane-limited vesicles (and tubules) appear on the plasma membrane over the acro somes of the spermatozoa. The number of vesicles appearing on the sperm brane reaches a maximum when the spermatozoa are in the proximal cauda epididymis. It declines sharply in the distal cauda epididymis. Spermatozoa in the vas deferens are free of the vesicles. The origin, chemical nature, and functional role of the vesicles that appear on the sperm surface during epididymal transit must be the subject of further investigation.
URL [本文引用: 2]
URLPMID:23613619 [本文引用: 3]

Mammalian spermatozoa are unique cells in many ways, and the acquisition of their main function, i.e. fertilization capacity, is a multistep process starting in the male gonad and ending near the female egg for the few cells reaching this point. Owing to the unique character of this cell, the molecular pathways necessary to achieve its maturation also show some specific characteristics. One of the most striking specificities of the spermatozoon is that its DNA is highly compacted after the replacement of histones by protamines, making the classical processes of transcription and translation impossible. The sperm cells are thus totally dependent on their extracellular environment for their protection against oxidative stress, for example, or for the molecular changes occurring during the transit of the epididymis; the first organ in which post-testicular maturation takes place. The molecular mechanisms underlying sperm maturation are still largely unknown, but it has been shown in the past three decades that extracellular vesicles secreted by the male reproductive tract are involved in this process. This review will examine the roles played by two types of naturally occurring extracellular vesicles, epididymosomes and prostasomes, secreted by the epididymis and the prostate respectively. We will also describe how the use of artificial vesicles, liposomes, contributed to the study of male reproductive physiology.
URLPMID:17589785 [本文引用: 5]

During epididymal transit, spermatozoa acquire new proteins. Some of these newly acquired proteins behave as integral membrane proteins, including glycosylphosphatidylinositol (GPI)-anchored proteins. This suggests that the secreted epididymal proteins are transferred to spermatozoa by an unusual mechanism. Within the epididymal lumen, spermatozoa interact with small membranous vesicles named epididymosomes. Many proteins are associated with epididymosomes and the protein composition of these vesicles varies along the excurrent duct and differs from soluble intraluminal proteins. Some epididymosome-associated proteins have been identified and their functions in sperm maturation hypothesized. These include P25b, a zona pellucida binding protein, macrophage migration inhibitory factor, enzymes of the polyol pathway, HE5/CD52, type 5 glutathione peroxidase, and SPAM1 or PH-20. The electrophoretic patterns of proteins associated to epididymosomes are complex and some of these proteins are transferred to defined surface domains of epididymal spermatozoa. Epididymosomes collected from different epididymal segments interact differently with spermatozoa. This protein transfer from epididymosomes to spermatozoa is time-dependent, temperature-dependent and pH-dependent, and is more efficient in the presence of zinc. Some proteins are segregated to lipid raft domains of epididymosomes and are selectively transferred to raft domains of the sperm plasma membrane. Some evidence is presented showing that epididymosomes are secreted in an apocrine manner by the epididymal epithelial cells. In conclusion, epididymosomes are small membranous vesicles secreted in an apocrine manner in the intraluminal compartment of the epididymis and play a major role in the acquisition of new proteins by the maturing spermatozoa.
URLPMID:26112481 [本文引用: 3]

A variety of glycosylphosphatidylinositol (GPI)-linked proteins are acquired on spermatozoa from epididymal luminal fluids (ELF) during sperm maturation. These proteins serve roles in immunoprotection and in key steps of fertilization such as capacitation, acrosomal exocytosis and sperm-egg interactions. Their acquisition on sperm cells is mediated both by membrane vesicles (epididymosomes, EP) which were first reported to dock on the sperm surface, and by lipid carriers which facilitate the transfer of proteins associated with the membrane-free fraction of ELF. While the nonvesicular fraction is more efficient, both pathways are dependent on hydrophobic interactions between the GPI-anchor and the external lipid layer of the sperm surface. More recently proteomic and hypothesis-driven studies have shown that EP from several mammals carry transmembrane (TM) proteins, including plasma membrane Ca2+-ATPase 4 (PMCA4). Synthesized in the testis, PMCA4 is an essential protein and the major Ca2+ efflux pump in murine spermatozoa. Delivery of PMCA4 to spermatozoa from bovine and mouse EP during epididymal maturation and in vitro suggests that the docking of EP on the sperm surface precedes fusion, and experimental evidence supports a fusogenic mechanism for TM proteins. Fusion is facilitated by CD9, which generates fusion ompetent sites on membranes. On the basis of knowledge of PMCA4's interacting partners a number of TM and membrane-associated proteins have been identified or are predicted to be present, in the epididymosomal cargo deliverable to spermatozoa. These Ca2+-dependent proteins, undetected in proteomic studies, play essential roles in sperm motility and fertility, and their detection highlights the usefulness of the hypothesis-driven approach.
URLPMID:2412382 [本文引用: 2]

The regional fine structure of the epithelium lining the epididymis of the has been investigated. Tall, prismatic principal cells constituted the major part of the epithelium. Their basal plasma lemma showed presence of and the surface was studded with stereocilis. Presence of numerous and between adjacent principal cells suggested the existence of blood epididymis barrier. Ultrastructural evidence is presented in support of high lysosomal turnover, absorptive and secretory functions in these cells. Apocrine was evident only in the principal cells of initial segment. Of the two types of basal cell, dark and pale, the latter revealed presence of lipofuscin pigment suggesting their scavenger role in the epithelium.
URLPMID:3235378 [本文引用: 1]

The origin and mechanism of the secretion of membrane-bound particles in bovine seminal plasma were studied with transmission (TEM) and scanning (SEM) electron microscopy of the epididymis, vas deferens, ampulla, and seminal vesicle of adult bulls. In the SEM study, all these organs were found to contain apical protrusions in the lining of the epithelial cells. Eventually the protrusions became detached and formed secretory bodies within the lumina of these organs. In the epididymis, the TEM study disclosed a granular and rather homogeneous content in the protrusions and bodies, whereas in the vas deferens they contained dilated cisternae of smooth endoplasmic reticulum. In the ampulla and seminal vesicle, the formation of the apical protrusions was associated with an accumulation of membrane-bound vesicles. These vesicles were found to be released from the storage bodies into the secretory fluid of the lumen. Both could be harvested from isolated seminal vesicle secretions by Percoll gradient centrifugation. It was concluded that various parts of the bovine reproductive organs discharge their secretory products at least partly by an apocrine mechanism. The membrane-bound particles in the seminal plasma, however, appear to be mainly derived from the ampulla and seminal vesicle.
URLPMID:12080033 [本文引用: 2]

During epididymal transit, spermatozoa acquire selected proteins secreted by epithelial cells. We recently showed that P25b, a protein with predictive properties for bull fertility, is transferred from prostasome-like particles present in the cauda epididymal fluid (PLPCd) to the sperm surface. To further characterize the interactions between PLPCd and epididymal spermatozoa, PLPCd were prepared by ultracentrifugation of bull epididymal fluid, then surface-exposed proteins were biotinylated and coincubated in different conditions with caput epididymal spermatozoa. Western blot analysis revealed that only selected proteins are transferred from PLPCd to spermatozoa. MALDI-TOF analysis revealed that these transferred proteins are closely related. The pattern of distribution of the PLPCd transferred varied from one sperm cell to the other, with a bias toward the acrosomal cap. This transfer appeared to be temperature sensitive, being more efficient at 32–37°C than at 22°C. Transfer of PLPCd proteins to spermatozoa was also pH dependant, the optimal pH for transfer being 6.0–6.5. The effect of divalent cations on PLPCd protein transfer to caput spermatozoa was investigated. Whereas Mgand Cahave no effect on the amount of proteins remaining associated with spermatozoa following coincubation, Znhad a beneficial effect. These results are discussed with regard to the function of PLPCd in epididymal sperm maturation.
URL [本文引用: 2]
URLPMID:10819445 [本文引用: 1]

ABSTRACT: Acidification of the epididymal lumen has been suggested to play an important role in sperm functions; however, the cell types, pumps, and mechanisms involved have not been fully addressed. In this study, carbonic anhydrase II (CA II) and a 67-kd subunit of Neurospora crassa vacuolar proton adenosinetriphosphatase (H+V-ATPase) pump were immunolocalized using light microscopy and electron microscopy (EM) in the epididymis of rats and mice. In both animals, narrow cells, identified in the initial segment and intermediate zone of the epididymis, contained numerous small vesicles in their apical region, often cup-shaped in appearance. In the mouse but not rat, these cells also possessed numerous cisternae of smooth endoplasmic reticulum, suggesting steroid synthesis; and cytoplasmic blebs of their apical cell surface, which appeared to detach, suggesting apocrine secretion. Anti-CA II antibody was immunocytochemically localized in the light microscope within narrow cells but not over any other cell types of the entire epididymis. Anti-H+V-ATPase antibody was also localized in narrow cells of the initial segment and intermediate zone; as well as clear cells of the caput, corpus, and cauda regions. Using EM, gold particles for anti-CA II and H+V-ATPase antibodies were noted in the apical region of narrow cells in relation to the numerous, small, cup-shaped vesicles. Although CA II was mainly located in the cytosol near these vesicles, H+V-ATPase appeared on their delimiting membrane and on the apical plasma membrane of these cells. A similar distribution was noted for H+V-ATPase in clear cells. The nature of the small vesicles of the apical region of narrow cells was examined with electron-dense fluid phase tracers that were introduced into the epididymal lumen. The tracers appeared within these vesicles and a few endosomes 1 hour after injection, suggesting that they contact the apical plasma membrane. Since these vesicles are also related to CA II and H+V-ATPase, the data suggests that, as the site of proton production, the vesicles recycle to and from the apical cell surface, and in this way, deliver protons to the epididymal lumen for acidification. Clear cells and their expression of H+V-ATPase may also serve in this function. In summary, both narrow and clear cells appear to be involved in luminal acidification, an activity that may be essential for sperm as they traverse and are stored in the epididymis.
URLPMID:7755184 [本文引用: 1]

Abstract Summary The epithelium of caput and cauda epididymidis of the rat was studied with transmission electron microscopy (TEM) and freeze-fracture techniques. In thin sections of both zones, the tissue consisted mainly of tall columnar cells (principal cells) with long stereocilia. Clusters of small membrane-bound vesicles were located in the lumen between or immediately over the stereocilia. Freeze-fracture replicas also displayed groups of smooth-surface vesicles in the same location. Membrane-bound vesicles isolated from the lumen of the rat epididymis were studied by TEM. In thin sections, some of them contained an electron dense material and others looked empty. In addition, the hydrolases: galactosidase, N-acetyl-glycosaminidase, mannosidase, aryl-sulfatase and glucuronidase were detectable in pellets of vesicles treated with Triton X-100. The results presented here indicate the presence of membrane-bound vesicles observed by two different methodologies in the rat epididymal fluid and demonstrate five glycosidases in their content.
URLPMID:1949991 [本文引用: 1]

Abstract BACKGROUND: The cytokine macrophage migration inhibitory factor (MIF), originally described as a T cell product, has recently been identified to mediate cellular interactions in several endocrine organs. Western blots analysis of rat epididymal homogenates using an anti-MIF antibody indicated the presence of substantial amounts of an immunoreactive protein with the apparent Mr of 12 kDa. Our study aimed to characterize the molecular nature of this immunoreactive factor. MATERIALS AND METHODS: The purified 12 kDa protein and a cloned cDNA fragment were characterized by sequence analysis. Furthermore, expression pattern and localization of the 12 kDa protein were investigated using in situ hybridization, immunohistochemistry, immunoelectron microscopy, and western blots experiments on epididymal sections, isolated epididymal vesicles, and outer dense fibers from spermatozoa. RESULTS: The N-terminal amino acid sequence analysis over 10 amino acids revealed a 100% homology of the 12 kDa protein to the N-terminus of the cytokine MIF. These data were confirmed by sequence analysis of a reverse transcription polymerase chain reaction (RT-PCR) amplified cDNA fragment from rat epididymis, which also showed complete homology to the MIF cDNA sequence. MIF protein and mRNA were localized in the epithelial cells of the epididymis in a regional distribution manner, with the expression maximal in the caput. Immune cells were not labeled. MIF is the first classical cytokine identified to be expressed by the epididymal epithelial cells. Immunoelectron microscopy detected MIF immunoreactivity in the cytoplasm, with no reaction visible in the Golgi complex and the cisternae of the endoplasmic reticulum. At the apical cell surface, MIF accumulated in stereocilia and vesicles that were pinched off from the plasma membrane. MIF detection in vesicles isolated from epididymal secretion together with the lack of a N-terminal signal sequence for translocation in the endoplasmic reticulum strongly suggested a nonclassical secretion mode. Furthermore, MIF was identified as a new component of the outer dense fibers (ODF), a cytoskeletal element of the mid- and principal piece of the sperm tail. CONCLUSION: The cytokine MIF was identified in substantial amounts in the epithelial cells of rat epididymis and in the outer dense fibers of rat epididymal spermatozoa. Our results indicate a nonclassical secretion mode for MIF and suggest a cell-to-cell transfer of MIF via vesicles to the sperm cells.
URLPMID:1926138 [本文引用: 1]

Epididymides of captive normal adult cats were studied by light and transmission electron microscopy. Release of apical portions of principal cells occured by a process of pinching-off. The membrane-bound bodies (spherules) formed were then found in the epididymal lumen. We postulate that this represents an apocrine secretion process. Such phenomenon were present in all segments of the epididymis, whether caput, corpus, or cauda. Rows of microvesicles similar to those described in other species were also observed between microvilli. The mechanism of formation of spherules and microvesicles seemed to be formed by a different mechanism.
URL [本文引用: 1]

Not Available
URL [本文引用: 5]
URLPMID:23803555 [本文引用: 11]

Epididymosomes are small membrane vesicles that are secreted by epididymal epithelial cells and are involved in posttesticular sperm maturation. Although their role in protein transfer to the sperm membrane is well documented, we report their capacity to transport microRNAs (miRNAs), which are potent regulators of posttranscriptional gene expression. Using a microperfusion technique combined with a global microarray approach, we demonstrated that epididymosomes from two discrete bovine epididymal regions (caput and cauda) possess distinct miRNA signatures. In addition, we also established that miRNA repertoires contained within epididymosomes differ from those of their parent epithelial cells, suggesting that miRNA populations released from the cells may be selectively sorted. Binding of DilC12-labeled epididymosomes to primary cultured epididymal cells was measured by flow cytometry, and the results indicated that epididymosomes from the median caput and their miRNA content may be incorporated into distal caput epithelial cells. Overall, these findings reveal that distinct miRNA repertoires are released into the intraluminal fluid in a region-specific manner and could be involved in a novel mechanism of intercellular communication throughout the epididymis via epididymosomes.
URLPMID:4994100 [本文引用: 3]

Recent evidence has shown that the sperm epigenome is vulnerable to dynamic modifications arising from a variety of paternal environment exposures and that this legacy can serve as an important determinant of intergenerational inheritance. It has been postulated that such exchange is communicated to maturing spermatozoa via the transfer of small non-protein-coding RNAs (sRNAs) in a mechanism mediated by epididymosomes; small membrane bound vesicles released by the soma of the male reproductive tract (epididymis). Here we confirm that mouse epididymosomes encapsulate an impressive cargo of >350 microRNAs (miRNAs), a developmentally important sRNA class, the majority (~60%) of which are also represented by the miRNA signature of spermatozoa. This includes >50 miRNAs that were found exclusively in epididymal sperm and epididymosomes, but not in the surrounding soma. We also documented substantial changes in the epididymosome miRNA cargo, including significant fold changes in almost half of the miRNAs along the length of the epididymis. Finally, we provide the first direct evidence for the transfer of several prominent miRNA species between mouse epididymosomes and spermatozoa to afford novel insight into a mechanism of intercellular communication by which the sRNA payload of sperm can be selectively modified during their post-testicular maturation.
URL [本文引用: 2]

ADAMs家族是含多结构域的跨膜蛋白.睾丸特异的ADAMs,在精子发生与附睾精子转运过 程中,经过蛋白水解成为成熟精子的分子形式,与精-卵质膜结合和融合有关.对于精-卵质膜相互作用,ADAMs去整合素域具有关键氨基酸残基和特殊模体. 模拟ADAM2和ADAM3去整合素域的短肽能用于鉴别特异性卵子识别蛋白.精子ADAMs去整合素域与卵子膜蛋白整合素β1、α4/α9、α6和CD9 相互作用,介导了精卵质膜的结合与融合.
URL [本文引用: 2]

ADAMs家族是含多结构域的跨膜蛋白.睾丸特异的ADAMs,在精子发生与附睾精子转运过 程中,经过蛋白水解成为成熟精子的分子形式,与精-卵质膜结合和融合有关.对于精-卵质膜相互作用,ADAMs去整合素域具有关键氨基酸残基和特殊模体. 模拟ADAM2和ADAM3去整合素域的短肽能用于鉴别特异性卵子识别蛋白.精子ADAMs去整合素域与卵子膜蛋白整合素β1、α4/α9、α6和CD9 相互作用,介导了精卵质膜的结合与融合.
[本文引用: 4]
URLPMID:12185102 [本文引用: 3]

The polyol metabolizing pathway, which consists of two enzymes, aldose reductase (AR) and sorbitol dehydrogenase (SDH), converts glucose to fructose. The enzymatic activities, expression, and localization of AR and SDH were studied in reproductive tracts and spermatozoa of male rats by immunohistochemistry, Western blotting, and enzyme assays. Immunoreactivity to an AR antibody was observed mainly in epithelia of epididymis, seminal vesicle, vas deferens, and prostate gland in adult rats. Similar staining profiles were observed for these tissues when an SDH antibody was used. However, in testis, the cells that express these 2 enzymes differed; whereas AR was expressed in Sertoli cells and to lesser extent in spermatogenic cells, SDH was detected in spermatogenic cells of seminiferous tubules. This cell type-specific gene expression was confirmed in primary cultured cells isolated from rat testes. SDH protein levels were higher during spermatid elongation, and large amounts of SDH were carried over to the spermatozoa. Because one of the functions of members of the aldo-keto reductase superfamily is to detoxify harmful carbonyl compounds, an intrinsic function of AR in Sertoli cells may be to catalyze the reduction of cytotoxic metabolites, such as lipid peroxidation products and steroid hormones, which are produced during spermatogenesis. Because uterine fluid and seminal plasma both contain sorbitol, it is likely that SDH in spermatozoa converts sorbitol to fructose for use as an energy source.
[本文引用: 2]
URLPMID:18384048 [本文引用: 2]

Sperm uptake of glycosyl phosphatidylinositol (GPI)-linked proteins from luminal fluids has been shown to occur in male and estrous female reproductive tracts. In males, this is attributed to membranous vesicles secreted into the epididymis and prostate. While epididymosomes have been characterized, there have been no reports of the presence of vesicles in female luminal fluids. Here we report the presence of vesicles, characterized as uterosomes, in the murine estrous female reproductive fluid; and use S perm A dhesion M olecule 1 (SPAM1/PH-20), a well-known hyaluronidase found in male and female fluids, as a model to investigate vesicle-mediated GPI-linked protein transfer to sperm. Epididymosomes and uterosomes isolated after ultracentrifugation of epididymal (ELF) and uterine luminal fluid (ULF) were analyzed by electron microscopy and shown to be 10-70 and 15-50 nm in diameter. The structural integrity of uterosomes was confirmed by their resistance to hypo-osmotic and freeze/thaw stresses; and immunogold labeling localized SPAM1 to their outer membrane surface, as was the case for epididymosomes. SPAM1 was acquired by caudal sperm during incubation in epididymosomes and uterosomes; uptake was abolished when the GPI anchor was enzymatically cleaved. Sperm analyzed by confocal and transmission electron microscopy (TEM) after incubation in fluorescently labeled vesicles revealed the label on the membrane over the acrosome and midpiece of the flagella, where SPAM1 normally resides. High magnification TEM images demonstrated vesicles juxtaposed to the sperm plasma membrane potentially transferring SPAM1. Taken together, these results implicate vesicular docking as the mechanism of vesicle-mediated GPI-linked protein transfer to sperm from murine reproductive fluids. Mol. Reprod. Dev. 75: 1627-1636, 2008. 2008 Wiley-Liss, Inc.
URLPMID:22719900 [本文引用: 1]

We report here that spermatozoa of mice lacking both the sperm nucleus glutathione peroxidase 4 (snGPx4) and the epididymal glutathione peroxidase 5 (GPx5) activities display sperm nucleus structural abnormalities including delayed and defective nuclear compaction, nuclear instability and DNA damage. We show that to counteract the GPx activity losses, the epididymis of the double KO animals mounted an antioxydant response resulting in a strong increase in the global H(2)O(2)-scavenger activity especially in the cauda epididymis. Quantitative RT-PCR data show that together with the up-regulation of epididymal scavengers (of the thioredoxin/peroxiredoxin system as well as glutathione-S-transferases) the epididymis of double mutant animals increased the expression of several disulfide isomerases in an attempt to recover normal disulfide-bridging activity. Despite these compensatory mechanisms cauda-stored spermatozoa of double mutant animals show high levels of DNA oxidation, increased fragmentation and greater susceptibility to nuclear decondensation. Nevertheless, the enzymatic epididymal salvage response is sufficient to maintain full fertility of double KO males whatever their age, crossed with young WT female mice.
URLPMID:19546506 [本文引用: 2]

The mammalian epididymis provides sperm with an environment that promotes their maturation and protects them from external stresses. For example, it harbors an array of antioxidants, including non-conventional glutathione peroxidase 5 (GPX5), to protect them from oxidative stress. To explore the role of GPX5 in the epididymis, we generated mice that lack epididymal expression of the enzyme. Histological analyses of Gpx5-/- epididymides and sperm cells revealed no obvious defects. Furthermore, there were no apparent differences in the fertilization rate of sexually mature Gpx5-/- male mice compared with WT male mice. However, a higher incidence of miscarriages and developmental defects were observed when WT female mice were mated with Gpx5-deficient males over 1 year old compared with WT males of the same age. Flow cytometric analysis of spermatozoa recovered from Gpx5-null and WT male mice revealed that sperm DNA compaction was substantially lower in the cauda epididymides of Gpx5-null animals and that they suffered from DNA oxidative attacks. Real-time PCR analysis of enzymatic scavengers expressed in the mouse epididymis indicated that the cauda epididymidis epithelium of Gpx5-null male mice mounted an antioxidant response to cope with an excess of ROS. These observations suggest that GPX5 is a potent antioxidant scavenger in the luminal compartment of the mouse cauda epididymidis that protects spermatozoa from oxidative injuries that could compromise their integrity and, consequently, embryo viability.
URL [本文引用: 2]

泛素-蛋白酶体途径(Ubiquitin-proteasome pathway,UPP)是真核细胞内蛋白质主要降解途径,通过调节蛋白质相互作用、蛋白活性、蛋白定位及信号转导,进而在细胞周期进程、细胞凋亡、应激反应及机体生长发育等过程发挥重要作用。研究表明,UPP 在人和动物精子生成中的顶体生物合成及精子尾部形成过程起着关键的调控作用,精子变态过程中 UPP 调控异常导致精子畸形及精子活力降低,并引发少精子症、不育及睾丸肿瘤等生殖系统疾病。本文综述了 UPP 在动物精子生成过程中的信号传导及调节机制,以期为后续相关研究提供参考。
URL [本文引用: 2]

泛素-蛋白酶体途径(Ubiquitin-proteasome pathway,UPP)是真核细胞内蛋白质主要降解途径,通过调节蛋白质相互作用、蛋白活性、蛋白定位及信号转导,进而在细胞周期进程、细胞凋亡、应激反应及机体生长发育等过程发挥重要作用。研究表明,UPP 在人和动物精子生成中的顶体生物合成及精子尾部形成过程起着关键的调控作用,精子变态过程中 UPP 调控异常导致精子畸形及精子活力降低,并引发少精子症、不育及睾丸肿瘤等生殖系统疾病。本文综述了 UPP 在动物精子生成过程中的信号传导及调节机制,以期为后续相关研究提供参考。
URLPMID:21872314 [本文引用: 3]

MicroRNAs (miRNAs) are involved in nearly every biological process examined to date, but little is known of the identity or function of miRNA in sperm cells or their potential involvement in spermatogenesis. The objective was to identify differences in miRNA expression between normal porcine sperm samples and those exhibiting high percentages of morphological abnormalities or low motility. Quantitative RT-PCR was performed on sperm RNA to compare expression levels of 10 specific miRNAs that are predicted to target genes that code for proteins involved in spermatogenesis, sperm structure, motility, or metabolism. There were increases in the expression of four miRNAs, let-7a, -7d, -7e, and miR-22, in the abnormal group (P < 0.05), whereas miR-15b was decreased compared to controls (P < 0.05). Two miRNAs, let-7d and let-7e, were increased in the low motility group when compared to controls (P < 0.05). Bioinformatic analyses revealed that messenger RNA targets of the differentially expressed miRNAs encode proteins previously described to play roles in sperm function. Although the precise role of miRNA in sperm remains to be determined, their changes as associated with morphology and motility signify a critical biological function. Perhaps they are remnants of spermatogenesis, stored for a later role in fertilization, or are delivered to the oocyte to influence early embryonic development. Although there is no single cause of male infertility, the identification of miRNAs associated with sperm motility, structural integrity, or metabolism could lead to the development of a microarray or real time-based diagnostic assay to provide an assessment of male fertility status.
URLPMID:23539611 [本文引用: 5]

Abstract STUDY QUESTION: Does vasectomy impact microRNA (miRNA) expression in the epididymis and seminal microvesicles (SMVs) in a non-reversible manner? SUMMARY ANSWER: The miRNA signature in the epididymis and SMVs is altered by vasectomy and only partially restored after vasovasostomy surgery. WHAT IS KNOWN ALREADY: Vasectomy modifies the epididymal transcriptome and triggers non-reversible changes that affect sperm function. Some vasovasostomized men experience a reduced fertility outcome. STUDY DESIGN, SIZE, DURATION: Human epididymides provided by three control donors and three vasectomized donors were collected under artificial circulation through Transplant Quebec (Quebec, QC, Canada). Semen from three normal, three vasectomized and five vasovasostomized donors was provided by the andrology clinic. PARTICIPANTS/MATERIALS, SETTING, METHODS: Epididymides and semen were collected from donors between 26 and 50 years of age with no known pathologies that could potentially affect reproductive function. After RNA extraction, epididymal miRNA profiles were determined by microarray (Affimetrix), compared by ANOVA and confirmed by real-time PCR. The correlation between miRNA and gene expression profiles was investigated by an integrated genomic approach. miRNA signature from purified SMVs was established by microarray. MAIN RESULTS AND THE ROLE OF CHANCE: Vasectomy significantly modified the expression of epididymal miRNAs, which were mainly correlated with mRNAs for transcription factors. Vasectomy also impacted the detection of 118 of the miRNAs found in SMVs from normal donors, including miRNAs of epididymal origin contained in epididymosomes. Among seminal miRNAs changes, 52 were reversible according to the expression levels of miRNA in the semen samples from vasovasostomized donors, while 66 were non-reversible. LIMITATIONS, REASONS FOR CAUTION: Identification of miRNAs responsive to vasectomy was determined with a limited number of samples due to the low number of human specimen samples available. WIDER IMPLICATIONS OF THE FINDINGS: According to the critical role played by miRNAs in all biological systems, we believe that miRNA changes occurring upstream and downstream of the vasectomy site may be related to the reduced fertility outcome reported following surgically successful vasectomy reversal. This study may provide new tools for predicting vasovasostomy success and open avenues for the identification of the molecular players involved in male infertility.
URLPMID:22194605 [本文引用: 2]

Cell proliferation often decreases gradually during postnatal development of some organs. However, the underlying molecular mechanisms remain unclear. Epididymis, playing important roles in sperm maturation, is a typical organ of this type, which displays a decreased proliferation during postnatal development and even ceased at the adult stage. Here, epididymis was employed as a model to explore the underlying mechanisms. We profiled the microRNA and mRNA expression of newborn (1 day) and adult (90 day) rat epididymis by microarray analysis, and found that the level of miR-29a was dramatically up-regulated during postnatal development of rat epididymis. Subsequent investigations demonstrated that overexpression of miR-29a inhibited the proliferation of epididymal epithelial cells in vitro. The nuclear autoantigenic sperm protein (NASP), a novel target of miR-29a, was significantly down-regulated during postnatal development of rat epididymis. Further analysis showed that silence of NASP mimicked the anti-proliferation effect of miR-29a, whereas overexpression of this protein attenuated the effect of miR-29a. As in rat epididymis, miR-29a was up-regulated and Nasp was down-regulated during postnatal development of mouse epididymis, heart, liver, and lung. Moreover, miR-29a can also inhibit the proliferation of cancer cells by targeting Nasp. Thus, an increase of miR-29a, and hence decrease of Nasp, may contribute to inhibit cell proliferation during postnatal organ development.
URLPMID:3721471 [本文引用: 2]

Abstract Activation of inflammatory pathways in the endothelium contributes to vascular diseases, including sepsis and atherosclerosis. We demonstrate that miR-146a and miR-146b are induced in endothelial cells upon exposure to pro-inflammatory cytokines. Despite the rapid transcriptional induction of the miR-146a/b loci, which is in part mediated by EGR-3, miR-146a/b induction is delayed and sustained compared to the expression of leukocyte adhesion molecules, and in fact coincides with the down-regulation of inflammatory gene expression. We demonstrate that miR-146 negatively regulates inflammation. Over-expression of miR-146a blunts endothelial activation, while knock-down of miR-146a/b in vitro or deletion of miR-146a in mice has the opposite effect. MiR-146 represses the pro-inflammatory NF-B pathway as well as the MAP kinase pathway and downstream EGR transcription factors. Finally, we demonstrate that HuR, an RNA binding protein that promotes endothelial activation by suppressing expression of endothelial nitric oxide synthase (eNOS), is a novel miR-146 target. Thus, we uncover an important negative feedback regulatory loop that controls pro-inflammatory signalling in endothelial cells that may impact vascular inflammatory diseases.
URLPMID:17382377 [本文引用: 2]

T cell sensitivity to antigen is intrinsically regulated during maturation to ensure proper development of immunity and tolerance, but how this is accomplished remains elusive. Here we show that increasing miR-181a expression in mature T cells augments the sensitivity to peptide antigens, while inhibiting miR-181a expression in the immature T cells reduces sensitivity and impairs both positive and negative selection. Moreover, quantitative regulation of T cell sensitivity by miR-181a enables mature T cells to recognize antagonists-the inhibitory peptide antigens-as agonists. These effects are in part achieved by the downregulation of multiple phosphatases, which leads to elevated steady-state levels of phosphorylated intermediates and a reduction of the T cell receptor signaling threshold. Importantly, higher miR-181a expression correlates with greater T cell sensitivity in immature T cells, suggesting that miR-181a acts as an intrinsic antigen sensitivity "rheostat" during T cell development.
URLPMID:4994100 [本文引用: 2]

Recent evidence has shown that the sperm epigenome is vulnerable to dynamic modifications arising from a variety of paternal environment exposures and that this legacy can serve as an important determinant of intergenerational inheritance. It has been postulated that such exchange is communicated to maturing spermatozoa via the transfer of small non-protein-coding RNAs (sRNAs) in a mechanism mediated by epididymosomes; small membrane bound vesicles released by the soma of the male reproductive tract (epididymis). Here we confirm that mouse epididymosomes encapsulate an impressive cargo of >350 microRNAs (miRNAs), a developmentally important sRNA class, the majority (~60%) of which are also represented by the miRNA signature of spermatozoa. This includes >50 miRNAs that were found exclusively in epididymal sperm and epididymosomes, but not in the surrounding soma. We also documented substantial changes in the epididymosome miRNA cargo, including significant fold changes in almost half of the miRNAs along the length of the epididymis. Finally, we provide the first direct evidence for the transfer of several prominent miRNA species between mouse epididymosomes and spermatozoa to afford novel insight into a mechanism of intercellular communication by which the sRNA payload of sperm can be selectively modified during their post-testicular maturation.
URLPMID:18667440 [本文引用: 2]

Human c-Myb proto-oncogene is highly expressed in hematopoietic progenitors as well as leukemia and certain solid tumor. However, the regulatory mechanisms of its expression and biological functions remain largely unclear. Recently, c-Myb has been shown to be targeted by microRNA-150 (miR-150) which thereby controls B cell differentiation in mice. In this study, we demonstrated that c-Myb is an evolutionary conserved target of miR-150 in human and zebrafish, using reporter assays. Ectopic expression of miR-150 in breast cancer and leukemic cells repressed endogenous c-Myb at both messenger RNA (mRNA) and protein levels. Among several leukemia cell lines, primary leukemia cells, and normal lymphocytes, expression levels of miR-150 inversely correlated with c-Myb. The miR-150 overexpression or c-Myb silencing in zebrafish zygotes led to similar and serious phenotypic defects in zebrafish, and the phenotypic aberrations induced by miR-150 could be reversed by coinjection of c-Myb mRNA. Our findings suggest that c-Myb is an evolutionally conserved target of miR-150 and miR-150/c-Myb interaction is important for embryonic development and possibly oncogenesis.
URLPMID:3777988 [本文引用: 2]

miRNAs are small RNAs directing many developmental processes by posttranscriptional regulation of protein-coding genes. We uncovered a new role for miR-1-1/133a-2 and miR-1-2/133a-1 clusters in the specification of embryonic cardiomyocytes allowing transition from an immature state characterized by expression of smooth muscle (SM) genes to a more mature fetal phenotype. Concomitant knockout of miR-1-1/133a-2 and miR-1-2/133a-1 released suppression of the transcriptional co-activator myocardin, a major regulator of SM gene expression, but not of its binding partner SRF. Overexpression of myocardin in the embryonic heart essentially recapitulated the miR-1/133a mutant phenotype at the molecular level, arresting embryonic cardiomyocytes in an immature state. Interestingly, the majority of postulated miR-1/133a targets was not altered in double mutant mice, indicating that the ability of miR-1/133a to suppress target molecules strongly depends on the cellular context. Finally, we show that myocardin positively regulates expression of miR-1/133a, thus constituting a negative feedback loop that is essential for early cardiac development.
URLPMID:21875428 [本文引用: 1]

During the epididymal maturation, spermatozoa interact with different populations of epididymosomes and sequentially acquire some epididymosome-associated proteins critical to sperm functions. Although very few proteins associated with epididymosomes have been identified, the physiological importance of these vesicles in the sperm maturation remains unclear. To document these relevant issues, lipid and protein analysis of epididymosomes from caput and cauda epididymal fluids was determined. Lipid analysis revealed a particular composition of specific phospholipids in these vesicles; the levels of phosphatidyl-ethanolamine, phosphatidyl-inositol and phosphatidyl-choline being higher in caput epididymosomes. From the 555 and 438 proteins identified in caput- and cauda-derived epididymosomes, respectively, 231 proteins were identified in both types of epididymosome. Proteins exclusively identified in caput and cauda epididymosomes are mainly enzymes and transporter molecules. The presence of several glycan-modifying enzymes is the hallmark of the caput epididymosomes proteome. Among the common proteins in both types of epididymosome, a subset of Rab and SNARE proteins implicated in vesicle trafficking and membrane fusion were identified. Together, these data suggest that epididymosome-associated proteins are involved in various molecular functions suggesting that during the epididymal transit, spermatozoa interact with different populations of epididymosomes, which could modify the male gamete in a sequential manner.
URL [本文引用: 3]
URLPMID:12826572 [本文引用: 2]

During the epididymal transit, mammalian spermatozoa acquire new surface proteins necessary for male gamete function. We have previously shown that membranous vesicles, called epididymosomes, interact with spermatozoa allowing the transfer of some proteins to sperm surface within the epididymal lumen. The protein composition of those vesicles has been investigated to document the mechanisms of protein transfer from epididymosomes to spermatozoa. Electrophoretic analysis revealed that protein composition is different from the epididymal soluble compartment as well as from similar vesicles present in the semen. Protein association with epididymosome is very strong as revealed by resistance to extraction with detergent. Matrix-assisted laser desorption ionization time-of-flight as well as immunodetection techniques have been used to identify some proteins associated to epididymosomes and spermatozoa. An aldose reductase known for its 20-hydroxysteroid dehydrogenase activity and the cytokine (macrophage migration inhibitory factor) have been identified. These two proteins have been immunolocalized in principal cells of the epididymal epithelium, a more intense signal being detected in the distal epididymal segment as well as in the vas deferens. Database search revealed that these two proteins are characterized by the lack of a signal peptide. These results are discussed with regard to a possible apocrine mode of secretion of these proteins acquired by spermatozoa during the epididymal transit.
URLPMID:16278369 [本文引用: 2]

Abstract Top of page Abstract Materials and Methods Results Discussion Acknowledgments References ABSTRACT: Two enzymes are involved in the polyol pathway: an aldose reductase that reduces glucose in sorbitol followed by its oxidation in fructose by sorbitol dehydrogenase. It has been previously shown that both enzymes are presented in the bovine epididymis, where they are associated with membranous vesicles called epididymosomes. Based on the distribution of these enzymes, it has been hypothesized that the polyol pathway can modulate sperm motility during the epididymal transit. In the present study, polyol pathway was investigated in semen and along the epididymis in humans in order to determine if sperm maturation can be associated with this sugar pathway. Western blot analysis shows that both aldose reductase and sorbitol dehydrogenase are associated with ejaculated spermatozoa and prostasomes in humans. These enzymes are also associated with epididymosomes collected during surgical vasectomy reversal. Western blot, Northern blot, and reverse transcription olymerase chain reaction analysis show that aldose reductase and sorbitol dehydrogenase are expressed at the transcriptional and translational levels along the human epididymis. Unlike what occurs in the bovine model, distribution of these enzymes is rather uniform along the human excurrent duct. Immunohistological studies together with Western blot analysis performed on epididymosomes preparations indicate that the polyol pathway enzymes are secreted by the epididymal epithelium. These results indicate that the polyol pathway plays a role in human sperm physiology.
URLPMID:16051683 [本文引用: 2]

Abstract During epididymal transit, mammalian spermatozoa acquire new proteins involved in the acquisition of motility and of male gamete fertilising ability. We have previously shown that membranous vesicles called epididymosomes are involved in the transfer of epididymal-originating proteins to spermatozoa. The cytokine macrophage migration inhibitory factor (MIF) is one of these proteins but the role played by MIF in relation to epididymal sperm maturation still remains unclear. As this protein has already been shown to bear different functions depending on its location, we investigated its distribution along the epididymis and in different compartments of human semen. Northern and Western blot analysis as well as immunohistochemical studies show that MIF is expressed all along the epididymis with a higher level of transcript in the proximal segment. MIF is associated with two types of membranous vesicles, i.e. epididymosomes and prostasomes, the latter being prostate-originating membranous vesicles present in the semen. In semen, MIF is associated with spermatozoa, prostasomes as well as the soluble fraction. The amount of MIF in the seminal fluid varies from one individual to another but does not correlate with the amount of MIF associated with ejaculated spermatozoa. There is a negative correlation between the amount of sperm-associated MIF and the percentage of motility in different semen samples. Sperm separation using discontinuous Percoll gradient centrifugation shows a higher amount of MIF associated with poorly motile spermatozoa compared to highly motile spermatozoa present in the lower Percoll fraction. These results are discussed with regards to the possible involvement of MIF in sperm motility acquisition during the epididymal transit.
URLPMID:18482993 [本文引用: 2]

The epididymal epithelium secretes membranous vesicles, called epididymosomes, with which a complex mixture of proteins is associated. These vesicles transfer to spermatozoa selected proteins involved in sperm maturation. Epididymosomes in the human excurrent duct have been described, but their protein composition and possible functions are unknown.Epididymosomes were collected during vasovasostomy procedures, purified and submitted to liquid chromatography with hybrid quadrupole time-of-flight mass spectrometry. From all the mass spectra generated, 1022 peptides allowed the identification of 146 different proteins. Identification of some of these proteins was confirmed by western blots. Furthermore, western blot showed that the protein composition of epididymosomes differed from that characterizing prostasomes; membranous vesicles secreted by the prostate. Organization of the epididymosomes proteome according to common functional features suggests that epididymosomes have multiple functions. In order to understand the origin of epididymosomes collected distally, microarray databases of caput, corpus and cauda epididymidis were analysed to determine where along the excurrent duct the encoded proteins associated to epididymosomes are synthesised. Results suggest that some proteins synthesized in the caput and corpus epididymidis are associated with epididymosomes collected distally.Epididymosomes thus transit along the excurrent duct, and vesicles collected distally represent a mixed population.
URLPMID:12297107 [本文引用: 1]

Abstract Hyperactivated motility, a swimming pattern of mammalian sperm in the oviduct, is essential for fertilization in vivo. It is characterized by high-amplitude flagellar waves and, usually, highly asymmetrical flagellar beating. It had been suggested, but not tested, that Ca2+ and cAMP switch on hyperactivation by directly affecting the flagellar axoneme. In this study, the direct affects of these agents on the axoneme were tested by using detergent-demembranated bull sperm. As confirmed by TEM, treatment of sperm with 0.2% Triton X-100 disrupted the plasma, acrosomal, and inner mitochondrial membranes, leaving axonemes intact. In the presence of 2 mM ATP, the percentage of reactivated sperm that were hyperactivated increased to 80% when free Ca2+ was increased from 50 to 400 nM. The effect of the Ca2+ in this range was to increase beat asymmetry by increasing the curvature of the principal bend. No additional increases were observed above 400 nM free Ca2+, but motility was suppressed at 1 mM. The ability of Ca2+ to produce hyperactivation depended on ATP availability, such that more ATP was required to produce the high amplitude flagellar bends characteristic of hyperactivated motility than to produce activated motility. Cyclic AMP was not required for reactivation, nor for hyperactivation. Production of hyperactivated motility also required an alkaline environment (pH 7.9-8.5). These results suggest that, provided sufficient ATP is present and pH is sufficiently alkaline, Ca2+ switches on hyperactivation by enabling curvature of the principal bends to increase.
URL [本文引用: 2]
URLMagsci [本文引用: 1]

哺乳动物的受精过程涉及到精子一系列的功能活动,如精子在雌性生殖道的运行、精子的超活化与获能、顶体反应以及精卵融合等。在精子经历的这一系列过程中,精子功能相关的蛋白质发挥着不可或缺的作用,这些蛋白分子的正常与否与雄性个体的繁殖力高低密切相关,因此精子功能相关的蛋白质能够作为评定哺乳动物精液受精能力的生物标记。文章主要对哺乳动物精子功能相关的蛋白质进行了综述,以阐述相关蛋白分子对精子运动活力、精子获能、顶体反应、透明带穿入和精卵融合等方面的重要作用以及这些蛋白分子在家畜遗传改良上的潜在应用。
URLMagsci [本文引用: 1]

哺乳动物的受精过程涉及到精子一系列的功能活动,如精子在雌性生殖道的运行、精子的超活化与获能、顶体反应以及精卵融合等。在精子经历的这一系列过程中,精子功能相关的蛋白质发挥着不可或缺的作用,这些蛋白分子的正常与否与雄性个体的繁殖力高低密切相关,因此精子功能相关的蛋白质能够作为评定哺乳动物精液受精能力的生物标记。文章主要对哺乳动物精子功能相关的蛋白质进行了综述,以阐述相关蛋白分子对精子运动活力、精子获能、顶体反应、透明带穿入和精卵融合等方面的重要作用以及这些蛋白分子在家畜遗传改良上的潜在应用。
URLPMID:19855936 [本文引用: 1]

During epididymal transit, mammalian sperm acquire selected proteins secreted by the epididymis. We previously showed that a disintegrin and metalloprotease (ADAM) 7 is expressed specifically in the epididymis and transferred to the sperm surface during epididymal transit. Here, we show that mouse ADAM7 secreted to the epididymal lumen is associated with membranous vesicles known as epididymosomes. Furthermore, we found that ADAM7 can be transferred directly from epididymal vesicles to sperm and that it is an integral plasma membrane protein in sperm. Thus, our study provides new information regarding the unique mode of secretion and interaction of ADAM7 during the epididymis-to-sperm transfer process.
URLPMID:12941270 [本文引用: 2]

We report the identification of SED1, a protein required for mouse sperm binding to the egg zona pellucida. SED1 is homologous to a small group of secreted cell-matrix adhesive proteins that contain Notch-like EGF repeats and discoidin/F5/8 type C domains. SED1 is expressed in spermatogenic cells and is secreted by the initial segment of the caput epididymis, resulting in SED1 localization on the sperm plasma membrane overlying the acrosome. SED1 binds specifically to the zona pellucida of unfertilized oocytes, but not to the zona of fertilized eggs. Recombinant SED1 and anti-SED1 antibodies competitively inhibit sperm-egg binding, as do truncated SED1 proteins containing a discoidin/C domain. SED1 null males are subfertile and their sperm are unable to bind to the egg coat in vitro. These studies illustrate that Notch-like EGF and discoidin/C domains, protein motifs that facilitate a variety of cellular interactions, participate in gamete recognition as well.
URLPMID:19498381 [本文引用: 1]

In multicellular organisms, communication between cells mainly involves the secretion of proteins that then bind to receptors on neighbouring cells. But another mode of intercellular communication 09” the release of membrane vesicles 09” has recently become the subject of increasing interest. Membrane vesicles are complex structures composed of a lipid bilayer that contains transmembrane proteins and encloses soluble hydrophilic components derived from the cytosol of the donor cell. These vesicles have been shown to affect the physiology of neighbouring recipient cells in various ways, from inducing intracellular signalling following binding to receptors to conferring new properties after the acquisition of new receptors, enzymes or even genetic material from the vesicles. This Review focuses on the role of membrane vesicles, in particular exosomes, in the communication between immune cells, and between tumour and immune cells.
URLPMID:20422713 [本文引用: 1]

SED1/MFG-E8, herein referred to as SED1, is a bimotif adhesive protein with ascribed functions in a range of cell-cell interactions, including sperm-egg binding. In the male reproductive tract, SED1 is secreted by the initial segment of the epididymis, where it coats sperm and subsequently facilitates binding to the egg zona pellucida. We have recently reported that SED1-null epididymides show an unexpected incidence of spermatic granulomas, reflecting breakdown of the epithelium and a consequent autoimmune response against sperm antigens. However, spermatic granulomas are most often manifest in the distal segments of the epididymis, whereas the bulk of SED1 is expressed in the proximal epididymis. In some models, the presence of granulomas in the distal epididymis is associated with an underlying defect in the maintenance of luminal fluid homeostasis. Herein, we report that SED1-null epididymal fluid is both hypo-osmotic and alkaline, relative to wildtype epididymal fluid. Furthermore, the SED1-null epididymal epithelium exhibits various hallmarks of disrupted fluid reabsorption and pH regulation, including altered morphology of clear cells, increased intracellular vesicles, and apical distribution of VATPase. Results indicate that the SED1-null epididymal pathologies are not the secondary consequences of defective testes or efferent ducts or of improper epididymal differentiation, unlike that seen in other epididymal models. The expression and distribution of various ion exchangers, channels, and enzymes that mediate fluid transport and pH regulation are examined in wildtype and SED1-null epididymides, and models to account for how SED1 functions in luminal fluid dynamics are discussed. Mol. Reprod. Dev. 77: 550-563, 2010. 漏 2010 Wiley-Liss, Inc.
URL [本文引用: 1]

SED1, also known as MFG-E8, is a secreted protein composed of two EGF repeats (the second of which contains an RGD motif) and two discoidin/Factor V/VIII C domains. SED1 is expressed by a wide range of cell types, where it participates in diverse cellular interactions, such as sperm binding to the egg coat and macrophage recognition of apoptotic lymphocytes. Although SED1 was originally identified as a milk protein, its function in the mammary gland remains unclear; suggested functions include inhibition of viral infection and clearance of apoptotic cells during mammary gland involution. We report here that SED1 has an unexpected obligatory role during mammary gland development. Unlike that seen in WT glands, SED1-null glands show severely reduced branching from epithelial ducts and from terminal end buds, which are thin and poorly developed. SED1 is expressed by both luminal and myoepithelial cells in the developing epithelial duct, and binds to $\alpha _{{\rm v}}$ integrin receptors on myoepithelial cells leading to MAPK activation and cell proliferation. The absence of SED1 leads to greatly reduced levels of activated MAPK and a concomitant reduction in cell proliferation and branching throughout the epithelial tree. These results suggest that SED1 contributes, at least partly, to the intercellular signaling between luminal and myoepithelial cells that is required for branching morphogenesis.
URLPMID:15363796 [本文引用: 1]

A prerequisite for successful fertilization is the species-specific binding of sperm to the extracellular coat of the egg. Gamete binding triggers the release of sperm hydrolytic enzymes that digest a path through the egg coat, thus bringing sperm into proximity with the egg plasma membrane where gamete fusion occurs. Although some components of the sperm membrane and the egg coat that participate in sperm–egg interactions have been identified, results from targeted deletions and gene substitutions indicate that other, as yet unidentified, gamete receptors must contribute to sperm–egg binding. Recent studies implicate the bi-motif protein, SED1, as being required for successful sperm–egg adhesion in mouse. SED1 contains Notch-like EGF repeats as well as discoidin/F5/8 complement domains — motifs that mediate a variety of cell–cell and cell–matrix interactions. SED1’s ability to promote gamete adhesion resides within its two discoidin/F5/802C domains, which are able to dock to substrates as diverse as phospholipid membranes and extracellular matrices. SED1 is also expressed in a wide range of tissues and epithelia, where it may function similarly as an adhesive protein facilitating cell–cell and/or cell–matrix interactions.
URLPMID:19240116 [本文引用: 1]

Abstract The epididymis is a highly convoluted tubule that connects the testis with the vas deferens, and in which mammalian sperm acquire the ability to fertilize eggs. The most proximal portion of the epididymis, or initial segment, secretes numerous factors that are critical for sperm maturation and storage. One such factor is SED1 (also known as MFG-E8) a bi-motif protein composed of two N-terminal EGF domains, the second of which contains an RGD motif, and two C-terminal discoidin domains (also known as F5/8 type C domains). Previous studies have reported that SED1 is secreted into the epididymal lumen, where it coats sperm and later facilitates sperm-egg binding. Herein, we report that SED1-null males also harbor unexpected epididymal pathologies, including detached epithelia and spermatic granulomas. We therefore examined whether SED1 has a tissue-intrinsic role in the epididymis, in addition to its role in sperm-egg adhesion. Improved fixation protocols revealed that SED1 is found in the basolateral domains of epididymal epithelial cells in vivo, and similarly, SED1 is secreted both apically and basally from polarized epididymal cells in vitro. The basolateral distribution of SED1 suggests that it may play a novel role in epididymal cell adhesion. Consistent with this, in vitro assays showed that SED1 supports epididymal cell adhesion via RGD binding to alphaV integrin receptors on epididymal epithelial cells. Finally, epididymal cells from SED1-null males showed reduced adhesion in vitro, a phenotype that can be rescued with exogenous SED1. These results suggest that SED1 facilitates epididymal cell adhesion, and that its loss leads to breakdown of the epididymal epithelium and consequent development of spermatic granulomas.
URLPMID:2631965 [本文引用: 1]

Protein-tyrosine sulfation is mediated by two Golgi tyrosyl-protein sulfotransferases (TPST-1 and TPST-2) that are widely expressed . However, the full substrate repertoire of this enzyme system is unknown and thus, our understanding of the biological role(s) of tyrosine sulfation is limited. We reported that whereas male mice have normal fertility, males are infertile despite normal spermatogenesis. However, sperm are severely defective in their motility in viscous media and in their ability to fertilize eggs. These findings suggest that sulfation of unidentified substrate(s) is crucial for normal sperm function. We therefore sought to identify tyrosine-sulfated proteins in the male genital tract using affinity chromatography on PSG2, an anti-sulfotyrosine monoclonal antibody, followed by mass spectrometry. Among the several candidate tyrosine-sulfated proteins identified, RNase 9 and Mfge8 were examined in detail. RNase 9, a catalytically inactive RNase A family member of unknown function, is expressed only in the epididymis after onset of sexual maturity. Mfge8 is expressed on mouse sperm and male mice are subfertile. Metabolic labeling coupled with sulfoamino acid analysis confirmed that both proteins are tyrosine-sulfated and both proteins are expressed at comparable levels in wild type, , and epididymides. However, we demonstrate that RNase 9 and Mfge8 are tyrosine-sulfated in wild type and , but not in mice. These findings suggest that lack of sulfation of one or both of these proteins may contribute mechanistically to the infertility of males.
URLPMID:16436526 [本文引用: 2]

Sperm adhesion molecule 1 (SPAM1), is a glycosyl phoshatidylinositol-linked sperm membrane protein that is dually expressed in testis and epididymis. Epididymal SPAM1 is secreted in all three regions of the epididymis in all mammalian species studied, including humans. It shares the same molecular mass and neutral hyaluronidase activity as the testicular and sperm isoforms that are responsible for the penetration of the cumulus during fertilization. Using wild-type (W/T) sperm and those from mice homozygous for either a null (Spam1-/-) or mutant Spam1 allele, which results in decreased mRNA and protein, we demonstrate that sperm binding of epididymal SPAM1 occurs in vitro after exposure to W/T sperm-free epididymal luminal fluid (ELF). Binding or adsorption that occurred after incubation at room temperature or 32 degrees C was detected immunocytochemically and confirmed quantitatively using flow cytometry. The localization of SPAM1 on the plasma membrane of Spam1-null sperm mimicked that seen in the W/T. The remarkable increase in binding on W/T caudal sperm indicates that they are not fully saturated with SPAM1 during storage, and suggests that uptake of epididymal SPAM1 in vivo augments testicular SPAM1. Spam1-null sperm exposed to W/T ELF for 45-60 min during in vitro capacitation to allow epididymal SPAM1 binding showed a highly significant (P < 0.001) increase in cumulus penetration after 6-7 h compared to those incubated in ELF from null males. Similarly, the number of cumulus-free oocytes was also highly significantly greater (P < 0.001) than that for sperm capacitated in W/T SPAM1-antibody-inhibited ELF. Because epididymal SPAM1 uptake significantly increases cumulus penetration, we conclude that it is a marker of sperm maturation.
URLPMID:8722621 [本文引用: 1]

The interaction of spermatozoa with the pellucida is a critical step of . Specific involved in this process can be added or modified during epididymal transit. We have previously described a 34-kDa epididymal sperm protein (P34H) that we proposed to be involved in sperm-pellucida interaction. In this study, Western blot analysis were performed to determine the level of P34H protein present on the spermatozoa of 16 men with idiopathic . These levels were compared with the amount of P34H protein found in men of proven fertility. In addition, a sperm-pellucida assay was performed with spermatozoa from fertile and infertile men. Spermatozoa obtained from different semen samples from a given individual had similar P34H levels. However, the amount of P34H varied from one to another. Nine of 16 infertile men had a P34H level that was less than 30% of the normal value based on a population of fertile men, while the remaining 7 males were in the normal range. Sperm from infertile subjects with a normal P43H determination bound to zonae pellucidae as efficiently as those from controls. However, spermatozoa from subjects with a low amount of P34H exhibited a dramatic diminution in their ability to interact with zonae pellucidae. Our results show that the quantity of the epididymal protein P34H varied from one male to another and that low levels of this epididymal sperm protein are associated with certain cases of idiopathic . Results are discussed with regard to the function of epididymis in sperm maturation.
URLPMID:23124857 [本文引用: 2]

Liprin α3 was reported for the first time using sperm proteomics. Present study reports its localization on sperm and immunochemical characterization. Liprin α3 is identified as a 13302kDa protein in testis and epididymal protein extracts. In testis, immunohistochemical localization was seen in pachytenes, diplotenes, round spermatids whereas it was localized in the epithelial cells and luminal sperm in all the three regions of epididymis. Protein was localized in acrosome of rat sperm, which was further confirmed by sequential treatment of sperm with hypertonic solution. In the spermatogenic cells the protein was found to be located in developing acrosome as evident by its co-localization with Golgi marker. Protein was found to be developmentally regulated. In silico analysis of Liprin α3 revealed presence of the estrogen responsive elements upstream to initiation site and its regulation by estrogen was experimentally validated using a tamoxifen treated rat model. Western blot analysis of epididymosomes showed the presence of Liprin α3, indicating its involvement in trafficking of vesicle. The protein expression was seen in both mouse and human sperm indicating conserved nature and a probable role in acrosome reaction.
URLPMID:24327330 [本文引用: 1]

Zona pellucida-based induction of acrosome reaction (AR) is a popular and well-accepted hypothesis. However, this hypothesis is being challenged in recent years and it has been proposed that the cumulus cells might be the site of AR. In our previous study, we reported the presence of a synaptic protein Liprin α3 on sperm acrosome, and proposed its role in AR. This study was designed to understand the role of Liprin α3 and its interacting proteins in regulation of AR. It is observed that the presence of anti-Liprin α3 antibody inhibits the process of AR. Colocalization experiments demonstrate the coexistence of leucocyte antigen related (LAR) protein, Rab-interacting molecule (RIM) and Liprin α3 on sperm acrosome thereby completing the identification of all the members of RIM/MUNC/Rab3A/liprinα complex required for membrane fusion. This study demonstrates the effect of LAR ligands such as Syndecans, Nidogens and LAR wedge domain peptide on AR. We could see an increase in AR in presence of these ligands. On the basis of these data, we speculate that in presence of ligands or wedge peptide, LAR undergoes dimerization leading to inhibition of phosphatase activity and increase in AR. The presence of one of the ligands Syndecan-1 on cumulus cells led us to hypothesize that it is Syndecan which induces AR in vivo and thus another site of AR could lie in cumulus.
URLPMID:19605784 [本文引用: 1]

Although sperm entry into the oocyte-cumulus complex and subsequent sperm penetration through the cumulus matrix to reach the oocyte zona pellucida are essential for mammalian fertilization, the molecular mechanism remains controversial. Previously, we have shown that mouse sperm lacking SPAM1 are capable of penetrating the cumulus matrix despite a delayed dispersal of cumulus cells. We also have identified another sperm hyaluronidase, HYAL5, as a candidate enzyme involved in sperm penetration through the cumulus. In the present study, we produced HYAL5-deficient mice to uncover the functional roles of HYAL5 and SPAM1 in fertilization. The HYAL5-deficient mice were fully fertile and yielded normal litter sizes. In vitro fertilization assays demonstrated that HYAL5-deficient epididymal sperm is functionally normal. We thus conclude that HYAL5 may be dispensable for fertilization. Comparative analysis among wild-type, HYAL5-deficient, and SPAM1-deficient epididymal sperm revealed that only SPAM1 is probably involved in sperm penetration through the cumulus matrix. Notably, the loss of SPAM1 resulted in a remarkably increased accumulation of sperm on the surface or outer edge of the cumulus. These data suggest that SPAM1 may function in sperm entry into the cumulus and sperm penetration through the cumulus matrix.
URLPMID:22750823 [本文引用: 1]

Abstract Changes that occur to mammalian sperm upon epididymal transit and maturation render these cells capable of moving progressively and capacitating. Signaling events leading to mammalian sperm capacitation depend on the modulation of proteins by phosphorylation and dephosphorylation cascades. Recent experiments have demonstrated that the Src family of kinases plays an important role in the regulation of these events. However, sperm from cSrc null mice display normal tyrosine phosphorylation associated with capacitation. We report here that, despite normal phosphorylation, sperm from cSrc null mice display a severe reduction in forward motility, and are unable to fertilize in vitro. Histological analysis of seminiferous tubules in the testes, caput and corpus epididymis do not reveal obvious defects. However, the cauda epididymis is significantly smaller, and expression of key transport proteins in the epithelial cells lining this region is reduced in cSrc null mice compared to wild type littermates. Although previously, we and others have shown the presence of cSrc in mature sperm from cauda epididymis, a closer evaluation indicates that this tyrosine kinase is not present in sperm from the caput epididymis, suggesting that this protein is acquired by sperm later during epididymal maturation. Consistent with this observation, cSrc is enriched in vesicles released by the epididymal epithelium known as epididymosomes. Altogether, these observations indicate that cSrc is essential for cauda epididymal development and suggest an essential role of this kinase in epididymal sperm maturation involving cSrc extracellular trafficking. Copyright 2012 Elsevier Inc. All rights reserved.
URLPMID:22552861 [本文引用: 1]

Abstract Glioma pathogenesis-related 1-like protein1 (GliPr1L1) was identified by liquid chromatography-tandem mass spectrometry analyses of proteins associated to bovine sperm lipid raft membrane domains. This protein belongs to the CAP superfamily including cysteine-rich secretory proteins, Antigen 5 and pathogenesis-related 1 protein. PCR analysis revealed that GliPr1L1 is expressed in testis and, at a much lower level, all along the epididymis. Western blotting showed a similar distribution of GliPr1L1 in testicular and epididymal tissue extracts. In the epididymal lumen, GliPr1L1 was associated with the maturing spermatozoa and epididymosomes all along the excurrent duct but was undetectable in the soluble fraction of epididymal fluid. The protein was detectable as multiple isoforms with a higher MW form in the testis and proximal caput. Treatments with PNGase F revealed that N -glycosylation was responsible of multiple bands detected on Western blots. These results suggest that the N -glycosylation moiety of GliPr1L1 is processed during the transit in the caput. Western blots demonstrated that GliPr1L1 was associated with the sperm plasma membrane preparation. GliPr1L1 is glycosyl phosphatidyl inositol (GPI) anchored to caput and cauda spermatozoa as demonstrated by the ability of phosphatidylinositol specific phospholipase C to release GliPr1L1 from intact sperm cells. Lipid raft membrane domains were separated from caput and cauda epididymal spermatozoa. GliPr1L1 was immunodetectable in the low buoyant density fractions where lipid rafts are distributed. GliPr1L1 was localized on sperm equatorial segment and neck. In vitro fertilization performed in presence of anti-GliPr1L1 showed that this protein is involved in sperm ona pellucida interaction. J. Cell. Physiol. 227: 3876 3886, 2012. 2012 Wiley Periodicals, Inc.
[本文引用: 1]
URL [本文引用: 1]

Differentiation of the testis and its subsequent hormonal function are pivotal events in development of the male, but there is increasing evidence that these events are susceptible to disruption by environmental/lifestyle factors which can then result in (common) reproductive disorders these are frequently referred to as testicular dysgenesis syndrome (TDS). In rats, fetal exposure to certain phthalates, such as dibutyl phthalate (DBP) can induce TDS disorders, and we have used this as a model system in which to elucidate the key effects and mechanisms. DBP exposure dose-dependently induces focal dysgenesis, manifest in fetal life by the appearance of ectopic Sertoli cells (SC) interspersed amongst fetal Leydig cells; remarkably, these ectopic SC do not differentiate until late (beyond e19.5) in gestation. After birth, these ectopic SC form seminiferous cords that are mis-shapen and within which (intratubular) Leydig cells also appear. Occurrence and severity of focal dysgenesis is closely interlinked with fetal Leydig cell (LC) dysfunction during the critical asculinization programming window (MPW; e15.5-e18.5), even though the dysgenesis does not become manifest until after the MPW. DBP-induced impairment of fetal LC steroidogenesis occurs because of a failure to relieve repression by COUP-TFII (chicken ovalbumin upstream promoter transcription factor-II), which normally occurs progressively during gestation; this mechanism is also susceptible to perturbation by hormones (estrogens, glucocorticoids). Altered expression of COUP-TFII may also be one factor involved in the origins of focal dysgenesis. To evaluate whether DBP/other phthalates might exert similar effects on the human fetal testis, we have developed a model in which pieces of 1st or 2nd trimester human fetal testes are xenografted under the back skin of nude mice, where they develop normally. In contrast to the fetal rat, neither DBP nor estrogen exposure impairs testosterone production by the fetal testis xenografts; this species difference is unexplained but fits with findings in vitro and with in vivo findings in the marmoset. In contrast, DBP exposure induces germ cell loss in human fetal testis xenografts analogous to that induced in rats, and this appears to selectively target the undifferentiated pluripotent germ cells; these cells may also be targets for other environmental/lifestyle factors. The mechanisms underlying germ cell loss are currently under investigation. The fetal testis also contains progenitor cells for adult Leydig cells and these cells are targets for androgen action. Emerging evidence suggests that disruption of testosterone production or action in the fetal rat testis, for example by DBP, may have consequences for adult Leydig cell numbers/function, effects that are presumed to be mediated via the progenitor cells.
URLPMID:26112475 [本文引用: 1]

Extracellular microvesicles present in the epididymal fluid have been named epididymosomes. Many epididymosome-associated proteins are transferred to spermatozoa during their maturation in the excurrent duct. Epididymosomes are heterogeneous, with their size varying between 50 and 250 nm. Two distinct population of epididymosomes characterized by different protein compositions and diameters have been isolated from the bovine epididymal fluid using different centrifugation protocols. One subpopulation of epididymosomes was characterized by CD9 and other tetraspanin partners. Transfer of proteins from these epididymosomes to maturing spermatozoa in co-incubation experiments was inhibited by antibodies against tetraspanin proteins. This suggests that this subpopulation of epididymosomes is involved in the acquisition of proteins involved in maturation by spermatozoa in the epididymis. The other population of epididymosomes was characterized by ELSPBP1 (epididymal sperm binding protein 1), known for its affinity for the phospholipid choline group. Flow cytometric analyses showed that ELSPBP1-positive epididymosomes only interacted with dying or dead epididymal spermatozoa in a Zn2 +-dependent manner. BLVRA (biliverdin reductase) was identified as a partner of ELSPBP1. This enzyme reduces biliverdin to bilirubin: two molecules with powerful anti-oxidant properties. We hypothesize that BLVRA is involved in an ROS-scavenging mechanism protecting live epididymal spermatozoa against detrimental molecules (ROS) released by dying cells. Therefore, it appears that there are at least two epididymosome population with distinct functions: targeting specific proteins to transiting spermatozoa by tetraspanin-mediated membrane fusion, and protection of epididymal spermatozoa against ROS released from dying cells. Further work is needed to understand functions of epididymosomes in epididymal physiology and sperm maturation and storage.
URL [本文引用: 1]
Magsci [本文引用: 2]

MicroRNA(miRNA)是一类长约22nt的非编码小RNA, 广泛存在于各种生物中, 调节生物体生长、发育和凋亡等过程。研究表明, miRNA在人和动物睾丸发育及精子生成等过程也起着重要的调控作用。但miRNA在不同种属的睾丸组织及其不同发育时间段均存在特异性表达。此外, miRNA在动物精子生成过程中也存在时空特异性。文章综述了睾丸发育和精子生成过程中miRNA的差异性表达、表达调控以及一些miRNA对精子生成的调节作用, 旨在为睾丸miRNA的进一步研究提供参考, 为利用miRNA调控和促进种公畜精液品质提供研究思路。
Magsci [本文引用: 2]

MicroRNA(miRNA)是一类长约22nt的非编码小RNA, 广泛存在于各种生物中, 调节生物体生长、发育和凋亡等过程。研究表明, miRNA在人和动物睾丸发育及精子生成等过程也起着重要的调控作用。但miRNA在不同种属的睾丸组织及其不同发育时间段均存在特异性表达。此外, miRNA在动物精子生成过程中也存在时空特异性。文章综述了睾丸发育和精子生成过程中miRNA的差异性表达、表达调控以及一些miRNA对精子生成的调节作用, 旨在为睾丸miRNA的进一步研究提供参考, 为利用miRNA调控和促进种公畜精液品质提供研究思路。
URLPMID:27694809 [本文引用: 1]

Once deemed heretical, emerging evidence now supports the notion that the inheritance of acquired characteristics can occur through ancestral exposures or experiences and that certain paternally acquired traits can be 'memorized' in the sperm as epigenetic information. The search for epigenetic factors in mammalian sperm that transmit acquired phenotypes has recently focused on RNAs and, more recently, RNA modifications. Here, we review insights that have been gained from studying sperm RNAs and RNA modifications, and their roles in influencing offspring phenotypes. We discuss the possible mechanisms by which sperm become acquisitive following environmental-somatic-germline interactions, and how they transmit paternally acquired phenotypes by shaping early embryonic development.
URLPMID:22652870 [本文引用: 1]

Small RNA has become a crucial regulator of during . Alterations in small RNA function prove to be detrimental to proper . As many patients suffer from idiopathic , understanding the molecular mechanisms of small RNA identifies possible causes of certain types of . With a comprehensive review of the history of miRNA and piRNA function and specificity in the testis from a wide range of studies offers a view of detrimental of small RNA. By combining a concise overview of small RNA mechanism and recent research we explain how some cases of can be a product of complications in specific small RNA functions. The future direction section offers insight into how treatment may be approached with a novel perspective.
URLPMID:24365599 [本文引用: 1]

61miR-200c is essential for Nanog expression/hESC renewal, and blocks EB formation.61miR-200c directly targets GATA4 3′UTR and inhibits GATA4 expression.61Downregulation of GATA4 partially restores hESC renewal upon miR-200c knockdown.61Overexpression of GATA4 rescues EB formation and three lineages differentiation.61miR-200c and activin A/Smad pathway can regulate each other's expression/function.
URL [本文引用: 1]

男性生殖道感染是引起男性不育的一个重要因素,然而其作用尚未完全清楚。脂多糖(Lipopolysaccharide,LPS)是革兰阴性菌细胞壁的主要组成成分,也是革兰阴性菌的主要致病因子,能够诱导机体的炎症反应,从而影响男性的生殖功能。男性生殖道细菌感染及其所引起的炎症反应均会影响男性的精液质量,从而导致男性生育能力降低。本文就泌尿生殖道感染及其导致的炎症以及氧化压力等方面对精液的影响来研究其与男性不育的相关性。
URL [本文引用: 1]

男性生殖道感染是引起男性不育的一个重要因素,然而其作用尚未完全清楚。脂多糖(Lipopolysaccharide,LPS)是革兰阴性菌细胞壁的主要组成成分,也是革兰阴性菌的主要致病因子,能够诱导机体的炎症反应,从而影响男性的生殖功能。男性生殖道细菌感染及其所引起的炎症反应均会影响男性的精液质量,从而导致男性生育能力降低。本文就泌尿生殖道感染及其导致的炎症以及氧化压力等方面对精液的影响来研究其与男性不育的相关性。
URLPMID:21320120 [本文引用: 1]

Summary Top of page Summary Introduction Materials and methods Results Discussion Conclusions Acknowledgements Disclosures References The innate immune response provides the initial defence mechanism against infection by other organisms. However, an excessive immune response will cause damage to host tissues. In an attempt to identify microRNAs (miRNAs) that regulate the innate immune response in inflammation and homeostasis, we examined the differential expression of miRNAs using microarray analysis in the spleens of mice injected intraperitoneally with lipopolysaccharide (LPS) and saline, respectively. Following challenge, we observed 19 miRNAs up-regulated (1·5-fold) in response to LPS. Among these miRNAs, miR-1224, whose expression level increased 5·7-fold 6hr after LPS injection and 2·3-fold after 24hr, was selected for further study. Tissue expression patterns showed that mouse miR-1224 is highly expressed in mouse spleen, kidney and lung. Transfection of miR-1224 mimics resulted in a decrease in basal tumour necrosis factor-α (TNF-α) promoter reporter gene activity and a down-regulation of LPS-induced TNF-α mRNA in RAW264.7 cells. With public databases of miRNA target prediction, miR-1224 was shown to bind to the 3′ untranslated region (UTR) of Sp1 mRNA, whose coding product controls TNF-α expression at the transcriptional level. Furthermore, we found that in HEK-293 cells, the activity of the luciferase reporter bearing Sp1 mRNA 3′ UTR was down-regulated significantly when transfected with miR-1224 mimics. After transfection of miR-1224 in RAW264.7 cells, nucleus Sp1 protein level decreased, and when endogenous miR-1224 was blocked, the decrease was abolished. Therefore, we initially speculated that miR-1224 was a negative regulator of TNF-α in an Sp1-dependent manner, which was confirmed in vivo by chromatin immunoprecipitation assay, and might be involved in regulating the LPS-mediated inflammatory responses.
URL [本文引用: 3]
URLPMID:4245725 [本文引用: 1]

Background Exosomes are membranous nanovesicles secreted into the extracellular milieu by diverse cell types. Exosomes facilitate intercellular communication, modulate cellular pheno/genotype, and regulate microbial pathogenesis. Although human semen contains exosomes, their role in regulating infection with viruses that are sexually transmitted remains unknown. In this study, we used semen exosomes purified from healthy human donors to evaluate the role of exosomes on the infectivity of different strains of HIV-1 in a variety of cell lines. Results We show that human semen contains a heterologous population of exosomes, enriched in mRNA encoding tetraspanin exosomal markers and various antiviral factors. Semen exosomes are internalized by recipient cells and upon internalization, inhibit replication of a broad array of HIV-1 strains. Remarkably, the anti- HIV-1 activity of semen exosomes is specific to retroviruses because semen exosomes blocked replication of the murine AIDS (mAIDS) virus complex (LP-BM5). However, exosomes from blood had no effect on HIV-1 or LP-BM5 replication. Additionally, semen and blood exosomes had no effect on replication of herpes simplex virus; types 1 and 2 (HSV1 and HSV2). Mechanistic studies indicate that semen exosomes exert a post-entry block on HIV-1 replication by orchestrating deleterious effects on particle-associated reverse transcriptase activity and infectivity. Conclusions These illuminating findings i) improve our knowledge of the cargo of semen exosomes, ii) reveal that semen exosomes possess anti-retroviral activity, and iii) suggest that semen exosome-mediated inhibition of HIV-1 replication may provide novel opportunities for the development of new therapeutics for HIV-1 .
URLPMID:25880110 [本文引用: 1]

Abstract Exosomes are membranous extracellular nanovesicles secreted by diverse cell types. Exosomes from healthy human semen have been shown to inhibit HIV-1 replication and to impair progeny virus infectivity. In this study, we examined the ability of healthy human semen exosomes to restrict HIV-1 and LP-BM5 murine AIDS virus transmission in three different model systems. We show that vaginal cells internalize exosomes with concomitant transfer of functional mRNA. Semen exosomes blocked the spread of HIV-1 from vaginal epithelial cells to target cells in our cell-to-cell infection model and suppressed transmission of HIV-1 across the vaginal epithelial barrier in our trans-well model. Our in vivo model shows that human semen exosomes restrict intravaginal transmission and propagation of murine AIDS virus. Our study highlights an antiretroviral role for semen exosomes that may be harnessed for the development of novel therapeutic strategies to combat HIV-1 transmission. Copyright 2015 Elsevier Inc. All rights reserved.
[本文引用: 1]
URLPMID:23093182 [本文引用: 1]

MicroRNA-mediated gene regulation is important in many physiological processes. Here we explore the roles of a microRNA, miR-941, in human evolution. We find that miR-941 emerged de novo in the human lineage, between six and one million years ago, from an evolutionarily volatile tandem repeat sequence. Its copy-number remains polymorphic in humans and shows a trend for decreasing copy-number with migration out of Africa. Emergence of miR-941 was accompanied by accelerated loss of miR-941-binding sites, presumably to escape regulation. We further show that miR-941 is highly expressed in pluripotent cells, repressed upon differentiation and preferentially targets genes in hedgehog- and insulin-signalling pathways, thus suggesting roles in cellular differentiation. Human-specific effects of miR-941 regulation are detectable in the brain and affect genes involved in neurotransmitter signalling. Taken together, these results implicate miR-941 in human evolution, and provide an example of rapid regulatory evolution in the human linage.
