 ,1,2, 张莹2, 周钢桥
,1,2, 张莹2, 周钢桥 ,2
,2The critical roles of TBC proteins in human diseases
Mengting Shi ,1,2, Ying Zhang2, Gangqiao Zhou
,1,2, Ying Zhang2, Gangqiao Zhou ,2
,2第一联系人:
收稿日期:2017-10-22修回日期:2017-12-12网络出版日期:--
| 基金资助: |
Received:2017-10-22Revised:2017-12-12Online:--
| Fund supported: |

摘要
关键词:
Abstract
Keywords:
PDF (636KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
施梦婷, 张莹, 周钢桥. TBC蛋白家族成员在人类疾病发生发展中的作用. 遗传[J], 2018, 40(1): 12-21 doi:10.16288/j.yczz.17-343
Mengting Shi, Ying Zhang, Gangqiao Zhou.
Rab是真核细胞中一类进化保守的小G蛋白(small GTPases),主要参与细胞内膜泡的形成、膜泡向靶细胞器的移动,以及与特定靶膜的锚定等一系列细胞内运输过程。该过程涉及Rab蛋白在激活状态Rab-GTP和失活状态Rab-GDP之间的循环。细胞质中GTP的水平较高,GTP优先占据鸟嘌呤核苷酸结合域;鸟嘌呤核苷酸交换因子(guaninenucleotide exchange factor, GEF)可促进GDP解离,加速GDP到GTP的转变,促使Rab-GTP的形成。Rab-GTP被结合到膜泡表面,与膜上特定的Rab效应分子(Rab effector)结合,并将膜泡锚定到靶膜上,进而发生膜泡融合。膜泡融合发生后,一类被称为GTP酶激活蛋白(GTPase activating protein, GAP)的分子将促进Rab-GTP水解为Rab-GDP,并将其释放到细胞质中,然后再进行下一轮的GDP-GTP交换、结合和水解循环(图1)。Rab蛋白家族共有70多个成员,具有相似的结构、功能及鸟苷酸结合能力。由于各自的亚细胞定位、Rab效应分子、GEFs和GAPs不同,Rab蛋白具有了不同的生物学功能[1]。
Rab效应分子和GEFs在结构上没有相似的结构特征,但GAPs普遍含有一个保守性蛋白结构域―Tre2-Bub2-Cdc16(TBC)。该结构域最早在酵母细胞中被发现,是癌基因tre-2、酵母的细胞周期调控蛋白Bub2及Cdc16的共同保守域[2],也是酵母细胞GAPs(也称为GYPs, GAP for Ypt proteins)的共有结构域。人们将含有TBC结构域的蛋白统称为TBC家族蛋白。本文对TBC家族蛋白的结构、功能及其在疾病中的作用和意义进行了综述。
图1
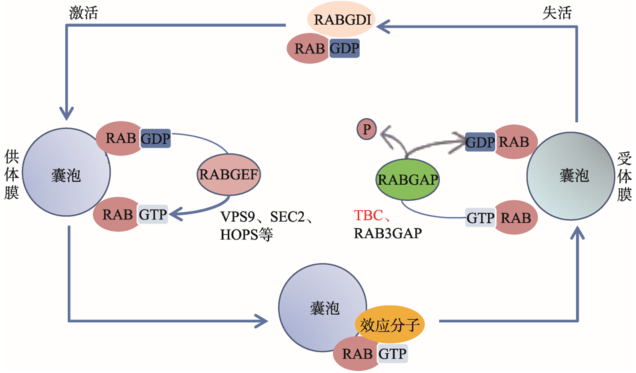 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1Rab循环
Rab在GDP结合的非活性状态和GTP结合的活性状态之间循环。两种状态之间的循环由激活酶GEF(如VPS9、SEC2、HOPS蛋白复合物)和失活酶GAP(如TBC、RAB3GAP蛋白)调节。Rab特异性GDP解离抑制剂(RABGDI)促进从受体膜向供体膜募集Rab-GDP。Rab特异性鸟嘌呤核苷酸交换因子(RABGEF)通过与细胞内丰富的GTP结合形成激活的Rab-GTP。Rab特异性GTP酶激活蛋白(RABGAP,如包含Tre2-Bub2-Cdc16(TBC)结构域的蛋白)可促进GTP水解。RABGAP:Rab特异性 GTP酶激活蛋白(Rab-specific GTPase activating protein);RABGDI:Rab特异性GDP解离抑制剂(Rab-specific GDP dissociation inhibitor);RABGEF:Rab特异性鸟嘌呤核苷酸交换因子(Rab-specific guanine nucleotide exchange factor)。
Fig. 1Rab cycle
1 TBC家族蛋白的结构特征
已有研究表明,TBC蛋白广泛存在于多种物种中,例如TBC1D15,分别在人(Homo sapiens)、小鼠(Mus musculus)和条纹斑竹鲨(Chiloscyllium plagiosum)等中被发现。同时,同一物种中含有多个TBC蛋白家族成员。通过序列同源性分析预测,人类中至少存在40种含有TBC结构域的蛋白(图2)。目前,其大部分成员的作用底物及功能尚不清楚。TBC结构域由约200个氨基酸残基组成。晶体学结构研究发现,该结构域中含有两个十分重要的催化残基精氨酸(R)和谷氨酰胺(Q),分别被称为R指和Q指。它们可将Rabs稳定在过渡态并催化Rabs的GTP水解,其中精氨酸是其Rab-GAP活性最重要的决定因素[3]。将鲨鱼TBC1D15-GAP结构域的R和Q分别突变成丙氨酸(A)和赖氨酸(K)后,其GAP活性消失[4]。一半以上的TBC家族成员均含有这两个指型结构,被称为经典型TBC蛋白(也称RQ型)。然而也有部分TBC蛋白不含有R指和/或Q指,这类蛋白被称为非经典的TBC蛋白。依据TBC蛋白所含有的结构域特征可细分为RX型、RR型、XR型、XQ型和XX型(其中X代表该区域不含有催化残基精氨酸和谷氨酰胺)。目前对非经典的TBC蛋白调控Rabs的机制还知之甚少,它们可能通过其他机制行使GAP活性的作用。
图2
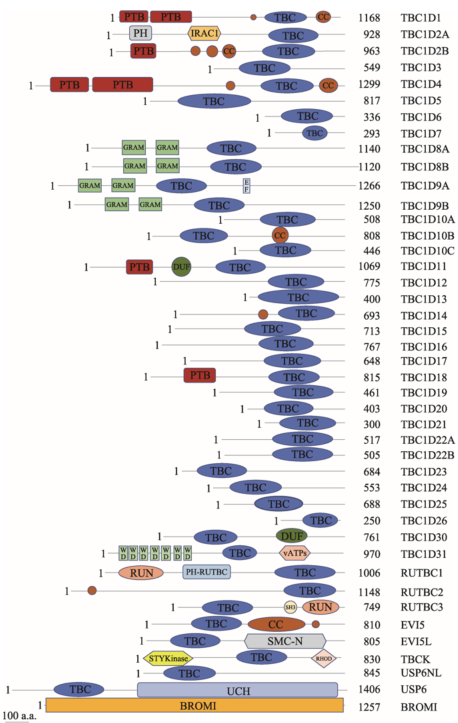 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2人类TBC蛋白的结构域示意图
通过使用HPRD(
Fig. 2Structure of human TBC proteins
TBC家族蛋白除了含有TBC结构域以外,通常还含有其他结构域,包括PH(pleckstrin homology domain)、CC(coiled-coil)、PTB(phosphotyrosine-binding domain)或者GRAM(gucosyl transferases)结构域等,这些结构域对TBC蛋白的亚细胞定位以及功能调节十分重要。TBC1D2C/ARMUS的PH结构域可与E钙粘素结合,将ARMUS定位到粘着连接处[5]。TBC1D2B的CC结构域可与Rab22A结合,这种结合并不促进Rab22A上GTP的水解,而是将TBC1D2B绑定到特定的囊泡中[6]。TBC1D11的PTB结构域可与Rab36结合,从而将TBC1D11绑定到含Rab36的核周囊泡或高尔基体上[6]。
2 TBC家族蛋白的主要生化功能、生物学作用及其特征
最初在酵母中的研究发现TBC蛋白具有GAP活性,能催化Rab-GTP水解为Rab-GDP,是Rab蛋白的负调控分子。随后,在哺乳动物细胞中也发现了大量的TBC蛋白,目前在人类中发现的TBC蛋白已多达40余种。大部分TBC蛋白是特异Rab蛋白的负调控分子,能选择性促进特定Rab蛋白的水解和失活。尽管最初酵母GYPs的体外生化实验显示其只有较弱的底物特异性,但遗传学和细胞生物学研究显示,不同的GYPs参与了完全不同的转运过程,其功能在体内具有明显的特异性。例如,在体外实验中,GYP7p与YPT7p的结合只有部分特异性,但在体内实验中GYP7p与蛋白激酶YCK3p一起调控YPT7p,参与液泡融合过程的调节[7,8]。此外,TBC蛋白也参与不同Rab之间或者Rab与其他小G蛋白之间的信号传递。由此可见,TBC家族成员参与了众多的生物学过程,包括纤毛的形成、大胞饮、免疫突触的形成、胞质分裂和细胞自噬等(表1)。Table 1
表1
表1 TBC蛋白的底物和功能
Table 1
| TBC | 底物 | 功能 | 疾病 | 参考文献 |
|---|---|---|---|---|
| TBC1D1 | Rab2A、Rab8A、Rab8B、 Rab10、Rab14 | GLUT4的运输 | 2型糖尿病;突变后易诱发肥胖 | [12~14] |
| TBC1D2 | Rab7 | E钙黏素的降解 | 在前列腺癌中高表达 | [5,15] |
| TBC1D2B | 与自噬小体相关 | [16] | ||
| TBC1D3 (PRC17) | Rab5 | 大胞饮; 抑制EGFR的降解 | 在前列腺癌和乳腺癌中高表达;骨髓增生异常综合症中存在基因扩增 | [17~20] |
| TBC1D4 | Rab2A、Rab8A、Rab10、Rab14 | GLUT4的运输; 胰腺β细胞PKB信号的效应分子 | 在过敏性皮肤炎患者的T细胞中表达上调 | [21~25] |
| TBC1D6 | 生长激素调节其表达 | [26] | ||
| TBC1D7 | 纤毛的形成;结合TSC1; 与自噬小体相关 | 在肺癌中高表达 | [16,27] | |
| TBC1D8 | 骨质疏松症的易感基因 | [28] | ||
| TBC1D9 | Rab7、Rab9 | [29] | ||
| TBC1D9B | Rab11a | 基底外侧到顶端的转胞吞作用 | [30] | |
| TBC1D10A | Rab27A、Rab35 | 黑色素体的运输;调节微绒毛结构和外泌体 | [31, 32] | |
| TBC1D10B | Rab3A、Rab3、Rab22A、Rab27A、Rab31、Rab27B | 黑色素体的运输;外泌体分泌;调节胰腺腺泡细胞中的胞吐作用 | 志贺氏毒素的吸收 | [33] |
| TBC1D10C | Rab35 | T细胞回收途径;免疫突触的形成;外泌体分泌 | [31,34] | |
| GAPCENA (TBC1D11) | Rab2、Rab4、Rab6、Rab11、Rab36 | 微管和高尔基体在细胞周期中的运动;调节纺锤体检测点 | [35,36] | |
| TBC1D12 | Rab11 | 神经突触的生长 | [37] | |
| TBC1D15 | Rab7、Rab11 | 与自噬小体相关;调节突触发育 | [38] | |
| TBC1D16 | Rab4A、Rab5C | 调节EGFR的降解、活化和信号 | 黑色素瘤中有扩增 | [39~41] |
| TBC1D17 | Rab21 | 志贺氏毒素的吸收 | [42] | |
| TBC1D18 | Rab22A、Rab34、Rab39B | [32] | ||
| TBC1D20 | Rab1、Rab2 | 内质网膜运输 | 丙肝病毒的复制;参与自噬体的成熟 | [43~45] |
| TBC1D23 | 卡氏肺孢子虫感染中表达下调;高微卫星不稳性肿瘤中有突变 | [46] | ||
| TBC1D24 | 调节神经突触的长度和分支 | 遗传性婴儿肌阵挛癫痫患者中有突变 | [47,48] | |
| TBC1D25 | Rab2A、Rab13、Rab34、Rab33A、Rab33B | 自噬体的成熟 | 滑膜肉瘤组织中有断点 | [49] |
| TBC1D30 | Rab8A | 纤毛的形成 | [50] | |
| RABGAP5 | Rab5A、Rab5B、Rab5C | 内吞作用 | [42] | |
| EVI5 | Rab35、Rab11 | 胞质分裂 | 志贺氏毒素的吸收 | [42,51] |
| EVI5-like | Rab4A、Rab7、Rab10、Rab23 | 纤毛的形成 | [50] | |
| TRE2 (USP6, TRE17) | 内吞作用 | 尤文氏肉瘤中有易位;动脉瘤样骨囊肿中表达上调 | [52] | |
| RN-TRE (USP6NL) | Rab1、Rab2、Rab3A、Rab5A、Rab28、Rab41、Rab43 | EGFR的内吞;大胞饮;志贺氏毒素的吸收 | [9,42] |
新窗口打开|下载CSV
TBC蛋白特异调控膜泡转运的过程和方式十分复杂,但大致具有以下两点特征:(1)同一TBC蛋白可能参与调控多个Rab蛋白的活性,而同一Rab蛋白也可能受到不同TBC蛋白的调节。例如,RN-tre是第一个被确定参与内吞过程的GAP,它调节Rab5的活性,并参与调控依赖于Rab5的EGFR内吞过程[9]。也有研究显示RN-tre可通过调控Rab43的活性,参与志贺氏毒素(Shiga toxin)的胞内转运过程[10]。RN-tre对上述两种特异性底物的选择性调节需要其他效应分子的参与。RabGAP-5是Rab5的另一个GAP蛋白,它具有高度的底物特异性[10,11]。RNA干扰和过表达实验显示,RabGAP-5可特异性阻断EGFR的转运过程,但对其他内吞作用无影响[11]。(2)同一TBC蛋白可参与多种生物学过程,同一生物学过程也可由多种不同的TBC蛋白完成。例如,RN-tre可调节大胞饮和EGFR的内吞作用;TBC1D10可调节微绒毛结构、外泌体的分泌和黑色素体的运输。TBC1D7、EVI5-L和TBC1D30可分别作为Rab17、Rab23和Rab8的GAP蛋白发挥作用,参与纤毛的形成。参与志贺氏毒素吸收的TBC蛋白则多达6种,包括EVI5、RN-tre/USP6NL、TBC1D10A-C和TBC1D17;另外,TBC1D3、RN-tre和TBC1D16均参与EGFR的胞内转运过程(表1)。
综上所述,每个TBC蛋白对特异Rab蛋白的调控作用十分复杂,该过程可能受到上游的信号通路、蛋白分子的亚细胞定位、其他蛋白分子的协同等多方面的共同影响。因此,全面解析TBC蛋白的调控作用还有待更多深入和全面的研究。
3 TBC家族蛋白在疾病发生发展中的作用
由Rab3A、Rab7、Rab23、Rab27A、Rab38和Rab39B等Rab蛋白或其效应分子GDI(GDP disso-ciation inhibitor)、REP(Rab escort protein)和GGTA (geranyl geranyl transferase α-subunit)等介导的膜泡转运功能障碍,已被广泛报道与人类疾病密切相关。TBC/Rab-GAPs蛋白的生物学功能十分重要,与多种信号转导通路联系紧密,其失调会导致肥胖[12,14]、病毒或细菌感染[10,44,45]、癫痫[53]、过敏性皮肤炎[23]和肿瘤[54,55,56,57]等疾病。TBC蛋白家族的部分基因在疾病中存在基因突变[46,58,59]、遗传学关联[18,28,60]、拷贝数扩增[19,55]或者表达异常[19,23,27],同时与病毒蛋白的结合[44,45]和磷酸化修饰[13]也会导致TBC/Rab- GAPs蛋白GAP活性的改变。TBC蛋白与疾病发生发展的相关性提示它可能作为疾病治疗的候选靶标。TBC1D4(AS160)和TBC1D1是哺乳动物中细胞学功能或生理学功能研究较清楚的TBC蛋白。它们对血液中葡萄糖水平的调节十分重要,其功能异常可影响胰岛素的作用和葡萄糖的摄取,从而引起肥胖或消瘦。TBC1D4是AKT的底物,参与了胰岛素刺激后的葡萄糖转运蛋白4(glucose transporter 4, GLUT4)向细胞质膜的运输[61]。GLUT4的运输对脂肪细胞中葡萄糖的摄入十分关键,可降低血液中葡萄糖的水平。突变分析证明AKT的磷酸化和TBC1D4的GAP活性是GLUT4转运过程所必需的[62]。在脂肪细胞3T3-L1中Rab10是TBC1D4的靶标[63,64],而肌细胞L6中Rab8A和Rab14参与GLUT4的转运[65],这表明TBC1D4的作用靶标具有细胞特异性。在静息状态下,TBC1D4通过不断促进Rab10失活来抑制GLUT4转运至质膜。胰岛素刺激后,AKT被激活,使TBC1D4磷酸化并失去Rab10-GAP活性,从而导致活化的Rab10-GTP介导GLUT4转运到质膜。TBC1D1是TBC1D4的同源物,在肌细胞中大量表达,其识别的特异性底物与TBC1D4相似,也参与肌细胞中GLUT4的转运[13]。在一种瘦小型小鼠中TBC1D1基因存在突变[66],胰岛素耐药患者中TBC1D4基因存在截短型突变[67]。
TBC1D20可与丙型肝炎病毒(hepatitis C virus, HCV)的NS5A蛋白相互作用[45],是Rab1的GAP蛋白(Rab1-GAP)。Rab1主要负责从内质网向高尔基体的运输,过表达TBC1D20可阻断内质网中水疱性口炎病毒(vesicular stomatitis virus, VSV)G蛋白的转运。敲低Rab1可显著降低细胞中HCV的RNA水平,提示Rab1在HCV复制中起重要作用[44]。敲低TBC1D20也可严重损害HCV的RNA复制,但对细胞生存无显著影响[45]。上述结果表明,NS5A与TBC1D20相互作用可促进HCV病毒的RNA复制,因此针对TBC1D20的靶向治疗可能成为抗HCV感染的新策略。
TBC1D24在大脑皮层发育和突触前神经传递中起重要作用,该基因的突变可导致早发性癫痫并伴随不同程度的智力障碍、听力损失、皮层肌阵挛和小脑共济失调,是DOORS综合征(具有耳聋、指甲营养不良、骨营养不良、智力低下和癫痫发作等症状)的致病因素之一[47,48,58]。果蝇中Skywalker蛋白是人类TBC1D24的直系同源物,蛋白质结构研究发现Skywalker的TBC结构域中含有一个阳离子口袋,可直接结合质膜上4、5位发生磷酸化的磷酸肌醇[PI(4,5)P2和PI(3,4,5)P3]。Skywalker基因的突变可影响其编码蛋白与质膜的结合,引起果蝇严重的神经缺陷,包括突触小泡的运输受损和癫痫[68]。
TBC家族蛋白还与多种肿瘤的发生发展密切相关,其中大多数发挥癌蛋白的功能,参与调控细胞的恶性转化或转移。TBC/Rab-GAPs在恶性肿瘤中的作用可能与其对Rabs的调控有直接的联系,这些调控可促进受体蛋白在细胞与细胞间、细胞与基质间的动态转运和回收,从而引起细胞发生侵袭。此外,很多癌基因信号通路的组分,如EGFR、RAS和RAC,均定位于胞吞泡或者需要被正确转运至细胞膜或粘附位点[20,69]。例如,TBC1D3(也称PRC17, prostate cancer gene 17)是Rab5的GAP,它在转移性前列腺癌中上调表达,过表达TBC1D3可促进NIH3T3细胞在体外或体内的生长,促进其接触抑制现象丢失。将TBC1D3的GAP结构域突变失活后,其上述促癌作用消失[55]。分子机制研究显示,TBC1D3过表达可抑制EGFR的泛素化、延迟降解,从而增强EGFR信号通路、促进细胞增殖[20]。再如,生物信息学分析显示TBC1D16为黑色素瘤的驱动基因,可促进黑色素瘤细胞生长[57]。表观遗传学研究显示,TBC1D16的甲基化状态与黑色素瘤的进程相关[39,40,41]。在转移性黑色素瘤中TBC1D16处于低甲基化状态,使得TBC1D16-47KD高表达,从而加速黑色素瘤细胞在体外和体内的生长和转移。TBC1D16-47KD对Rab4A和Rab5C具有GAP活性,促进EGFR的降解。TBC1D16-47KD启动子区甲基化水平低的黑色素瘤患者预后更差,但对BRAF和MEK抑制剂的敏感性更高[40]。又如,TBC1D7是Rab17的GAP,能显著促进肺癌细胞的生长、在肺癌组织中显著高表达、与患者的不良预后显著相关[27]。TBC1D7可与TSC1(Tuberous sclerosis complex 1,结节性硬化症1)相互作用,这种相互作用对TBC1D7蛋白的稳定十分重要[27]。含有TBC1D7第152~171位氨基酸的多肽可以抑制TBC1D7-TSC1复合体的形成,有效抑制肺癌细胞的生长。因此,抑制TBC1D7或者TBC1D7-TSC1复合体的形成有可能成为肺癌治疗的新策略[27]。
4 结语与展望
近年来,针对TBC家族蛋白的研究越来越多。人们逐渐认识到,TBC蛋白不仅可作为Rab的负调控分子,还可在膜泡运输,以及不同信号转导通路的协调中发挥效应分子或支架蛋白的作用。解析细胞中受TBC蛋白特异识别的Rab蛋白或与TBC蛋白相互作用的蛋白,有助于人们全面认识TBC的时空调节特征。更重要的是,TBC家族蛋白功能异常是多种疾病的重要驱动因素之一。鉴于某些TBC蛋白可选择性调节某些关键的膜泡转运过程,它们有成为生物标志物或治疗靶标的潜能。然而,迄今为止尚无针对TBC蛋白的特异性药物被开发出来。TBC蛋白的活性可能受到翻译后修饰、蛋白质定位变化、GAP结构域失活突变等影响,针对这些过程的研究,有可能为靶向TBC蛋白的疾病治疗药物的研发开辟新的方向。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
URLPMID:5053131 [本文引用: 1]

A large group of small Rab GTPases which mediate secretory and endosomal membrane transport, as well as autophagosome biogenesis, are essential components of vesicle trafficking machinery. Specific Rab protein together with the cognate effectors coordinates the dynamics of trafficking pathway and determines the cargo proteins destination. Functional impairments of Rab proteins by mutations or post-translational modifications disrupting the regulatory network of vesicle trafficking have been implicated in tumorigenesis. Therefore, the vesicle transport regulators play essential roles in the mediation of cancer cell biology, including uncontrolled cell growth, invasion and metastasis. The context-dependent role of the same Rab to act as either an oncoprotein or tumor suppressor in different cancers is found. Such discrepancies may be due in part to the interaction of specific Rab protein with different effectors or cargos in various tumors. Here, we review recent advances in the roles of Rab GTPases in communicating with other effectors in tumor progression. In this review, we also emphasize dysregulation of Rab-mediated membrane delivery shifting normal cell behaviors toward malignancy. Thus, recovery of the dysregulated vesicle trafficking systems in cancer cells may provide future directions for potential strategy to restrain tumor progression.
URLPMID:7566974 [本文引用: 1]

Abstract In an effort to identify genes that are differentially regulated during mast cell development, subtracted cDNA prepared from wild-type murine P815 mastocytoma cells and a P815 subline that exhibits properties of mast cell differentiation was used to screen mast cell cDNA libraries. Several known mast cell-specific cDNAs were isolated including mast cell carboxypeptidase A (MC-CPA), murine mast cell protease-5 (MMCP-5), and gp49. A novel cDNA, designated Tbc1, was identified that showed differential expression in the two mast cell lines. The amino acid sequence predicted from the cDNA contains a 200 amino acid domain that is homologous to regions in the tre-2 oncogene and the yeast regulators of mitosis, BUB2 and cdc16. The N-terminal region contains a number of cysteine and histidine residues, potentially encoding a zinc finger domain. Tbc1 is a nuclear protein and is expressed in highest levels in hematopoietic cells, testis and kidney. Within these tissues, expression of Tbc1 is cell- and stage-specific. Based on sequence similarity, pattern of expression and subcellular localization, Tbc1 may play a role in the cell cycle and differentiation of various tissues.
URLPMID:16855591 [本文引用: 1]

Abstract Rab GTPases regulate membrane trafficking by cycling between inactive (GDP-bound) and active (GTP-bound) conformations. The duration of the active state is limited by GTPase-activating proteins (GAPs), which accelerate the slow intrinsic rate of GTP hydrolysis. Proteins containing TBC (Tre-2, Bub2 and Cdc16) domains are broadly conserved in eukaryotic organisms and function as GAPs for Rab GTPases as well as GTPases that control cytokinesis. An exposed arginine residue is a critical determinant of GAP activity in vitro and in vivo. It has been expected that the catalytic mechanism of TBC domains would parallel that of Ras and Rho family GAPs. Here we report crystallographic, mutational and functional analyses of complexes between Rab GTPases and the TBC domain of Gyp1p. In the crystal structure of a TBC-domain-Rab-GTPase-aluminium fluoride complex, which approximates the transition-state intermediate for GTP hydrolysis, the TBC domain supplies two catalytic residues in trans, an arginine finger analogous to Ras/Rho family GAPs and a glutamine finger that substitutes for the glutamine in the DxxGQ motif of the GTPase. The glutamine from the Rab GTPase does not stabilize the transition state as expected but instead interacts with the TBC domain. Strong conservation of both catalytic fingers indicates that most TBC-domain GAPs may accelerate GTP hydrolysis by a similar dual-finger mechanism.
URLPMID:28168758 [本文引用: 1]

react-text: 202 investigate the relationship between γ-secretase and it AD-causing substrate-APP and C99 /react-text react-text: 203 /react-text
URLPMID:20116244 [本文引用: 1]

Our data indicate that active Rac1 recruits Armus to locally inactivate Rab7 and facilitate E-cadherin degradation in lysosomes. Thus, the integration of Rac1 and Rab7 activities by Armus provides an important regulatory node for E-cadherin turnover and stability of cell-cell contacts.
URLPMID:20070612 [本文引用: 2]

Abstract The Rab family belongs to the Ras-like small GTPase superfamily and is implicated in membrane trafficking through interaction with specific effector molecules. Because of the large number of Rab isoforms in mammals, however, the effectors of most of the mammalian Rabs are yet to be identified. In this study, we systematically screened five different cell or tissue lysates for novel Rab effectors by a combination of glutathione S-transferase (GST) pull-down assay with 60 different mammalian Rabs and mass spectroscopic analysis. Three of the 21 Rab-binding proteins we identified, mKIAA1055/TBC1D2B (Rab22-binding protein), GAPCenA/TBC1D11 (Rab36-binding protein) and centaurin β2/ACAP2 (Rab35-binding protein), are GTPase-activating proteins (GAPs) for Rab or Arf. Although it has recently been proposed that the Rab–GAP (Tre-2 /Bub2/Cdc16) domain physically interacts with its substrate Rab, these three GAPs interacted with specific Rabs via a domain other than a GAP domain, e.g. centaurin β2 binds GTP-Rab35 via the ankyrin repeat (ANKR) domain. Although centaurin β2 did not exhibit any Rab35–GAP activity in vitro , the Rab35-binding ANKR domain of centaurin β2 was found to be required for its plasma membrane localization and regulation of Rab35-dependent neurite outgrowth of PC12 cells through inactivation of Arf6. These findings suggest a novel mode of interaction between Rab and GAP.
URLPMID:11118206 [本文引用: 1]

Abstract Top of page Abstract Introduction Results Discussion Materials and methods Acknowledgements References Homotypic vacuole fusion occurs by sequential priming, docking and fusion reactions. Priming frees the HOPS complex (Vps 11, 16, 18, 33, 39 and 41) to activate Ypt7p for docking. Here we explore the roles of the GDP and GTP states of Ypt7p using Gdi1p (which extracts Ypt7:GDP), Gyp7p (a GTPase-activating protein for Ypt7p:GTP), GTP纬S or GppNHp (non-hydrolyzable nucleotides), and mutant forms of Ypt7p that favor either GTP or GDP states. GDP-bound Ypt7p on isolated vacuoles can be extracted by Gdi1p, although only the GTP-bound state allows docking. Ypt7p is converted to the GTP-bound state after priming and stably associates with HOPS. Gyp7p can cause Ypt7p to hydrolyze bound GTP to GDP, driving HOPS release and accelerating Gdi1p-mediated release of Ypt7p. Ypt7p extraction does not inhibit the Ca 2+ -triggered cascade that leads to fusion. However, in the absence of Ypt7p, fusion is still sensitive to GTP纬S and GppNHp, indicating that there is a second specific GTPase that regulates the calcium flux and hence fusion. Thus, two GTPases sequentially govern vacuole docking and fusion.
URLPMID:2542475 [本文引用: 1]

Rab guanosine triphosphatases (GTPases) are pivotal regulators of membrane identity and dynamics, but the in vivo pathways that control Rab signaling are poorly defined. Here, we show that the GTPase-activating protein Gyp7 inactivates the yeast vacuole Rab Ypt7 in vivo. To efficiently terminate Ypt7 signaling, Gyp7 requires downstream assistance from an inhibitory casein kinase I, Yck3. Yck3 mediates phosphorylation of at least two Ypt7 signaling targets: a tether, the Vps-C/homotypic fusion and vacuole protein sorting (HOPS) subunit Vps41, and a SNARE, Vam3. Phosphorylation of both substrates is opposed by Ypt7-guanosine triphosphate (GTP). We further demonstrate that Ypt7 binds not one but two Vps-C/HOPS subunits: Vps39, a putative Ypt7 nucleotide exchange factor, and Vps41. Gyp7-stimulated GTP hydrolysis on Ypt7 therefore appears to trigger both passive termination of Ypt7 signaling and active kinase-mediated inhibition of Ypt7's downstream targets. We propose that signal propagation through the Ypt7 pathway is controlled by integrated feedback and feed-forward loops. In this model, Yck3 enforces a requirement for the activated Rab in docking and fusion.
URLPMID:11099046 [本文引用: 1]

Abstract How epidermal growth factor receptor (EGFR) signalling is linked to EGFR trafficking is largely unknown. Signalling and trafficking involve small GTPases of the Rho and Rab families, respectively. But it remains unknown whether the signalling relying on these two classes of GTPases is integrated, and, if it is, what molecular machinery is involved. Here we report that the protein Eps8 connects these signalling pathways. Eps8 is a substrate of the EGFR, which is held in a complex with Sos1 by the adaptor protein E3bl (ref. 2), thereby mediating activation of Rac. Through its src homology-3 domain, Eps8 interacts with RN-tre. We show that RN-tre is a Rab5 GTPase-activating protein, whose activity is regulated by the EGFR. By entering in a complex with Eps8, RN-tre acts on Rab5 and inhibits internalization of the EGFR. Furthermore, RN-tre diverts Eps8 from its Rac-activating function, resulting in the attenuation of Rac signalling. Thus, depending on its state of association with E3b1 or RN-tre, Eps8 participates in both EGFR signalling through Rac, and trafficking through Rab5.
URLPMID:2064371 [本文引用: 3]

Rab family guanosine triphosphatases (GTPases) together with their regulators define specific pathways of membrane traffic within eukaryotic cells. In this study, we have investigated which Rab GTPase-activating proteins (GAPs) can interfere with the trafficking of Shiga toxin from the cell surface to the Golgi apparatus and studied transport of the epidermal growth factor (EGF) from the cell surface to endosomes. This screen identifies 6 (EVI5, RN-tre/USP6NL, TBC1D10A-C, and TBC1D17) of 39 predicted human Rab GAPs as specific regulators of Shiga toxin but not EGF uptake. We show that Rab43 is the target of RN-tre and is required for Shiga toxin uptake. In contrast, RabGAP-5, a Rab5 GAP, was unique among the GAPs tested and reduced the uptake of EGF but not Shiga toxin. These results suggest that Shiga toxin trafficking to the Golgi is a multistep process controlled by several Rab GAPs and their target Rabs and that this process is discrete from ligand-induced EGF receptor trafficking.
URLPMID:1608601316086013 [本文引用: 2]

Rab-family GTPases are conserved regulators of membrane trafficking that cycle between inactive GDP-bound and activated GTP-bound states. A key determinant of Rab function is the lifetime of the GTP-bound state. As Rabs have a low intrinsic rate of GTP hydrolysis, this process is under the control of GTP-hydrolysis-activating proteins (GAPs). Due to the large number of Rabs and GAPs that are encoded by the human genome, it has proven difficult to assign specific functional relationships to these proteins. Here, we identify a Rab5-specific GAP (RabGAP-5), and show that RN-Tre (previously described as a Rab5 GAP) acts on Rab41. RabGAP-5 overexpression triggers a loss of the Rab5 effector EEA1 from endosomes and blocks endocytic trafficking. By contrast, depletion of RabGAP-5 results in increased endosome size, more endosome-associated EEA1, and disrupts the trafficking of EGF and LAMP1. RabGAP-5 therefore limits the amount of activated Rab5, and thereby regulates trafficking through endosomes.
URLPMID:18325908 [本文引用: 1]

Stone . previously reported an association between the gene variant R125W (rs35859249) and severe obesity in women from US pedigrees. We attempted to replicate this result in 9714 French Caucasian individuals, combining family-based and general population studies. We confirmed an association with familial obesity (defined as body mass index (BMI) 鈮 97th percentile) in women from 1109 obesity-selected pedigrees (Z-score = 2.70, = 0.008). Analysis of 16 microsatellite markers on chromosome 4 restricted to the 42 pedigrees carrying the R125W variant allele also revealed a suggestive evidence of linkage with obesity (maximum likelihood binomial LOD of 2.73, = 0.0002) on chromosome 4p14, where resides . In contrast, R125W variant was neither associated with BMI nor with obesity in a large population-based cohort. These results confirm a putative role of R125W variant in familial obesity predisposition.
URLPMID:19740738 [本文引用: 2]

Insulin stimulates the translocation of the glucose transporter GLUT4 from intracellular locations to the plasma membrane in adipose and muscle cells. Prior studies have shown that Akt phosphorylation of the Rab GTPase-activating protein, AS160 (160-kDa Akt substrate; also known as TBC1D4), triggers GLUT4 translocation, most likely by suppressing its Rab GTPase-activating protein activity. However, the regulation of a very similar protein, TBC1D1 (TBC domain family, member 1), which is mainly found in muscle, in insulin-stimulated GLUT4 translocation has been unclear. In the present study, we have identified likely Akt sites of insulin-stimulated phosphorylation of TBC1D1 in C2C12 myotubes. We show that a mutant of TBC1D1, in which several Akt sites have been converted to alanine, is considerably more inhibitory to insulin-stimulated GLUT4 translocation than wild-type TBC1D1. This result thus indicates that similar to AS160, Akt phosphorylation of TBC1D1 enables GLUT4 translocation. We also show that in addition to Akt activation, activation of the AMP-dependent protein kinase partially relieves the inhibition of GLUT4 translocation by TBC1D1. Finally, we show that the R125W variant of TBC1D1, which has been genetically associated with obesity, is equally inhibitory to insulin-stimulated GLUT4 translocation, as is wild-type TBC1D1, and that healthy and type 2 diabetic individuals express approximately the same level of TBC1D1 in biopsies of vastus lateralis muscle. In conclusion, phosphorylation of TBC1D1 is required for GLUT4 translocation. Thus, the regulation of TBC1D1 resembles that of its paralog, AS160.
URLPMID:16893906 [本文引用: 1]

The molecular etiology of predisposition is largely unknown. Here, we present evidence that genetic variation in confers risk for in females. We identified a coding variant (R125W) in that segregated with the disease in 4p15-14-linked pedigrees. In cases derived from pedigrees with the strongest linkage evidence, the variant was significantly associated with (P=0.000007) and carrying R125W accounted for the majority of the evidence that originally linked 4p15-14 with the disease. In addition, by selecting families that segregated R125W with , we were able to generate highly significant linkage evidence for an predisposition locus at 4q34-35. This result provides additional and confirming evidence that R125W affects susceptibility, delimits the location of an gene at 4q34-35 and identifies a gene/gene interaction that influences the risk for predisposition. Finally, although the function of is unknown, the is structurally similar to a known regulator of -mediated translocation.
URLPMID:11785977

Identifying immunogenic tumor antigens plays a critical role in developing efficient diagnostic and therapeutic strategies for treatment of cancer. Using a recently developed technology, serological identification of antigens by recombinant expression cloning (SEREX), we identified a total of 8 genes whose expression elicited antibody responses in prostate cancer patients. Of the 8 genes, 5 represented known genes in the GenBank database, 2 were previously uncharacterized genes, and 1 showed sequence homology to a mouse gene. The sequence feature and the expression of one of the novel genes, prostate antigen recognized and identified by SEREX (PARIS-1), are determined in this study. The PARIS-1 cDNA is 3257 bp in length and contains a complete open reading frame of 2751 bp encoding for a primary translation product of 917 amino acids. Using Northern blot hybridization assay, we detected a single species of approximately 3.3 kb PARIS-1 mRNA that is differentially expressed in prostate normal and cancer cells. Western blot analysis confirmed the expression of the PARIS-1 protein in these cells. Structure analysis revealed that PARIS-1 protein contains a TBC domain that is conserved in the family of cell cycle-regulatory and Rab GTPase-activating proteins (Rab-GAP). Thus, the PARIS-1 protein may play a role in regulation of cell differentiation and growth or represent a new member of the Rab-GAP family.
URLPMID:20562859

Autophagy, the process by which proteins and organelles are sequestered in autophagosomal vesicles and delivered to the lysosome/vacuole for degradation, provides a primary route for turnover of stable and defective cellular proteins. Defects in this system are linked with numerous human diseases. Although conserved protein kinase, lipid kinase and ubiquitin-like protein conjugation subnetworks controlling autophagosome formation and cargo recruitment have been defined, our understanding of the global organization of this system is limited. Here we report a proteomic analysis of the autophagy interaction network in human cells under conditions of ongoing (basal) autophagy, revealing a network of 751 interactions among 409 candidate interacting proteins with extensive connectivity among subnetworks. Many new autophagy interaction network components have roles in vesicle trafficking, protein or lipid phosphorylation and protein ubiquitination, and affect autophagosome number or flux when depleted by RNA interference. The six ATG8 orthologues in humans (MAP1LC3/GABARAP proteins) interact with a cohort of 67 proteins, with extensive binding partner overlap between family members, and frequent involvement of a conserved surface on ATG8 proteins known to interact with LC3-interacting regions in partner proteins. These studies provide a global view of the mammalian autophagy interaction landscape and a resource for mechanistic analysis of this critical protein homeostasis pathway.
URLPMID:18199687

Abstract The generation of novel genes and proteins throughout evolution has been proposed to occur as a result of whole genome and gene duplications, exon shuffling, and retrotransposition events. The analysis of such genes might thus shed light into the functional complexity associated with highly evolved species. One such case is represented by TBC1D3, a primate-specific gene, harboring a TBC domain. Because TBC domains encode Rab-specific GAP activities, TBC-containing proteins are predicted to play a major role in endocytosis and intracellular traffic. Here, we show that the TBC1D3 gene originated late in evolution, likely through a duplication of the RNTRE locus, and underwent gene amplification during primate speciation. Despite possessing a TBC domain, TBC1D3 is apparently devoid of Rab-GAP activity. However, TBC1D3 regulates the optimal rate of epidermal growth factor-mediated macropinocytosis by participating in a novel pathway involving ARF6 and RAB5. In addition, TBC1D3 binds and colocalize to GGA3, an ARF6-effector, in an ARF6-dependent manner, and synergize with it in promoting macropinocytosis, suggesting that the two proteins act together in this process. Accordingly, GGA3 siRNA-mediated ablation impaired TBC1D3-induced macropinocytosis. We thus uncover a novel signaling pathway that appeared after primate speciation. Within this pathway, a TBC1D3:GGA3 complex contributes to optimal propagation of signals, ultimately facilitating the macropinocytic process.
URLPMID:16863688 [本文引用: 1]

TBC1D3 is a member of the TBC1 domain family of proteins that stimulates the intrinsic GTPase activity of RAB5A, an essential actor in early endosome trafficking. Oncogenic properties of TBC1D3 have been demonstrated previously both in vitro and in mouse models. Although the oncogenic mechanism of TBC1D3 has yet to be elucidated, the TBC1D3 locus (chromosome 17q12) is amplified in 15% of primary prostate tumors. Here, we describe eight highly related TBC1D3 paralogues located within that genomic region, potentially encoding six variant TBC1D3 proteins. We found that human tissues display specific transcription patterns of these paralogues. Furthermore, that pattern was altered in several primary prostate tumors in comparison to healthy prostate tissues. Potential TBC1D3 oncogenic mechanisms are discussed in light of these results.
[本文引用: 2]
URLPMID:2442359 [本文引用: 2]

Hominoid- and human-specific genes may have evolved to modulate signaling pathways of a higher order of complexity. TBC1D3 is a hominoid-specific oncogene encoded by a cluster of eight paralogs on chromosome 17. Initial work indicates that TBC1D3 is widely expressed in human tissues ( Hodzic, D., Kong, C., Wainszelbaum, M. J., Charron, A. J., Su, X., and Stahl, P. D. (2006) Genomics 88, 731-736 ). In this study, we show that TBC1D3 expression has a powerful effect on cell proliferation that is further enhanced by epidermal growth factor (EGF) in both human and mouse cell lines. EGF activation of the Erk and protein kinase B/Akt pathways is enhanced, both in amplitude and duration, by TBC1D3 expression, whereas RNA interference silencing of TBC1D3 suppresses the activation. Light microscopy and Western blot experiments demonstrate that increased signaling in response to EGF is coupled with a significant delay in EGF receptor (EGFR) trafficking and degradation, which significantly extends the life span of EGFR. Moreover, TBC1D3 suppresses polyubiquitination of the EGFR and the recruitment of c-Cbl. Using the Ras binding domain of Raf1 to monitor GTP-Ras we show that TBC1D3 expression enhances Ras activation in quiescent cells, which is further increased by EGF treatment. We speculate that TBC1D3 may alter Ras GTP loading. We conclude that the expression of TBC1D3 generates a delay in EGFR degradation, a decrease in ubiquitination, and a failure to recruit adapter proteins that ultimately dysregulate EGFR signal transduction and enhance cell proliferation. Altered growth factor receptor trafficking and GTP-Ras turnover may be sites where recently evolved genes such as TBC1D3 selectively modulate signaling in hominoids and humans.
URLPMID:18276765

OBJECTIVE--Protein kinase B/Akt plays a central role in [beta]-cells, but little is known regarding downstream Akt substrates in these cells. Recently, Rab GTPase-activating protein AS160, a substrate of Akt, was shown to be involved in insulin modulation of GLUT4 trafficking in skeletal muscle and adipose tissue. The aim of this study was to investigate the expression and potential role of AS160 in [beta]-cells. RESEARCH DESIGN AND METHODS--AS160 mRNA expression was measured in mouse and human islets and fluorescence-activated cell sorted [beta]-cells and compared in islets from control subjects versus individuals with type 2 diabetes. For knockdown experiments, transformed mouse insulin-secreting MIN6B1 cells were transfected with pSUPER-GFP plasmid encoding a small hairpin RNA against insulin receptor substrate (IRS)-2, AS160, or a negative control. Primary mouse islet cells were transfected with AS160 small interfering RNA. RESULTS--AS160 was expressed in human and mouse pancreatic [beta]-cells and phosphorylated after glucose stimulation. AS160 mRNA expression was downregulated in pancreatic islets from individuals with type 2 diabetes. In MIN6B1 cells, glucose induced phosphorylation of Akt and AS160, and this was mediated by insulin receptor/IRS-2/phosphatidylinositol 3-kinase independently of changes in cytosolic [Ca.sup.2+]. Knockdown of AS160 resulted in increased basal insulin secretion, whereas glucose-stimulated insulin release was abolished. Furthermore, [beta]-cells with decreased AS160 showed increased apoptosis and loss of glucose-induced proliferation. CONCLUSIONS--This study shows for the first time that AS160, previously recognized as a key player in insulin signaling in skeletal muscle and adipose tissue, is also a major effector of protein kinase B/Akt signaling in the [beta]-cell.
URLPMID:16154996

Insulin stimulates the translocation of the glucose transporter GLUT4 from intracellular vesicles to the plasma membrane. In the present study we have conducted a comprehensive proteomic analysis of affinity-purified GLUT4 vesicles from 3T3-L1 adipocytes to discover potential regulators of GLUT4 trafficking. In addition to previously identified components of GLUT4 storage vesicles including the insulin-regulated aminopeptidase insulin-regulated aminopeptidase and the vesicle soluble N-ethylmaleimide factor attachment protein (v-SNARE) VAMP2, we have identified three new Rab proteins, Rab10, Rab11, and Rab14, on GLUT4 vesicles. We have also found that the putative Rab GTPase-activating protein AS160 (Akt substrate of 160 kDa) is associated with GLUT4 vesicles in the basal state and dissociates in response to insulin. This association is likely to be mediated by the cytosolic tail of insulin-regulated aminopeptidase, which interacted both in vitro and in vivo with AS160. Consistent with an inhibitory role of AS160 in the basal state, reduced expression of AS160 in adipocytes using short hairpin RNA increased plasma membrane levels of GLUT4 in an insulin-independent manner. These findings support an important role for AS160 in the insulin regulated trafficking of GLUT4.
URLPMID:15304337 [本文引用: 2]

We have analyzed transcription profiles in peripheral blood CD3+ cells from patients with allergic diseases to better understand the genes that are involved. Transcription levels of the gene KIAA0603/AS160 in CD3+ cells from patients with atopic dermatitis (AD) were significantly higher than in normal individuals. The KIAA0603 gene encodes a 1299 amino acid protein with two phosphotyrosine interaction domains at the N-terminal region and a TBC domain at the C-terminal region. The region containing the TBC domain has a 31% homology to human rab6 GTPase activating protein (GAP). When human primary CD3+ cells were stimulated with anti-CD3 or calcium ionophore, the KIAA0603 transcript level was upregulated. The marked upregulation of KIAA0607 was accompanied by activation induced cell death of primary CD3+ cells. KIAA0603 is likely to be a Rab GAP that participates in the regulation of activated T cells, especially helper memory T cells. Expression of KIAA0603 in T cells may be involved in pathogenesis of AD.
URLPMID:15971998

Recently, we described a 160 kDa protein (designated AS160, for Akt substrate of 160 kDa) with a predicted Rab GAP (GTPase-activating protein) domain that is phosphorylated on multiple sites by the protein kinase Akt. Phosphorylation of AS160 in adipocytes is required for insulin-stimulated translocation of the glucose transporter GLUT4 to the plasma membrane. The aim of the present study was to determine whether AS160 is in fact a GAP for Rabs, and, if so, what its specificity is. We first identified a group of 16 Rabs in a preparation of intracellular vesicles containing GLUT4 by MS. We then prepared the recombinant GAP domain of AS160 and examined its activity against many of these Rabs, as well as several others. The GAP domain was active against Rabs 2A, 8A, 10 and 14. There was no significant activity against 14 other Rabs. GAP activity was further validated by the finding that the recombinant GAP domain with the predicted catalytic arginine residue replaced by lysine was inactive. Finally, it was found by immunoblotting that Rabs 2A, 8A and 14 are present in GLUT4 vesicles. These results indicate that AS160 is a Rab GAP, and suggest novel Rabs that may participate in GLUT4 translocation.
URLPMID:21041651

Skeletal muscle is the primary site of dietary glucose disposal, a function that depends on insulin-mediated exocytosis of GLUT4 vesicles to its cell surface. In skeletal muscle and adipocytes, this response involves Akt signaling to the Rab-GAP (GTPase-activating protein) AS160/TBC1D4. Intriguingly, the AS160-targeted Rabs appear to differ, with Rab8A participating in GLUT4 exocytosis in muscle cells and Rab10 in adipocytes, and their activation by insulin is unknown. Rabs 8A, 10, and 13 belong to the same subfamily of Rab-GTPases. Here we show that insulin promotes GTP loading of Rab13 and Rab8A but not Rab10 in rat L6 muscle cells, Rab8A activation preceding that of Rab13. siRNA-mediated Rab13 knockdown blocked the insulin-induced increase of GLUT4 at the muscle cell surface that was rescued by a Rab13 ortholog but not by Rab8A. Constitutively active AS160 lowered basal and insulin-stimulated levels of surface GLUT4, effects that were reversed by overexpressing Rab8A or Rab13, suggesting that both Rabs are targets of AS160-GAP activity in the context of GLUT4 traffic. Rab13 had a broader intracellular distribution compared with the perinuclear restriction of Rab8A, and insulin promoted Rab13 colocalization with GLUT4 at the cell periphery. We conclude that Rab13 and Rab8A are Rab-GTPases activated by insulin, and that downstream of AS160 they regulate traffic of GLUT4 vesicles, possibly acting at distinct steps and sites. These findings close in on the series of events regulating muscle GLUT4 traffic in response to insulin, crucial for whole-body glucose homeostasis.
URLPMID:11564724

Abstract An in vitro model of GH-responsive cells was subjected to microarray analysis to identify a novel gene regulated by GH. This 258 amino acid protein, we term GH Regulated TBC Protein-1 (GRTP1), contains the TBC signature motif of GTPase activator proteins of Rab-like small GTPases. Northern blot analysis revealed a 1.3 kb major mRNA species, most abundant in testes. TaqMan assay confirmed that in the mouse, Grtp1 is expressed at highest levels in testes, with lesser abundance in intestine, kidney, lung, and liver. In the testis, expression of Grtp1 significantly increases post-pubertally. Administration of GH to mice increased levels of GRTP1 mRNA in testes (140%), but decreased GRTP1 mRNA abundance in kidney (50%) and liver (25%). Grtp1 was localized to mouse proximal chromosome 8. Orthologs of this protein are present in human, mouse, rat, and drosophila suggesting that GRTP1 has an important biological role(s).
URLPMID:20095038 [本文引用: 4]

Abstract To develop novel biomarkers and therapeutic agents for lung cancers, we screened molecules that were highly expressed in lung cancers by means of cDNA microarray analysis and found an elevated expression of TBC1 domain family, member 7 (TBC1D7) in the majority of lung cancers. Northern-blot analysis using mRNAs from 16 normal tissues detected its expression only in testis. Immunohistochemical staining using tumor tissue microarrays consisting of 261 archived non-small cell lung cancer (NSCLC) specimens suggested an association of TBC1D7 expression with poor prognosis for NSCLC patients (P = 0.0063). Treatment of lung cancer cells using siRNA against TBC1D7, suppressed its expression and resulted in inhibition of the cell growth. Furthermore, the induction of exogenous expression of TBC1D7 conferred growth-promoting activity at in vitro and in vivo conditions. We also identified TBC1D7 to interact with TSC1 protein in lung cancer cells. TSC1 introduction into cells increased the level of TBC1D7 protein, whereas knockdown of TSC1 expression decreased the level of TBC1D7 protein, suggesting that TBC1D7 is stabilized probably through interaction with TSC1. In addition, inhibition of the binding between TBC1D7 and TSC1 by a TBC1D7-derived 20-amino acid cell-permeable peptide (11R-TBC1D7(152-171)), which corresponded to the binding domain to TSC1, effectively suppressed growth of lung cancer cells. Selective suppression of TBC1D7 and/or inhibition of the TBC1D7-TSC1 complex formation could be promising therapeutic strategies for lung cancer therapy.
URLPMID:20548944 [本文引用: 1]

Abstract Osteoporosis is a complex disorder and commonly leads to fractures in elderly persons. Genome-wide association studies (GWAS) have become an unbiased approach to identify variations in the genome that potentially affect health. However, the genetic variants identified so far only explain a small proportion of the heritability for complex traits. Due to the modest genetic effect size and inadequate power, true association signals may not be revealed based on a stringent genome-wide significance threshold. Here, we take advantage of SNP and transcript arrays and integrate GWAS and expression signature profiling relevant to the skeletal system in cellular and animal models to prioritize the discovery of novel candidate genes for osteoporosis-related traits, including bone mineral density (BMD) at the lumbar spine (LS) and femoral neck (FN), as well as geometric indices of the hip (femoral neck-shaft angle, NSA; femoral neck length, NL; and narrow-neck width, NW). A two-stage meta-analysis of GWAS from 7,633 Caucasian women and 3,657 men, revealed three novel loci associated with osteoporosis-related traits, including chromosome 1p13.2 (RAP1A, p = 3.6x10(-8)), 2q11.2 (TBC1D8), and 18q11.2 (OSBPL1A), and confirmed a previously reported region near TNFRSF11B/OPG gene. We also prioritized 16 suggestive genome-wide significant candidate genes based on their potential involvement in skeletal metabolism. Among them, 3 candidate genes were associated with BMD in women. Notably, 2 out of these 3 genes (GPR177, p = 2.6x10(-13); SOX6, p = 6.4x10(-10)) associated with BMD in women have been successfully replicated in a large-scale meta-analysis of BMD, but none of the non-prioritized candidates (associated with BMD) did. Our results support the concept of our prioritization strategy. In the absence of direct biological support for identified genes, we highlighted the efficiency of subsequent functional characterization using publicly available expression profiling relevant to the skeletal system in cellular or whole animal models to prioritize candidate genes for further functional validation.
URLPMID:4637379

Membrane trafficking in male germ cells contributes to their development via cell morphological changes and acrosome formation. TBC family proteins work as Rab GTPase accelerating proteins (GAPs), which negatively regulate Rab proteins, to mediate membrane trafficking. In this study, we analyzed the expression of a Rab GAP, TBC1D9, in mouse organs and the intracellular localization of the gene products. Tbc1d9 showed abundant expression in adult mice testis. We found that the Tbc1d9 mRNA was expressed in primary and secondary spermatocytes, and that the TBC1D9 protein was expressed in spermatocytes and round spermatids. In 293T cells, TBC1D9-GFP proteins were localized in the endosome and Golgi apparatus. Compartments that were positive for the constitutive active mutants of Rab7 and Rab9 were also positive for TBC1D9 isoform 1. In addition, TBC1D9 proteins were associated with Rab7 and Rab9, respectively. These results indicate that TBC1D9 is expressed mainly in spermatocytes, and suggest that TBC1D9 regulates membrane trafficking pathways related to Rab9- or Rab7-positive vesicles.
URLPMID:4230784

Rab11a is a key modulator of vesicular trafficking processes, but there is limited information about the guanine nucleotide-exchange factors and GTPase-activating proteins (GAPs) that regulate its GTP-GDP cycle. We observed that in the presence of Mg(2+) (2.5 mM), TBC1D9B interacted via its Tre2-Bub2-Cdc16 (TBC) domain with Rab11a, Rab11b, and Rab4a in a nucleotide-dependent manner. However, only Rab11a was a substrate for TBC1D9B-stimulated GTP hydrolysis. At limiting Mg(2+) concentrations (<0.5 mM), Rab8a was an additional substrate for this GAP. In polarized Madin-Darby canine kidney cells, endogenous TBC1D9B colocalized with Rab11a-positive recycling endosomes but less so with EEA1-positive early endosomes, transferrin-positive recycling endosomes, or late endosomes. Overexpression of TBC1D9B, but not an inactive mutant, decreased the rate of basolateral-to-apical IgA transcytosis--a Rab11a-dependent pathway--and shRNA-mediated depletion of TBC1D9B increased the rate of this process. In contrast, TBC1D9B had no effect on two Rab11a-independent pathways--basolateral recycling of the transferrin receptor or degradation of the epidermal growth factor receptor. Finally, expression of TBC1D9B decreased the amount of active Rab11a in the cell and concomitantly disrupted the interaction between Rab11a and its effector, Sec15A. We conclude that TBC1D9B is a Rab11a GAP that regulates basolateral-to-apical transcytosis in polarized MDCK cells.
URLPMID:20404108

Oligodendrocytes secrete vesicles into the extracellular space, where they might play a role in neuron—glia communication. These exosomes are small vesicles with a diameter of 50-100 nm that are formed within multivesicular bodies and are released after fusion with the plasma membrane. The intracellular pathways that generate exosomes are poorly defined. Because Rab family guanosine triphosphatases (GTPases) together with their regulators are important membrane trafficking organizers, we investigated which Rab GTPase-activating proteins interfere with exosome release. We find that TBC1D10A—C regulate exosome secretion in a catalytic activity—dependent manner. We show that Rab35 is the target of TBC1D10A—C and that the inhibition of Rab35 function leads to intracellular accumulation of endosomal vesicles and impairs exosome secretion. Rab35 localizes to the surface of oligodendroglia in a GTP-dependent manner, where it increases the density of vesicles, suggesting a function in docking or tethering. These findings provide a basis for understanding the biogenesis and function of exosomes in the central nervous system.
URLPMID:16923123

It has recently been proposed that the TBC (Tre2/Bub2/Cdc16) domain functions as a GAP (GTPase-activating protein) domain for small GTPase Rab. Because of the large number of Rab proteins in mammals, however, most TBC domains have never been investigated for Rab-GAP activity. In this study we established panels of the GTP-fixed form of 60 different Rabs constructed in pGAD-C1, a yeast two-hybrid bait vector. We also constructed a yeast two-hybrid prey vector (pGBDU-C1) that harbors the cDNA of 40 distinct TBC proteins. Systematic investigation of 2400 combinations of 60 GTP-fixed Rabs and 40 TBC proteins by yeast two-hybrid screening revealed that seven TBC proteins specifically and differentially interact with specific Rabs (e.g. OATL1 interacts with Rab2A; FLJ12085 with Rab5A/B/C; and Evi5-like with Rab10). Measurement of in vitro Rab-GAP activity revealed that OATL1 and Evi5-like actually possess significant Rab2A- and Rab10-GAP activity, respectively, but that FLJ12085 do not display Rab5A-GAP activity at all. These results indicate that specific interaction between TBC protein and Rab would be a useful indicator for screening for the target Rabs of some TBC/Rab-GAP domains, but that there is little correlation between the Rab-binding activity and Rab-GAP activity of other TBC proteins.
URLPMID:3707656

The small GTPase Rab27B localizes to the zymogen granule membranes and plays an important role in regulating protein secretion by pancreatic acinar cells, as does Rab3D. A common guanine nucleotide exchange factor (GEF) for Rab3 and Rab27 has been reported; however, the GTPase-activating protein (GAP) specific for Rab27B has not been identified. In this study, the expression in mouse pancreatic acini of two candidate Tre-2/Bub2/Cdc16 (TBC) domain-containing proteins, EPI64 (TBC1D10A) and EPI64B (TBC1D10B), was first demonstrated. Their GAP activity on digestive enzyme secretion was examined by adenovirus-mediated overexpression of EPI64 and EPI64B in isolated pancreatic acini. EPI64B almost completely abolished the GTP-bound form of Rab27B, without affecting GTP-Rab3D. Overexpression of EPI64B also enhanced amylase release. This enhanced release was independent of Rab27A, but dependent on Rab27B, as shown using acini from genetically modified mice. EPI64 had a mild effect on both GTP-Rab27B and amylase release. Co-overexpression of EPI64B with Rab27B can reverse the inhibitory effect of Rab27B on amylase release. Mutations that block the GAP activity decreased the inhibitory effect of EPI64B on the GTP-bound state of Rab27B and abolished the enhancing effect of EPI64B on the amylase release. These data suggest that EPI64B can serve as a potential physiological GAP for Rab27B and thereby participate in the regulation of exocytosis in pancreatic acinar cells.
URLPMID:18450757

Upon antigen recognition, T-cell receptor (TCR/CD3) and other signaling molecules become enriched in a specialized contact site between the T cell and antigen-presenting cell, i.e. the immunological synapse (IS). Enrichment occurs via mechanisms that include polarized secretion from recycling endosomes, but the Rabs and RabGAPs that regulate this are unknown. EPI64C (TBC1D10C) is an uncharacterized candidate RabGAP we identified by mass spectrometry as abundant in human peripheral blood T cells that is preferentially expressed in hematopoietic cells. EPI64C is a Rab35-GAP based both on in vitro Rab35-specific GAP activity and findings in transfection assays. EPI64C and Rab35 dominant negative (DN) constructs each impaired transferrin export from a recycling pathway in Jurkat T-cells and induced large vacuoles marked by transferrin receptor, TCR, and SNAREs implicated in TCR-polarized secretion. Rab35 localized to the plasma membrane and to intracellular vesicles where it substantially colocalized with TfR and with TCR. Rab35 was strongly recruited to the IS. Conjugate formation was impaired by transfection with Rab35-DN or EPI64C and by EPI64C knock down. TCR enrichment at the IS was impaired by Rab35-DN. Thus, EPI64C and Rab35 regulate a recycling pathway in T cells and contribute to IS formation, most likely by participating in TCR transport to the IS.
URLPMID:16395330

The two isoforms of the Rab6 GTPase, Rab6A and Rab6A', regulate a retrograde transport route connecting early endosomes and the endoplasmic reticulum via the Golgi complex in interphasic cells. Here we report that when Rab6A' function is altered cells are unable to progress normally through mitosis. Such cells are blocked in metaphase, despite displaying a normal Golgi fragmentation and with the Mad2-spindle checkpoint activated. Furthermore, the Rab6 effector p150(Glued), a subunit of the dynein/dynactin complex, remains associated with some kinetochores. A similar phenotype was observed when GAPCenA, a GTPase-activating protein of Rab6, was depleted from cells. Our results suggest that Rab6A' likely regulates the dynamics of the dynein/dynactin complex at the kinetochores and consequently the inactivation of the Mad2-spindle checkpoint. Rab6A', through its interaction with p150(Glued) and GAPCenA, may thus participate in a pathway involved in the metaphase/anaphase transition.
URLPMID:28384198

Abstract Recycling endosomes are generally thought to play a central role in endocytic recycling, but recent evidence has indicated that they also participate in other cellular events, including cytokinesis, autophagy, and neurite outgrowth. Rab small GTPases are key regulators in membrane trafficking, and although several Rab isoforms, e.g., Rab11, have been shown to regulate recycling endosomal trafficking, the precise mechanism by which these Rabs regulate recycling endosomes is not fully understood. In this study, we focused on a Rab-GTPase-activating protein (Rab-GAP), one of the key regulators of Rabs, and comprehensively screened 43 mammalian Tre-2/Bub2/Cdc16 (TBC)/Rab-GAP-domain-containing proteins (TBC proteins) for proteins that specifically localize on recycling endosomes in mouse embryonic fibroblasts (MEFs). Four of the 43 mammalian TBC proteins screened, i.e., TBC1D11, TBC1D12, TBC1D14, and EVI5, were found to colocalize well with transferrin receptor, a well-known recycling endosome marker. We further investigated the biochemical properties of TBC1D12, a previously uncharacterized TBC protein. The results showed that TBC1D12 interacted with active Rab11 through its middle region and that it did not display Rab11-GAP activity in vitro. The recycling endosomal localization of TBC1D12 was found to depend on the expression of Rab11. We also found that TBC1D12 expression had no effect on common Rab11-dependent cellular events, e.g., transferrin recycling, in MEFs and that it promoted neurite outgrowth, a specialized Rab11-dependent cellular event, of PC12 cells independently of its GAP activity. These findings indicated that TBC1D12 is a novel Rab11-binding protein that modulates neurite outgrowth of PC12 cells.
URLPMID:23812537

Members of the Tre-2/Bub2/Cdc16 (TBC) family of proteins are believed to function as GTPase-activating proteins (GAPs) for Rab GTPases, which play pivotal roles in intracellular membrane trafficking. Although membrane trafficking is fundamental to neuronal morphogenesis and function, the roles of TBC-family Rab GAPs have been poorly characterized in the nervous system. In this paper, we provide genetic evidence that Tbc1d15-17, the Drosophila homolog of mammalian Rab7-GAP TBC1d15, is required for normal presynaptic growth and postsynaptic organization at the neuromuscular junction (NMJ). A loss-of-function mutation in Tbc1d15-17 or its presynaptic knockdown leads to an increase in synaptic bouton number and NMJ length. Tbc1d15-17 mutants are also defective in the distribution of the postsynaptic scaffold Discs-large (Dlg) and in the level of the postsynaptic glutamate subunit GluRIIA. These postsynaptic phenotypes are recapitulated by postsynaptic knockdown of Tbc1d15-17. We also show that presynaptic overexpression of a constitutively active Rab7 mutant in a wild-type background causes a synaptic overgrowth phenotype resembling that of Tbc1d15-17 mutants, while a dominant-negative form of Rab7 has the opposite effect. Together, our findings establish a novel role for Tbc1d15-17 and its potential substrate Rab7 in regulating synaptic development.
URLPMID:28276657 [本文引用: 1]

Abstract OBJECTIVES: Suicidal behavior (SB) is a major cause of mortality for patients diagnosed with bipolar disorder (BD). In this study, we investigated epigenetic differences in BD participants with and without a history of SB. METHODS: We used suicidality scores constructed from Schedule for Clinical Assessments in Neuropsychiatry (SCAN) interview questions about suicidal thought and behavior to identify individuals from a BD cohort of n=452; participants with the most extreme high (H-SB, n=18) and most extreme low (L-SB, n=22) scores were used as cases and controls, respectively. Epigenome-wide DNA methylation patterns were compared between the two groups using the Illumina Infinium Human Methylation 450 BeadChip microarray. DNA methylation age was compared to chronological tissue age. RESULTS: We observed highly significant differences in methylation between cases and controls in three genomic regions enriched for epigenetic modifications corresponding to gene regulatory regions. BD participants with a history of SB showed less overall methylation in the 5' untranslated region of Membrane palmitoylated protein 4 (MPP4) (P=7.42脙聴10 -7 ) and in intron 3 of TRE2/BUB2/CDC16 domain family member 16 (TBC1D16) (P=6.47脙聴10 -7 ), while exon 1 of Nucleoporin 133 (NUP133) was less methylated in controls (P=1.17x10 -6 ). Moreover, we observed a greater correlation between DNA methylation age and tissue age in controls (r=.91, P<.0001) than in the H-SB group (r=.83, P<.0001). CONCLUSIONS: We report significant findings at three loci based on a methylome scan of participants with BD for an SB phenotype, and potentially altered molecular aging in suicide attempters. Despite the small sample size, our proof-of-concept study highlights the potential for epigenetic factors to be useful in understanding the molecular underpinnings of suicide with the ultimate aim of its prevention. 脗漏 2017 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
URLPMID:26030178 [本文引用: 2]

Abstract Metastasis is responsible for most cancer-related deaths, and, among common tumor types, melanoma is one with great potential to metastasize. Here we study the contribution of epigenetic changes to the dissemination process by analyzing the changes that occur at the DNA methylation level between primary cancer cells and metastases. We found a hypomethylation event that reactivates a cryptic transcript of the Rab GTPase activating protein TBC1D16 (TBC1D16-47 kDa; referred to hereafter as TBC1D16-47KD) to be a characteristic feature of the metastatic cascade. This short isoform of TBC1D16 exacerbates melanoma growth and metastasis both in vitro and in vivo. By combining immunoprecipitation and mass spectrometry, we identified RAB5C as a new TBC1D16 target and showed that it regulates EGFR in melanoma cells. We also found that epigenetic reactivation of TBC1D16-47KD is associated with poor clinical outcome in melanoma, while conferring greater sensitivity to BRAF and MEK inhibitors.
URLPMID:5458482 [本文引用: 1]

Cutaneous melanoma is the deadliest skin cancer, with an increasing incidence and mortality rate. Currently, staging of patients with primary melanoma is performed using histological biomarkers such as tumor thickness and ulceration. As disruption of the epigenomic landscape is recognized as a widespread feature inherent in tumor development and progression, we aimed to identify novel biomarkers providing additional clinical information over current factors using unbiased genome-wide DNA methylation analyses. We performed a comprehensive DNA methylation analysis during all progression stages of melanoma using Infinium HumanMethylation450 BeadChips on a discovery cohort of benign nevi (n65=6514) and malignant melanoma from both primary (n65=6533) and metastatic (n65=6528) sites, integrating the DNA methylome with gene expression data. We validated the discovered biomarkers in three independent validation cohorts by pyrosequencing and immunohistochemistry. We identified and validated biomarkers for, and pathways involved in, melanoma development (e.g.,HOXA9DNA methylation) and tumor progression (e.g.,TBC1D16DNA methylation). In addition, we determined a prognostic signature with potential clinical applicability and validatedPON3DNA methylation and OVOL1 protein expression as biomarkers with prognostic information independent of tumor thickness and ulceration. Our data underscores the importance of epigenomic regulation in triggering metastatic dissemination through the inactivation of central cancer-related pathways. Inactivation of cell-adhesion and differentiation unleashes dissemination, and subsequent activation of inflammatory and immune system programs impairs anti-tumoral defense pathways. Moreover, we identify several markers of tumor development and progression previously unrelated to melanoma, and determined a prognostic signature with potential clinical utility. The online version of this article (doi:10.1186/s12916-017-0851-3) contains supplementary material, which is available to authorized users.
URLPMID:2064371

Rab family guanosine triphosphatases (GTPases) together with their regulators define specific pathways of membrane traffic within eukaryotic cells. In this study, we have investigated which Rab GTPase-activating proteins (GAPs) can interfere with the trafficking of Shiga toxin from the cell surface to the Golgi apparatus and studied transport of the epidermal growth factor (EGF) from the cell surface to endosomes. This screen identifies 6 (EVI5, RN-tre/USP6NL, TBC1D10A-C, and TBC1D17) of 39 predicted human Rab GAPs as specific regulators of Shiga toxin but not EGF uptake. We show that Rab43 is the target of RN-tre and is required for Shiga toxin uptake. In contrast, RabGAP-5, a Rab5 GAP, was unique among the GAPs tested and reduced the uptake of EGF but not Shiga toxin. These results suggest that Shiga toxin trafficking to the Golgi is a multistep process controlled by several Rab GAPs and their target Rabs and that this process is discrete from ligand-induced EGF receptor trafficking.
URLPMID:27487390

In humans, loss of TBC1D20 (TBC1 domain family, member 20) protein function causes Warburg Micro syndrome 4 (WARBM4), an autosomal recessive disorder characterized by congenital eye, brain, and genital abnormalities. TBC1D20-deficient mice exhibit ocular abnormalities and male infertility. TBC1D20 is a ubiquitously expressed member of the family of GTPase-activating proteins (GAPs) that increase the intrinsically slow GTP-hydrolysis rate of small RAB-GTPases when bound to GTP. Biochemical studies have established TBC1D20 as a GAP for RAB1B and RAB2A. However, the cellular role of TBC1D20 still remains elusive, and there is little information about how the functional loss of TBC1D20 causes clinical manifestations in WARBM4-affected children. Here we evaluate the role of TBC1D20 in cells carrying a null mutant allele, as well as TBC1D20-deficient mice, which display eye and testicular abnormalities. We demonstrate that TBC1D20, via its RAB1B GAP function, is a key regulator of autophagosome maturation, a process required for maintenance of autophagic flux and degradation of autophagic cargo. Our results provide evidence that TBC1D20-mediated autophagosome maturation maintains lens transparency by mediating the removal of damaged proteins and organelles from lens fiber cells. Additionally, our results show that in the testes TBC1D20-mediated maturation of autophagosomes is required for autophagic flux, but is also required for the formation of acrosomes. Furthermore TBC1D20-deficient mice, while not mimicking severe developmental brain abnormalities identified in WARBM4 affected children, display disrupted neuronal autophagic flux resulting in adult-onset motor dysfunction. In summary, we show that TBC1D20 has an essential role in the maturation of autophagosomes and a defect in TBC1D20 function results in eye, testicular, and neuronal abnormalities in mice implicating disrupted autophagy as a mechanism that contributes to WARBM4 pathogenesis.
URLPMID:17901050 [本文引用: 3]

Abstract Like other viruses, productive hepatitis C virus (HCV) infection depends on certain critical host factors. We have recently shown that an interaction between HCV nonstructural protein NS5A and a host protein, TBC1D20, is necessary for efficient HCV replication. TBC1D20 contains a TBC (Tre-2, Bub2, and Cdc16) domain present in most known Rab GTPase-activating proteins (GAPs). The latter are master regulators of vesicular membrane transport, as they control the activity of membrane-associated Rab proteins. To better understand the role of the NS5A-TBC1D20 interaction in the HCV life cycle, we used a biochemical screen to identify the TBC1D20 Rab substrate. TBC1D20 was found to be the first known GAP for Rab1, which is implicated in the regulation of anterograde traffic between the endoplasmic reticulum and the Golgi complex. Mutation of amino acids implicated in Rab GTPase activation by other TBC domain-containing GAPs abrogated the ability of TBC1D20 to activate Rab1 GTPase. Overexpression of TBC1D20 blocked the transport of exogenous vesicular stomatitis virus G protein from the endoplasmic reticulum, validating the involvement of TBC1D20 in this pathway. Rab1 depletion significantly decreased HCV RNA levels, suggesting a role for Rab1 in HCV replication. These results highlight a novel mechanism by which viruses can hijack host cell machinery and suggest an attractive model whereby the NS5A-TBC1D20 interaction may promote viral membrane-associated RNA replication.
URLPMID:17686842 [本文引用: 4]

Hepatitis C virus (HCV) is an important cause of liver disease worldwide. Current therapies are inadequate for most patients. Using a two-hybrid screen, we isolated a novel cellular binding partner interacting with the N terminus of HCV nonstructural protein NS5A. This partner contains a TBC Rab-GAP (GTPase-activating protein) homology domain found in all known Rab-activating proteins. As the first described interaction between such a Rab-GAP and a viral protein, this finding suggests a new mechanism whereby viruses may subvert host cell machinery for mediating the endocytosis, trafficking, and sorting of their own proteins. Moreover, depleting the expression of this partner severely impairs HCV RNA replication with no obvious effect on cell viability. These results suggest that pharmacologic disruption of this NS5A-interacting partner can be contemplated as a potential new antiviral strategy against a pathogen affecting nearly 3% of the world's population.
URLPMID:20824714 [本文引用: 1]

Abstract Frameshift mutations at coding mononucleotide repeats (cMNR) are frequent in high-microsatellite instability (MSI-H) cancers. Frameshift mutations in cMNR result in the formation of a premature termination codon (PTC) in the transcribed mRNA, and these abnormal mRNAs are generally degraded by nonsense mediated mRNA decay (NMD). We have identified novel genes that are frequently mutated at their cMNR by blocking NMD in two MSI-H cancer cell lines. After blocking NMD, we screened for differentially expressed genes using DNA microarrays, and then used database analysis to select 28 candidate genes containing cMNR with more than 9 nucleotide repeats. cMNR mutations have not been previously reported in MSI-H cancers for 15 of the 28 genes. We analyzed the cMNR mutation of each of the 15 genes in 10 MSI-H cell lines and 21 MSI-H cancers, and found frequent mutations of 12 genes in MSI-H cell lines and cancers, but not in microsatellite stable (MSS) cancers. Among these genes, the most frequently mutated in MSI-H cell lines were MLL3 (70%), PHACTR4 (70%), RUFY2 (50%) and TBC1D23 (50%). MLL3, which has already been implicated in cancer, had the highest mutation frequency in MSI-H cancers (48%). Our combined approach of NMD block, database search, and mutation analysis has identified a large number of genes mutated in their cMNR in MSI-H cancers. The identified mutations are expected to contribute to MSI-H tumorigenesis by causing an absence of gene expression or low gene dosage effects. Copyright 漏 2010 UICC.
URLPMID:3895324 [本文引用: 1]

Deafness, onychodystrophy, osteodystrophy, mental retardation, and seizures (DOORS) syndrome is a rare autosomal recessive disorder of unknown cause. We aimed to identify the genetic basis of this syndrome by sequencing most coding exons in affected individuals.Through a search of available case studies and communication with collaborators, we identified families that included at least one individual with at least three of the five main features of the DOORS syndrome: deafness, onychodystrophy, osteodystrophy, intellectual disability, and seizures. Participants were recruited from 26 centres in 17 countries. Families described in this study were enrolled between Dec 1, 2010, and March 1, 2013. Collaborating physicians enrolling participants obtained clinical information and DNA samples from the affected child and both parents if possible. We did whole-exome sequencing in affected individuals as they were enrolled, until we identified a candidate gene, and Sanger sequencing to confirm mutations. We did expression studies in human fibroblasts from one individual by real-time PCR and western blot analysis, and in mouse tissues by immunohistochemistry and real-time PCR.26 families were included in the study. We did exome sequencing in the first 17 enrolled families; we screened for TBC1D24 by Sanger sequencing in subsequent families. We identified TBC1D24 mutations in 11 individuals from nine families (by exome sequencing in seven families, and Sanger sequencing in two families). 18 families had individuals with all five main features of DOORS syndrome, and TBC1D24 mutations were identified in half of these families. The seizure types in individuals with TBC1D24 mutations included generalised tonic-clonic, complex partial, focal clonic, and infantile spasms. Of the 18 individuals with DOORS syndrome from 17 families without TBC1D24 mutations, eight did not have seizures and three did not have deafness. In expression studies, some mutations abrogated TBC1D24 mRNA stability. We also detected Tbc1d24 expression in mouse phalangeal chondrocytes and calvaria, which suggests a role of TBC1D24 in skeletogenesis.Our findings suggest that mutations in TBC1D24 seem to be an important cause of DOORS syndrome and can cause diverse phenotypes. Thus, individuals with DOORS syndrome without deafness and seizures but with the other features should still be screened for TBC1D24 mutations. More information is needed to understand the cellular roles of TBC1D24 and identify the genes responsible for DOORS phenotypes in individuals who do not have a mutation in TBC1D24.US National Institutes of Health, the CIHR (Canada), the NIHR (UK), the Wellcome Trust, the Henry Smith Charity, and Action Medical Research.
URLPMID:27541164 [本文引用: 1]

Abstract TBC1D24-related disorders include a wide phenotypic ranging from mild to lethal seizure disorders, non-syndromic deafness, and composite syndromes such as DOORS (deafness, onychodystrophy, osteodystrophy, mental retardation, and seizures). The TBC1D24 gene has a role in cerebral cortex development and in presynaptic neurotransmission. Here, we present a familial case of a lethal early-onset epileptic encephalopathy, associated with two novel compound heterozygous missense variants on the TBC1D24 gene, which were detected by exome sequencing. The detailed clinical data of the three siblings is summarized in order to support the variability of the phenotype, severity, and progression of this disorder among these family members. Functional studies demonstrated that the identified novel missense mutations result in a loss of expression of the protein, suggesting a correlation between residual expression, and the disease severity. This indicates that protein expression analysis is important for interpreting genetic results when novel variants are found, as well as for complementing clinical assessment by predicting the functional impact. Further analysis is necessary to delineate the clinical presentation of individuals with TBC1D24 pathogenic variants, as well as to develop markers for diagnosis, prognosis, and potential targeted treatments. 漏 2016 Wiley Periodicals, Inc.
URL
URLPMID:17646400

Primary cilia are sensory structures involved in morphogen signalling during development, liquid flow in the kidney, mechanosensation, sight, and smell (Badano, J.L., N. Mitsuma, P.L. Beales, and N. Katsanis. 2006. Annu. Rev. Genomics Hum. Genet. 7:125–148; Singla, V., and J.F. Reiter. 2006. Science. 313:629–633.). Mutations that affect primary cilia are responsible for several diseases, including neural tube defects, polycystic kidney disease, retinal degeneration, and cancers (Badano et al., 2006; Singla and Reiter, 2006). Primary cilia formation and function requires tight integration of the microtubule cytoskeleton with membrane trafficking (Singla and Reiter, 2006), and this is poorly understood. We show that the Rab GTPase membrane trafficking regulators Rab8a, -17, and -23, and their cognate GTPase-activating proteins (GAPs), XM_037557, TBC1D7, and EVI5like, are involved in primary cilia formation. However, other human Rabs and GAPs are not. Additionally, Rab8a specifically interacts with cenexin/ODF2, a basal body and microtubule binding protein required for cilium biogenesis (Ishikawa, H., A. Kubo, S. Tsukita, and S. Tsukita. 2005. Nat. Cell Biol. 7:517–524), and is the sole Rab enriched at primary cilia. These findings provide a basis for understanding how specific membrane trafficking pathways cooperate with the microtubule cytoskeleton to give rise to the primary cilia.
URLPMID:16764853

EVI5 has been shown to be a novel centrosomal protein in interphase cells. In this report, we demonstrate using immunofluorescence microscopy that EVI5 has a dynamic distribution during mitosis, being associated with the mitotic spindle through anaphase and remaining within the midzone and midbody until completion of cytokinesis. Knockdown of EVI5 using siRNA results in a multinucleate phenotype, which is consistent with an essential role for this protein in the completion of cytokinesis. The EVI5 protein also undergoes posttranslational modifications during the cell cycle, which involve phosphorylation in early mitosis and proteolytic cleavage during late mitosis and cytokinesis. Since the subcellular distribution of the EVI5 protein was similar to that characteristic of chromosomal passenger proteins during the terminal stages of cytokinesis, we used immunoprecipitation and GST pull-down approaches to demonstrate that EVI5 is associated with the aurora B kinase protein complex (INCENP, aurora B kinase and survivin). Together, these data provide evidence that EVI5 is an essential component of the protein machinery facilitating the final stages of cell septation at the end of mitosis.
URLPMID:15509780

TBC (Tre-2/Bub2/Cdc16) domains are predicted to encode GTPase-activating proteins (GAPs) for Rab family G proteins. While approximately 50 TBC proteins are predicted to exist in humans, little is known about their substrate specificity. Here we show that TRE17 (also called Tre-2 and USP6), a founding member of the TBC family, targets the Arf family GTPase Arf6, which regulates plasma membrane-endosome trafficking. Surprisingly, TRE17 does not function as a GAP for Arf6 but rather promotes its activation in vivo. TRE17 associates directly with Arf6 in its GDP- but not GTP-bound state. Mapping experiments pinpoint the site of interaction to the TBC domain of TRE17. Forced expression of TRE17 promotes the localization of Arf6 to the plasma membrane, leading to Arf6 activation, presumably due to facilitated access to membrane-associated guanine nucleotide exchange factors (GEFs). Furthermore, TRE17 cooperates with Arf6 GEFs to induce GTP loading of Arf6 in vivo. Finally, short interfering RNA-mediated loss of TRE17 leads to attenuated Arf6 activation. These studies identify TRE17 as a novel regulator of the Arf6-regulated plasma membrane recycling system and reveal an unexpected function for TBC domains.
[本文引用: 1]
URLPMID:15026324 [本文引用: 1]

Abstract Aneurysmal bone cyst (ABC) is a locally aggressive osseous lesion that typically occurs during the first two decades of life. ABC was regarded historically as a nonneoplastic process, but recent cytogenetic data have shown clonal rearrangements of chromosomal bands 16q22 and 17p13, indicating a neoplastic basis in at least some ABCs. Herein we show that a recurring ABC chromosomal translocation t(16;17)(q22;p13) creates a fusion gene in which the osteoblast cadherin 11 gene (CDH11) promoter region on 16q22 is juxtaposed to the entire ubiquitin-specific protease USP6 (Tre2) coding sequence on 17p13. CDH11-USP6 fusion transcripts were demonstrated only in ABC with t(16;17) but other ABCs had CDH11 or USP6 rearrangements resulting from alternate cytogenetic mechanisms. CDH11 is expressed strongly in bone, and our findings implicate a novel oncogenic mechanism in which deregulated USP6 transcription results from juxtaposition to the highly active CDH11 promoter.
URLPMID:12359748 [本文引用: 3]

We used cDNA-based genomic microarrays to examine DNA copy number changes in a panel of prostate tumors and found a previously undescribed amplicon on chromosome 17 containing a novel overexpressed gene that we termed prostate cancer gene 17 (PRC17). When overexpressed in 3T3 mouse fibroblast cells, PRC17 induced growth in low serum, loss of contact inhibition, and tumor formation in nude mice. The PRC17 gene product contains a GTPase-activating protein (GAP) catalytic core motif found in various Rab/Ypt GAPs, including RN-Tre. Similar to RN-Tre, we found that PRC17 protein interacts directly with Rab5 and stimulates its GTP hydrolysis. Point mutations that alter conserved amino acid residues within the PRC17 GAP domain abolished its transforming abilities, suggesting that GAP activity is essential for its oncogenic function. Whereas PRC17 is amplified in 15% of prostate cancers, it is highly overexpressed in approximately one-half of metastatic prostate tumors. The potent oncogenic activity of PRC17 is likely to influence the tumorigenic phenotype of these prostate cancers.
URLPMID:1565468 [本文引用: 1]

Tre is a recombinant gene isolated from NIH3T3 cells transfected with human Ewing's sarcoma DNA. It is composed of three major genetic elements derived, 5' to 3', from human chromosomes 5, 18 and 17. We report here on transcripts from the 3' domain of tre. The transcripts were cloned from a cDNA library of cytoplasmic poly(A)+ RNA from tre-transfected NIH3T3 tumor cells. The complete cDNA sequence, 8201 nucleotides, possessed an unusually long non-coding region and a translatable region with two open reading frames. In one cDNA clone, the presence of two insertions suggested the possibility of alternative splicing. The sequence mapped to the centromere-proximal region of 17q. Transfection-tumorigenicity assays with the open reading frames subcloned into expression vectors were positive for the reading frame adjacent to the 5' non-coding region and negative for the second, downstream, reading frame and the possible alternatively spliced versions of both reading frames. Analysis of the 786 amino acid sequence deduced from the 5' reading frame predicted a highly hydrophilic protein with two charge clusters suggesting nucleic acid-binding properties. When used as probe, the cloned sequence detected RNA transcripts in a wide variety of human cancer cells regardless of their lineage of origin from different tissues, but not in human cells from normal tissue.
URLPMID:21129771 [本文引用: 2]

Systematic characterization of cancer genomes has revealed a staggering number of diverse aberrations that differ among individuals, such that the functional importance and physiological impact of most tumor genetic alterations remain poorly defined. We developed a computational framework that integrates chromosomal copy number and gene expression data for detecting aberrations that promote cancer progression. We demonstrate the utility of this framework using a melanoma data set. Our analysis correctly identified known drivers of melanoma and predicted multiple tumor dependencies. Two dependencies, TBC1D16 and RAB27A , confirmed empirically, suggest that abnormal regulation of protein trafficking contributes to proliferation in melanoma. Together, these results demonstrate the ability of integrative Bayesian approaches to identify candidate drivers with biological, and possibly therapeutic, importance in cancer.
URLPMID:20797691 [本文引用: 2]

We characterized an autosomal-recessive syndrome of focal epilepsy, dysarthria, and mild to moderate intellectual disability in a consanguineous Arab-Israeli family associated with subtle cortical thickening. We used multipoint linkage analysis to map the causative mutation to a 3.2 Mb interval within 16p13.3 with a LOD score of 3.86. The linked interval contained 160 genes, many of which were considered to be plausible candidates to harbor the disease-causing mutation. To interrogate the interval in an efficient and unbiased manner, we used targeted sequence enrichment and massively parallel sequencing. By prioritizing unique variants that affected protein translation, a pathogenic mutation was identified in TBC1D24 (p.F251L), a gene of unknown function. It is a member of a large gene family encoding TBC domain proteins with predicted function as Rab GTPase activators. We show that TBC1D24 is expressed early in mouse brain and that TBC1D24 protein is a potent modulator of primary axonal arborization and specification in neuronal cells, consistent with the phenotypic abnormality described.
[本文引用: 1]
URLPMID:16555005 [本文引用: 1]

The product of the Tre2 oncogene, structurally related to the Ypt/RabGTPase-activating proteins (Ypt/RabGAP), is involved in various human cancers, including Ewing's sarcoma. In order to identify proteins interacting with the GAP part of this protein, we performed yeast two-hybrid screening of two libraries. Two components of the cytoskeleton were thus identified, whose interaction with the GAP region was confirmed by GST-pulldown, co-immunoprecipitation, and colocalisation experiments. The proteins found to interact with the GAP region are the light regulatory chain of myosin II (Myl2) and LOC91256, a protein containing ankyrin repeats.
URLPMID:11994271 [本文引用: 1]

This study describes a method for the identification of the substrates of specific serine kinases. An antibody specific for the phosphomotif generated by the kinase is used to isolate phosphorylated substrates by immunoprecipitation, and the isolated proteins are identified by tandem mass spectrometry of peptides. This method was applied to the identification of substrates for the protein kinase Akt, which specifically phosphorylates the RXRXXS/T motif. 3T3-L1 adipocytes were treated with insulin to activate Akt, and the putative Akt substrate proteins were isolated by immunoprecipitation with an antibody against the phospho form of this motif. This led to the identification of a novel 160-kDa substrate for Akt. The 160-kDa substrate for Akt, which was designated AS160, has a Rab GAP domain. Recombinant AS160 was shown to be a substrate for Akt, and two sites of phosphorylation, both in RXRXXS/T motifs, were identified by mass spectrometry and mutation. Insulin treatment of adipocytes caused AS160 to redistribute from the low density microsomes to the cytosol.
URLPMID:12637568 [本文引用: 1]

Abstract Insulin stimulates the rapid translocation of intracellular glucose transporters of the GLUT4 isotype to the plasma membrane in fat and muscle cells. The connections between known insulin signaling pathways and the protein machinery of this membrane-trafficking process have not been fully defined. Recently, we identified a 160-kDa protein in adipocytes, designated AS160, that is phosphorylated by the insulin-activated kinase Akt. This protein contains a GTPase-activating domain (GAP) for Rabs, which are small G proteins required for membrane trafficking. In the present study we have identified six sites of in vivo phosphorylation on AS160. These sites lie in the motif characteristic of Akt phosphorylation, and insulin treatment increased phosphorylation at five of the sites. Expression of AS160 with two or more of these sites mutated to alanine markedly inhibited insulin-stimulated GLUT4 translocation in 3T3-L1 adipocytes. Moreover, this inhibition did not occur when the GAP function in the phosphorylation site mutant was inactivated by a point mutation. These findings strongly indicate that insulin-stimulated phosphorylation of AS160 is required for GLUT4 translocation and that this phosphorylation signals translocation through inactivation of the Rab GAP function.
URLPMID:18076383 [本文引用: 1]

In fat and muscle cells, insulin stimulates the movement to and fusion of intracellular vesicles containing GLUT4 with the plasma membrane, a process referred to as GLUT4 translocation. Previous studies have indicated that Akt [also known as PKB (protein kinase B)] phosphorylation of AS160, a GAP (GTPase-activating protein) for Rabs, is required for GLUT4 translocation. The results suggest that this phosphorylation suppresses the GAP activity and leads to the elevation of the GTP form of one or more Rabs required for GLUT4 translocation. Based on their presence in GLUT4 vesicles and activity as AS160 GAP substrates, Rabs 8A, 8B, 10 and 14 are candidate Rabs. Here, we provide further evidence that Rab10 participates in GLUT4 translocation in 3T3-L1 adipocytes. Among Rabs 8A, 8B, 10 and 14, only the knockdown of Rab10 inhibited GLUT4 translocation. In addition, we describe the subcellular distribution of Rab10 and estimate the fraction of Rab10 in the active GTP form in vivo. Approx. 5% of the total Rab10 was present in GLUT4 vesicles isolated from the low-density microsomes. In both the basal and the insulin state, 90% of the total Rab10 was in the inactive GDP state. Thus, if insulin increases the GTP form of Rab10, the increase is limited to a small portion of the total Rab10. Finally, we report that the Rab10 mutant considered to be constitutively active (Rab10 Q68L) is a substrate for the AS160 GAP domain and, hence, cannot be used to deduce rigorously the function of Rab10 in its GTP form.
URLPMID:17403373 [本文引用: 1]

Abstract GLUT4 trafficking to the plasma membrane of muscle and fat cells is regulated by insulin. An important component of insulin-regulated GLUT4 distribution is the Akt substrate AS160 rab GTPase-activating protein. Here we show that Rab10 functions as a downstream target of AS160 in the insulin-signaling pathway that regulates GLUT4 translocation in adipocytes. Overexpression of a mutant of Rab10 defective for GTP hydrolysis increased GLUT4 on the surface of basal adipocytes. Rab10 knockdown resulted in an attenuation of insulin-induced GLUT4 redistribution to the plasma membrane and a concomitant 2-fold decrease in GLUT4 exocytosis rate. Re-expression of a wild-type Rab10 restored normal GLUT4 translocation. The basal increase in plasma-membrane GLUT4 due to AS160 knockdown was partially blocked by knocking down Rab10 in the same cells, further indicating that Rab10 is a target of AS160 and a positive regulator of GLUT4 trafficking to the cell surface upon insulin stimulation.
URLPMID:18650435 [本文引用: 1]

Insulin increases glucose uptake into muscle by enhancing the surface recycling of GLUT4 transporters. In myoblasts, insulin signals bifurcate downstream of phosphatidylinositol 3-kinase into separate Akt and Rac/actin arms. Akt-mediated Rab-GAP AS160 phosphorylation and Rac/actin are required for net insulin gain of GLUT4, but the specific steps (vesicle recruitment, docking or fusion) regulated by Rac, actin dynamics, and AS160 target Rab8A are unknown. In L6 myoblasts expressing GLUT4myc, blocking vesicle fusion by tetanus toxin cleavage of VAMP2 impeded GLUT4myc membrane insertion without diminishing its build-up at the cell periphery. Conversely, actin disruption by dominant negative Rac or Latrunculin B abolished insulin-induced surface and submembrane GLUT4myc accumulation. Expression of non-phosphorylatable AS160 (AS160-4P) abrogated membrane insertion of GLUT4myc and partially reduced its cortical build-up, an effect magnified by selective Rab8A knockdown. We propose that insulin-induced actin dynamics participates in GLUT4myc vesicle retention beneath the membrane, whereas AS160 phosphorylation is essential for GLUT4myc vesicle-membrane docking/fusion and also contributes to GLUT4myc cortical availability through Rab8A.
URLPMID:18931681 [本文引用: 1]

Nat Genet. 2008 Nov;40(11):1354-9. doi: 10.1038/ng.244. Epub 2008 Oct 19. Research Support, Non-U.S. Gov't
URLPMID:2695078 [本文引用: 1]

Tre-2, BUB2, CDC16, 1 domain family member 4 (TBC1D4) (AS160) is a Rab-GTPase activating protein implicated in insulin-stimulated glucose transporter 4 (GLUT4) translocation in adipocytes and myotubes. To determine whether loss-of-function mutations in TBC1D4 might impair GLUT4 translocation and cause insulin resistance in humans, we screened the coding regions of this gene in 156 severely insulin-resistant patients. A female presenting at age 11 years with acanthosis nigricans and extreme postprandial hyperinsulinemia was heterozygous for a premature stop mutation (R363X) in TBC1D4. After demonstrating reduced expression of wild-type TBC1D4 protein and expression of the truncated protein in lymphocytes from the proband, we further characterized the biological effects of the truncated protein in 3T3L1 adipocytes. Prematurely truncated TBC1D4 protein tended to increase basal cell membrane GLUT4 levels (P = 0.053) and significantly reduced insulin-stimulated GLUT4 cell membrane translocation (P < 0.05). When coexpressed with wild-type TBC1D4, the truncated protein dimerized with full-length TBC1D4, suggesting that the heterozygous truncated variant might interfere with its wild-type counterpart in a dominant negative fashion. Two overweight family members with the mutation also manifested normal fasting glucose and insulin levels but disproportionately elevated insulin levels following an oral glucose challenge. This family provides unique genetic evidence of TBC1D4 involvement in human insulin action.
URLPMID:27669036 [本文引用: 1]

Mutations in TBC1D24 cause severe epilepsy and DOORS syndrome, but the molecular mechanisms underlying these pathologies are unresolved. We solved the crystal structure of the TBC domain of the Drosophila ortholog Skywalker, revealing an unanticipated cationic pocket conserved among TBC1D24 homologs. Cocrystallization and biochemistry showed that this pocket binds phosphoinositides phosphorylated at the 4 and 5 positions. The most prevalent patient mutations affect the phosphoinositide-binding pocket and inhibit lipid binding. Using in vivo photobleaching of Skywalker-GFP mutants, including pathogenic mutants, we showed that membrane binding via this pocket restricts Skywalker diffusion in presynaptic terminals. Additionally, the pathogenic mutations cause severe neurological defects in flies, including impaired synaptic-vesicle trafficking and seizures, and these defects are reversed by genetically increasing synaptic PI(4,5)P2 concentrations through synaptojanin mutations. Hence, we discovered that a TBC domain affected by clinical mutations directly binds phosphoinositides through a cationic pocket and that phosphoinositide binding is critical for presynaptic function.
URLPMID:18614017 [本文引用: 1]

The small GTPases, Rab5 and Rac, are essential for endocytosis and actin remodeling, respectively. Coordination of these processes is critical to achieve spatial restriction of intracellular signaling, which is essential for a variety of polarized functions. Here, we show that clathrin- and Rab5-mediated endocytosis are required for the activation of Rac induced by motogenic stimuli. Rac activation occurs on early endosomes, where the RacGEF Tiam1 is also recruited. Subsequent recycling of Rac to the plasma membrane ensures localized signaling, leading to the formation of actin-based migratory protrusions. Thus, membrane trafficking of Rac is required for the spatial resolution of Rac-dependent motogenic signals. We further demonstrate that a Rab5-to-Rac circuitry controls the morphology of motile mammalian tumor cells and primordial germinal cells during zebrafish development, suggesting that this circuitry is relevant for the regulation of migratory programs in various cells, in both in vitro settings and whole organisms.
