朱峰1,,
1.中国科学院遗传与发育生物学研究所农业资源研究中心/河北省土壤生态学重点实验室/中国科学院农业水资源重点实验室 石家庄 050022
2.中国科学院大学 北京 100049
基金项目:中国科学院科技服务网络计划项目(KFJ-STS-QYZD-160)和中国科学院“****”项目资助
详细信息
作者简介:毛梦雪, 主要从事植物介导地上地下互作机制研究。E-mail: maomengxue18@mails.ucas.ac.cn
通讯作者:朱峰, 主要从事植物介导地上地下互作机制研究。E-mail: zhufeng@sjziam.ac.cn
中图分类号:S181计量
文章访问数:305
HTML全文浏览量:56
PDF下载量:141
被引次数:0
出版历程
收稿日期:2021-05-30
录用日期:2021-07-16
网络出版日期:2021-08-20
刊出日期:2021-10-01
Progress and perspective in research on plant resistance mediated by root exudates
MAO Mengxue1, 2,,ZHU Feng1,,
1. Center for Agricultural Resources Research, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences / Hebei Key Laboratory of Soil Ecology / Key Laboratory of Agricultural Water Resources, Chinese Academy of Sciences, Shijiazhuang 050022, China
2. University of Chinese Academy of Sciences, Beijing 100049, China
Funds:This study was supported by the Science and Technology Service Network Initiative of Chinese Academy of Sciences (KFJ-STS-QYZD-160) and the 100-Talent Project of Chinese Academy of Sciences
More Information
Corresponding author:E-mail: zhufeng@sjziam.ac.cn
摘要
HTML全文
图
参考文献
相关文章
施引文献
资源附件
访问统计
摘要
摘要:根系分泌物是由植物根系主动或被动分泌的多种生物化学物质, 在介导植物根际微环境间的物质交换、能量传递和信息交流中具有重要作用, 是植物响应外界胁迫的重要途径。生物和非生物胁迫会改变根系分泌物的组成和数量, 使植物根系分泌物中的防御性化合物含量增加。植物运用不同的根系分泌物模式抵御生物和非生物胁迫, 包括释放有毒物质直接防御、释放挥发性物质吸引天敌以及与微生物互作抵御生物胁迫; 释放具有渗透调节功能及抗氧化能力的根系分泌物以及协同激素信号抵抗非生物胁迫。此外, 根系分泌物的流动局部地提高了许多常见代谢物的浓度, 不仅可以改变土壤的理化性质及微生物活性, 还会影响土壤-植物界面的许多生理生化过程, 直接或间接地提高植物抗逆性。本文综述了生物与非生物胁迫对植物根系分泌物组成和数量的影响, 总结了根系分泌物介导植物防御生物与非生物胁迫的方式, 并对未来的研究方向进行了展望, 旨在为更深层次地研究植物在逆境胁迫下的适应性机制提供参考。
关键词:根系分泌物/
生物胁迫/
非生物胁迫/
土壤微生物/
植物抗逆性
Abstract:Root exudates are a variety of biochemical substances actively or passively secreted by plant roots that play an important role in mediating material exchange, energy transfer and information exchange in plant rhizosphere microenvironments, as well as in plant responses to environmental stresses. Biotic and abiotic stresses can change the composition and quantity of root exudates and increase the content of defensive compounds in plant root exudates. Plants use different root exudates to resist biotic and abiotic stresses, including releasing toxic substances for direct defense, releasing volatile substances to attract natural enemies, interacting with microorganisms to resist biotic stresses, releasing root exudates with osmotic regulation and antioxidant capacity and coordinating hormone signals to resist abiotic stress. Additionally, root exudate flow increases the concentration of many common metabolites, changing the soil physical and chemical properties and microbial activities, and affecting the physiological and biochemical processes at the soil-plant interface, thereby, directly or indirectly improving plant stress resistance. In this paper, the effects of biotic and abiotic stresses on the composition and quantity of plant root exudates were reviewed, the mechanisms of plant defense against biotic and abiotic stresses mediated by root exudates were summarized, and the aspects needed to be further studied were also suggested, to provide a reference for further research on the adaptive mechanism of plants under stress.
Key words:Root exudates/
Biotic stress/
Abiotic stress/
Soil microorganism/
Plant resistance
HTML全文
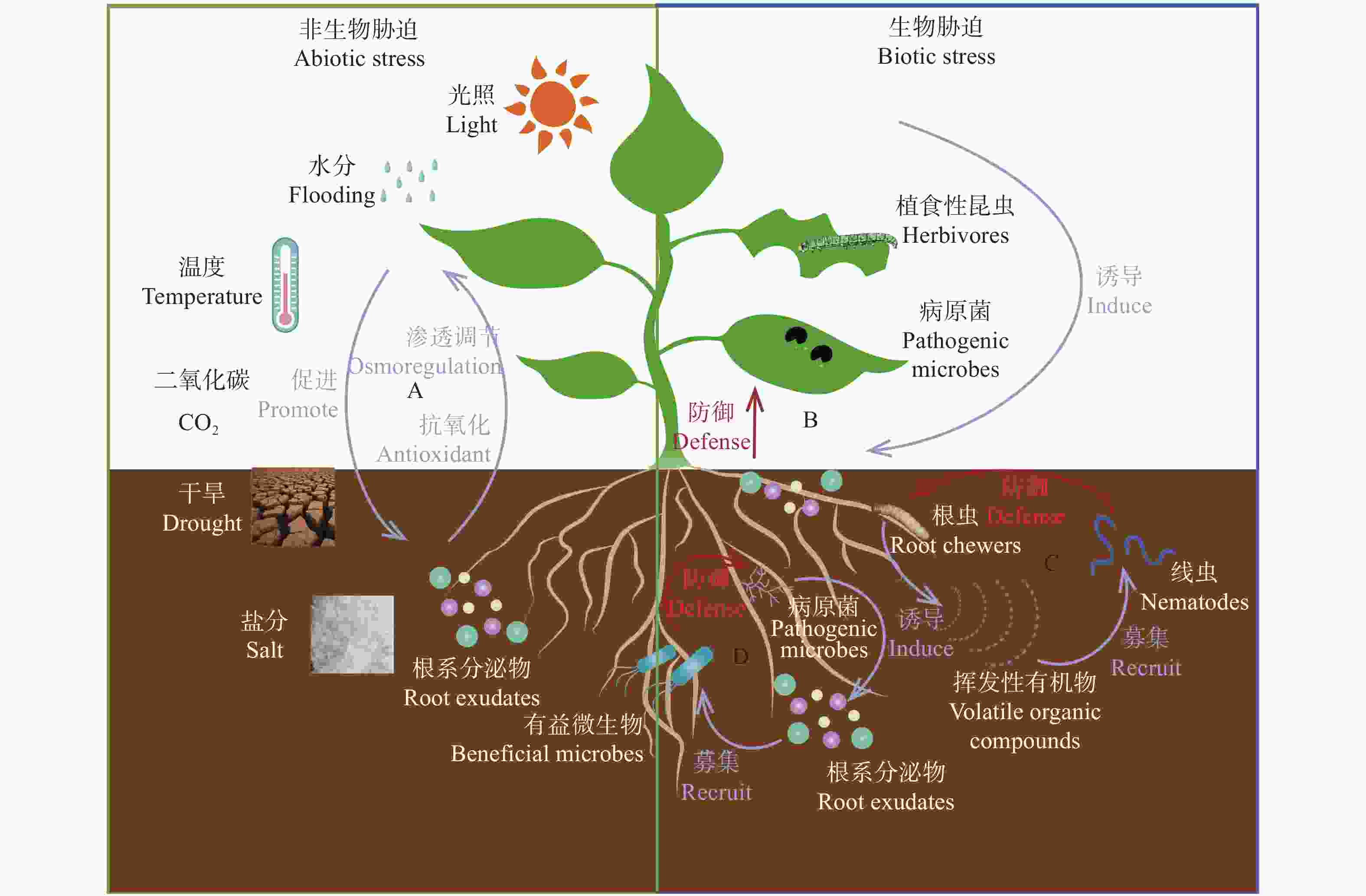
图1根系分泌物介导的植物对生物与非生物胁迫响应模型
根系分泌物介导植物对环境胁迫的应答机制。A: 根系分泌物介导植物抵御非生物胁迫。B: 植物根系分泌物介导植物对地上植食性昆虫、病原菌的防御。C: 植物根系分泌物介导植物对地下植食性昆虫的防御。D: 植物根系分泌物介导植物对地下病原菌的防御。蓝色箭头表示诱导/促进作用, 红色箭头表示防御作用。Root exudates mediate the response mechanism of plants to environmental stress. A: root exudates-mediated plant resistance to abiotic stresses; B: root exudates-mediated plant defense against aboveground herbivores and pathogens; C: root exudates-mediated plant defense against underground herbivores; D: root exudates-mediated plant defense against underground pathogens. Blue arrows indicate induction/promotion, the red arrow indicates defense.
Figure1.Model of root exudates-mediated plant responses to biotic and abiotic stresses
 下载: 全尺寸图片幻灯片
下载: 全尺寸图片幻灯片参考文献
| [1] | BAETZ U, MARTINOIA E. Root exudates: the hidden part of plant defense[J]. Trends in Plant Science, 2014, 19(2): 90?98 doi: 10.1016/j.tplants.2013.11.006 |
| [2] | WEN F S, VANETTEN H D, TSAPRAILIS G, et al. Extracellular proteins in pea root tip and border cell exudates[J]. Plant Physiology, 2007, 143(2): 773?783 doi: 10.1104/pp.106.091637 |
| [3] | CANARINI A, KAISER C, MERCHANT A, et al. Root exudation of primary metabolites: mechanisms and their roles in plant responses to environmental stimuli[J]. Frontiers in Plant Science, 2019, 10: 157 |
| [4] | SANCHEZ-ARCOS C, KAI M, SVATO? A, et al. Untargeted metabolomics approach reveals differences in host plant chemistry before and after infestation with different pea aphid host races[J]. Frontiers in Plant Science, 2019, 10: 188 doi: 10.3389/fpls.2019.00188 |
| [5] | VIVES-PERIS V, GóMEZ-CADENAS A, PéREZ-CLEMENTE R M. Citrus plants exude proline and phytohormones under abiotic stress conditions[J]. Plant Cell Reports, 2017, 36(12): 1971?1984 doi: 10.1007/s00299-017-2214-0 |
| [6] | CALVO O C, FRANZARING J, SCHMID I, et al. Root exudation of carbohydrates and cations from barley in response to drought and elevated CO2[J]. Plant and Soil, 2019, 438(1/2): 127?142 |
| [7] | CARVALHAIS L C, DENNIS P G, FEDOSEYENKO D, et al. Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency[J]. Journal of Plant Nutrition and Soil Science, 2011, 174(1): 3?11 doi: 10.1002/jpln.201000085 |
| [8] | LOMBARDI N, VITALE S, TURRà D, et al. Root exudates of stressed plants stimulate and attract Trichoderma soil fungi[J]. Molecular Plant-Microbe Interactions?, 2018, 31(10): 982?994 |
| [9] | SURENDER REDDY P, JOGESWAR G, RASINENI G K, et al. Proline over-accumulation alleviates salt stress and protects photosynthetic and antioxidant enzyme activities in transgenic sorghum |
| [10] | BADRI D V, VIVANCO J M. Regulation and function of root exudates[J]. Plant, Cell & Environment, 2009, 32(6): 666?681 |
| [11] | 吴彩霞, 傅华. 根系分泌物的作用及影响因素[J]. 草业科学, 2009, 26(9): 24?29 doi: 10.3969/j.issn.1001-0629.2009.09.005 WU C X, FU H. Effects and roles of root exudates[J]. Pratacultural Science, 2009, 26(9): 24?29 doi: 10.3969/j.issn.1001-0629.2009.09.005 |
| [12] | GERA HOL W H, MACEL M, VAN VEEN J A, et al. Root damage and aboveground herbivory change concentration and composition of pyrrolizidine alkaloids of Senecio jacobaea[J]. Basic and Applied Ecology, 2004, 5(3): 253?260 |
| [13] | HOYSTED G A, BELL C A, LILLEY C J, et al. Aphid colonization affects potato root exudate composition and the hatching of a soil borne pathogen[J]. Frontiers in Plant Science, 2018, 9: 1278 doi: 10.3389/fpls.2018.01278 |
| [14] | BEZEMER T M, VAN DAM N M. Linking aboveground and belowground interactions via induced plant defenses[J]. Trends in Ecology & Evolution, 2005, 20(11): 617?624 |
| [15] | HU L F, ROBERT C A M, CADOT S, et al. Root exudate metabolites drive plant-soil feedbacks on growth and defense by shaping the rhizosphere microbiota[J]. Nature Communications, 2018, 9: 2738 doi: 10.1038/s41467-018-05122-7 |
| [16] | BEZEMER T M, WAGENAAR R, VAN DAM N M, et al. Above- and below-ground terpenoid aldehyde induction in cotton, Gossypium herbaceum, following root and leaf injury[J]. Journal of Chemical Ecology, 2004, 30(1): 53?67 doi: 10.1023/B:JOEC.0000013182.50662.2a |
| [17] | MARAK H B, BIERE A, VAN DAMME J M M. Systemic, genotype-specific induction of two herbivore-deterrent iridoid glycosides in Plantago lanceolata L. in response to fungal infection by Diaporthe adunca (rob.) Niessel[J]. Journal of Chemical Ecology, 2002, 28(12): 2429?2448 |
| [18] | ROBERT C A M, VEYRAT N, GLAUSER G, et al. A specialist root herbivore exploits defensive metabolites to locate nutritious tissues[J]. Ecology Letters, 2012, 15(1): 55?64 doi: 10.1111/j.1461-0248.2011.01708.x |
| [19] | RASMANN S, K?LLNER T G, DEGENHARDT J, et al. Recruitment of entomopathogenic nematodes by insect-damaged maize roots[J]. Nature, 2005, 434(7034): 732?737 doi: 10.1038/nature03451 |
| [20] | ALI J G, ALBORN H T, STELINSKI L L. Subterranean herbivore-induced volatiles released by Citrus roots upon feeding by Diaprepes abbreviatus recruit entomopathogenic nematodes[J]. Journal of Chemical Ecology, 2010, 36(4): 361?368 doi: 10.1007/s10886-010-9773-7 |
| [21] | LEE B, LEE S, RYU C M. Foliar aphid feeding recruits rhizosphere bacteria and primes plant immunity against pathogenic and non-pathogenic bacteria in pepper[J]. Annals of Botany, 2012, 110(2): 281?290 doi: 10.1093/aob/mcs055 |
| [22] | SONG G C, LEE S, HONG J, et al. Aboveground insect infestation attenuates belowground Agrobacterium-mediated genetic transformation[J]. The New Phytologist, 2015, 207(1): 148?158 doi: 10.1111/nph.13324 |
| [23] | EISENHAUER N, LANOUE A, STRECKER T, et al. Root biomass and exudates link plant diversity with soil bacterial and fungal biomass[J]. Scientific Reports, 2017, 7(1): 1?8 doi: 10.1038/s41598-016-0028-x |
| [24] | YUAN J, ZHAO J, WEN T, et al. Root exudates drive the soil-borne legacy of aboveground pathogen infection[J]. Microbiome, 2018, 6(1): 156 doi: 10.1186/s40168-018-0537-x |
| [25] | PHILLIPS D A, FOX T C, KING M D, et al. Microbial products trigger amino acid exudation from plant roots[J]. Plant Physiology, 2004, 136(1): 2887?2894 doi: 10.1104/pp.104.044222 |
| [26] | OZAN A, SAFIR G R, NAIR M G. Persistence of isoflavones formononetin and biochanin A in soil and their effects on soil microbe populations[J]. Journal of Chemical Ecology, 1997, 23(2): 247?258 doi: 10.1023/B:JOEC.0000006357.18358.14 |
| [27] | DARDANELLI M S, DE CóRDOBA F J F, ESTéVEZ J, et al. Changes in flavonoids secreted by Phaseolus vulgaris roots in the presence of salt and the plant growth-promoting rhizobacterium Chryseobacterium balustinum[J]. Applied Soil Ecology, 2012, 57: 31?38 doi: 10.1016/j.apsoil.2012.01.005 |
| [28] | HENRY A, DOUCETTE W, NORTON J, et al. Changes in crested wheatgrass root exudation caused by flood, drought, and nutrient stress[J]. Journal of Environmental Quality, 2007, 36(3): 904?912 doi: 10.2134/jeq2006.0425sc |
| [29] | KSOURI R, MEGDICHE W, DEBEZ A, et al. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima[J]. Plant Physiology and Biochemistry, 2007, 45(3/4): 244?249 |
| [30] | NAVARRO J M, FLORES P, GARRIDO C, et al. Changes in the contents of antioxidant compounds in pepper fruits at different ripening stages, as affected by salinity[J]. Food Chemistry, 2006, 96(1): 66?73 |
| [31] | DE ABREU I N, MAZZAFERA P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy[J]. Plant Physiology and Biochemistry, 2005, 43(3): 241?248 doi: 10.1016/j.plaphy.2005.01.020 |
| [32] | GOMEZ-CADENAS A, VIVES V, ZANDALINAS S I, et al. Abscisic acid: a versatile phytohormone in plant signaling and beyond[J]. Current Protein & Peptide Science, 2015, 16(5): 413?434 |
| [33] | VIVES-PERIS V, MOLINA L, SEGURA A, et al. Root exudates from citrus plants subjected to abiotic stress conditions have a positive effect on rhizobacteria[J]. Journal of Plant Physiology, 2018, 228: 208?217 doi: 10.1016/j.jplph.2018.06.003 |
| [34] | ZHOU Y, TANG N Y, HUANG L J, et al. Effects of salt stress on plant growth, antioxidant capacity, glandular trichome density, and volatile exudates of Schizonepeta tenuifolia briq[J]. International Journal of Molecular Sciences, 2018, 19(1): E252 doi: 10.3390/ijms19010252 |
| [35] | HUGHES M, DONNELLY C, CROZIER A, et al. Effects of the exposure of roots of Alnus glutinosa to light on flavonoids and nodulation[J]. Canadian Journal of Botany, 1999, 77(9): 1311?1315 |
| [36] | JONES D L. Organic acids in the rhizosphere—a critical review[J]. Plant and Soil, 1998, 205(1): 25?44 doi: 10.1023/A:1004356007312 |
| [37] | RYALLS J M W, MOORE B D, RIEGLER M, et al. Amino acid-mediated impacts of elevated carbon dioxide and simulated root herbivory on aphids are neutralized by increased air temperatures[J]. Journal of Experimental Botany, 2015, 66(2): 613?623 doi: 10.1093/jxb/eru439 |
| [38] | MITH?FER A, BOLAND W. Plant defense against herbivores: chemical aspects[J]. Annual Review of Plant Biology, 2012, 63(1): 431?450 doi: 10.1146/annurev-arplant-042110-103854 |
| [39] | MAFFEI M E, MITH?FER A, BOLAND W. Insects feeding on plants: Rapid signals and responses preceding the induction of phytochemical release[J]. Phytochemistry, 2007, 68(22/23/24): 2946?2959 |
| [40] | TU X B, LIU Z K, ZHANG Z H. Comparative transcriptomic analysis of resistant and susceptible alfalfa cultivars (Medicago sativa L.) after thrips infestation[J]. BMC Genomics, 2018, 19(1): 1?8 doi: 10.1186/s12864-017-4368-0 |
| [41] | RASOOL S, VIDKJAER N H, HOOSHMAND K, et al. Seed inoculations with entomopathogenic fungi affect aphid populations coinciding with modulation of plant secondary metabolite profiles across plant families[J]. The New Phytologist, 2021, 229(3): 1715?1727 doi: 10.1111/nph.16979 |
| [42] | VAN TOL R W H M, VAN DER SOMMEN A T C, BOFF M I C, et al. Plants protect their roots by alerting the enemies of grubs[J]. Ecology Letters, 2001, 4(4): 292?294 doi: 10.1046/j.1461-0248.2001.00227.x |
| [43] | HU L, MATEO P, YE M, et al. Plant iron acquisition strategy exploited by an insect herbivore[J]. Science, 2018, 361(6403): 694?697 |
| [44] | VAN DE MORTEL J E, DE VOS R C H, DEKKERS E, et al. Metabolic and transcriptomic changes induced in Arabidopsis by the rhizobacterium Pseudomonas fluorescens SS101[J]. Plant Physiology, 2012, 160(4): 2173?2188 doi: 10.1104/pp.112.207324 |
| [45] | BADRI D V, CHAPARRO J M, ZHANG R F, et al. Application of natural blends of phytochemicals derived from the root exudates of Arabidopsis to the soil reveal that phenolic-related compounds predominantly modulate the soil microbiome[J]. Journal of Biological Chemistry, 2013, 288(7): 4502?4512 doi: 10.1074/jbc.M112.433300 |
| [46] | MATILLA M A, RAMOS J L, BAKKER P A, et al. Pseudomonas putida KT2440 causes induced systemic resistance and changes in Arabidopsis root exudation[J]. Environmental Microbiology Reports, 2010, 2(3): 381?388 |
| [47] | PIETERSE C M, ZAMIOUDIS C, BERENDSEN R L, et al. Induced systemic resistance by beneficial microbes[J]. Annual Review of Phytopathology, 2014, 52: 347?375 doi: 10.1146/annurev-phyto-082712-102340 |
| [48] | BROECKLING C D, BROZ A K, BERGELSON J, et al. Root exudates regulate soil fungal community composition and diversity[J]. Applied and Environmental Microbiology, 2008, 74(3): 738?744 doi: 10.1128/AEM.02188-07 |
| [49] | RAY S, MISHRA S, BISEN K, et al. Modulation in phenolic root exudate profile of Abelmoschus esculentus expressing activation of defense pathway[J]. Microbiological Research, 2018, 207: 100?107 |
| [50] | RUDRAPPA T, CZYMMEK K J, PARE? P W, et al. Root-secreted malic acid recruits beneficial soil bacteria[J]. Plant Physiology, 2008, 148(3): 1547?1556 doi: 10.1104/pp.108.127613 |
| [51] | YANG J W, YI H S, KIM H, et al. Whitefly infestation of pepper plants elicits defence responses against bacterial pathogens in leaves and roots and changes the below-ground microflora[J]. Journal of Ecology, 2011, 99(1): 46?56 doi: 10.1111/j.1365-2745.2010.01756.x |
| [52] | TU X B, ZHAO H L, ZHANG Z H. Transcriptome approach to understand the potential mechanisms of resistant and susceptible alfalfa (Medicago sativa L.) cultivars in response to aphid feeding[J]. Journal of Integrative Agriculture, 2018, 17(11): 2518?2527 doi: 10.1016/S2095-3119(17)61843-4 |
| [53] | ZHAO Y, WEI X H, JI X Z, et al. Endogenous NO-mediated transcripts involved in photosynthesis and carbohydrate metabolism in alfalfa (Medicago sativa L.) seedlings under drought stress[J]. Plant Physiology and Biochemistry, 2019, 141: 456?465 doi: 10.1016/j.plaphy.2019.06.023 |
| [54] | PER T S, KHAN N A, REDDY P S, et al. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics[J]. Plant Physiology and Biochemistry, 2017, 115: 126?140 doi: 10.1016/j.plaphy.2017.03.018 |
| [55] | RUAN Y L. Sucrose metabolism: gateway to diverse carbon use and sugar signaling[J]. Annual Review of Plant Biology, 2014, 65: 33?67 doi: 10.1146/annurev-arplant-050213-040251 |
| [56] | SAFTNER R A, WYSE R E. Effect of plant hormones on sucrose uptake by sugar beet root tissue discs[J]. Plant Physiology, 1984, 74(4): 951?955 doi: 10.1104/pp.74.4.951 |
| [57] | PARIDA A K, DAS A B. Salt tolerance and salinity effects on plants: a review[J]. Ecotoxicology and Environmental Safety, 2005, 60(3): 324?349 doi: 10.1016/j.ecoenv.2004.06.010 |
| [58] | OGAWA K. Glutathione-associated regulation of plant growth and stress responses[J]. Antioxidants & Redox Signaling, 2005, 7(7/8): 973?981 |
| [59] | TEH C Y, MAHMOOD M, SHAHARUDDIN N A, et al. In vitro rice shoot apices as simple model to study the effect of NaCl and the potential of exogenous proline and glutathione in mitigating salinity stress[J]. Plant Growth Regulation, 2015, 75(3): 771?781 doi: 10.1007/s10725-014-9980-2 |
| [60] | WANG N Q, KONG C H, WANG P, et al. Root exudate signals in plant-plant interactions[J]. Plant, Cell & Environment, 2021, 44(4): 1044?1058 |
| [61] | IQBAL N, UMAR S, KHAN N A, et al. A new perspective of phytohormones in salinity tolerance: Regulation of proline metabolism[J]. Environmental and Experimental Botany, 2014, 100: 34?42 doi: 10.1016/j.envexpbot.2013.12.006 |
| [62] | PYE M F, DYE S M, RESENDE R S, et al. Abscisic acid as a dominant signal in tomato during salt stress predisposition to Phytophthora root and crown rot[J]. Frontiers in Plant Science, 2018, 9: 525 doi: 10.3389/fpls.2018.00525 |
| [63] | KHAN M I R, IQBAL N, MASOOD A, et al. Salicylic acid alleviates adverse effects of heat stress on photosynthesis through changes in proline production and ethylene formation[J]. Plant Signaling & Behavior, 2013, 8(11): e26374 |
| [64] | CANARINI A, MERCHANT A, DIJKSTRA F A. Drought effects on Helianthus annuus and Glycine max metabolites: from phloem to root exudates[J]. Rhizosphere, 2016, 2: 85?97 doi: 10.1016/j.rhisph.2016.06.003 |
| [65] | OBURGER E, GRUBER B, SCHINDLEGGER Y, et al. Root exudation of phytosiderophores from soil-grown wheat[J]. New Phytologist, 2014, 203(4): 1161?1174 doi: 10.1111/nph.12868 |
