 ,1,2, 孙冷雪2, 郑嘉敏2, 蔡灿军2, 王蓓2, 李开拓2, 潘腾飞1,2, 佘文琴
,1,2, 孙冷雪2, 郑嘉敏2, 蔡灿军2, 王蓓2, 李开拓2, 潘腾飞1,2, 佘文琴 ,1,2, 陈桂信1,2, 潘东明1,2
,1,2, 陈桂信1,2, 潘东明1,2Purification, Characterization and Expression of Ionically Bound Peroxidase in Litchi Pericarp during Coloration and Maturation of Fruit
GUO ZhiXiong ,1,2, SUN LengXue2, ZHENG JiaMin2, CAI CanJun2, WANG Bei2, LI KaiTuo2, PAN TengFei1,2, SHE WenQin
,1,2, SUN LengXue2, ZHENG JiaMin2, CAI CanJun2, WANG Bei2, LI KaiTuo2, PAN TengFei1,2, SHE WenQin ,1,2, CHEN GuiXin1,2, PAN DongMing1,2
,1,2, CHEN GuiXin1,2, PAN DongMing1,2通讯作者:
责任编辑: 赵伶俐
收稿日期:2020-09-18接受日期:2020-12-8
| 基金资助: |
Received:2020-09-18Accepted:2020-12-8
作者简介 About authors
郭志雄,Tel:0591-83789241;E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (2841KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
郭志雄, 孙冷雪, 郑嘉敏, 蔡灿军, 王蓓, 李开拓, 潘腾飞, 佘文琴, 陈桂信, 潘东明. 荔枝果皮BPox的分离纯化及其在果实成熟过程中的表达[J]. 中国农业科学, 2021, 54(16): 3502-3513 doi:10.3864/j.issn.0578-1752.2021.16.012
GUO ZhiXiong, SUN LengXue, ZHENG JiaMin, CAI CanJun, WANG Bei, LI KaiTuo, PAN TengFei, SHE WenQin, CHEN GuiXin, PAN DongMing.
开放科学(资源服务)标识码(OSID)

0 引言
【研究意义】过氧化物酶(Pox)主要催化H2O2与各种氢(电子)供体之间的氧化还原反应,普遍存在于动物、植物和微生物。植物Pox超家族可分为I类、II类和III类等3种不同类型。源于高等植物的第III类Pox(EC 1.11.1.7)是细胞系统的守护者(caretaker),具有广泛的生理功能,通过分泌至液泡或者胞外,参与植物诸如胁迫反应、生长素代谢、木质素合成、细胞壁代谢、自由基清除等各种反应及各个过程[1,2,3]。有研究发现,Pox参与果实的着色、成熟[4,5]。探讨Pox参与果实发育的作用机制,对果实品质形成及其调控研究意义显著。【前人研究进展】植物Pox存在可溶态、结合态以及束缚态等3种不同的形式,束缚态以共价结合方式存在于细胞壁[6]。在荔枝中,由于Pox与采后果皮迅速酶促褐变的密切关联性使其颇受关注[7,8,9,10,11,12,13],但前人对荔枝Pox的研究都集中于可溶态部分,对果皮中的结合态Pox(BPox)只有初步的分析[13,14]。笔者前期的研究发现,前人采用去垢剂(Triton X-100)提取荔枝果皮的BPox很不充分,只能获得其中的个别组分[15]。前期的研究还发现,果皮BPox表现为碱性,组分丰富;动态观测显示,幼果期果皮BPox活性微弱,转色期开始,BPox骤然表现活跃,活性迅速上升直至果实成熟。【本研究切入点】目前的研究已初步表明,BPox与荔枝果实的发育、成熟关系密切,但其特性、作用机制不明。【拟解决的关键问题】本研究以‘乌叶’荔枝果实为材料,开展果皮BPox组分的分离纯化、生化特性分析、质谱鉴定和cDNA克隆研究,分析其在果实转色和成熟过程中的基因表达变化,为进一步揭示其参与荔枝果实的发育、成熟及果实采后衰老、褐变的机制奠定基础。1 材料与方法
试验于2016—2017年在福建农林大学进行。1.1 材料
试验所使用的‘乌叶’荔枝果实采自福建省漳州市,分别于盛花后58、69、76、80和90 d采摘不同发育阶段的果实。果实于当天早晨采摘,立即夹冰运回实验室。每次选取发育一致的果实,取果皮,液氮速冻3—5 min,置于-80℃冰箱保存备用。1.2 BPox组分的分离纯化
荔枝果皮BPox的提取参照王蓓等[15]的方法,做适当调整。取盛花后90 d果实的果皮100 g,经反复提取、离心去除可溶性蛋白(包括可溶性Pox)。在残渣中加入BPox提取缓冲液(0.1 mol∙L-1 Tris- HCl(pH 8.0),20%(v/v)甘油,3.0 mol∙L-1 NaCl,0.1%(v/v)Triton X-100)500 mL,16℃振荡提取1 h;4℃下19 000×g离心20 min。上清液为BPox粗酶液。Streamline Phenyl柱(Amersham Phamarcia产品,2.6 cm×15 cm)以BPox提取缓冲液平衡4—5个柱体积。粗酶液上柱,以2个柱体积平衡缓冲液洗柱,以10 mmol∙L-1 Tris-HCl(pH 8.0),20%甘油,0.1% Triton X-100洗脱获得BPox活性部分并透析除盐48 h。
CM-52柱(Whatman产品,1.6 cm×15 cm)以10 mmol∙L-1 Tris-HCl(pH 8.0),20%甘油缓冲液平衡。将透析后的酶液上柱,平衡缓冲液洗柱,以0—0.3 mol∙L-1 NaCl进行线性梯度洗脱。分部收集,检测活性。分别收集合并两个主要活性峰,并命名为BPox-2和BPox-3,用于Phenyl疏水层析纯化。
Phenyl Sepharose HP柱(Amersham Phamarcia产品,1.6 cm×15 cm)以40 mmol∙L-1 Tris-HCl(pH 8.0),25%甘油,1.1 mol∙L-1 (NH4)2SO4缓冲液平衡4—5个柱体积。将BPox-2和BPox-3对平衡液按1﹕20稀释,分别上柱。洗柱,以1.1—0 mol∙L-1 (NH4)2SO4逆梯度洗脱。分别检测活性,合并活性峰管。采用Ultra 4(Millipore产品,10K)进行离心超滤,浓缩至400—500 μL,用于分子筛色谱。
Superdex 200柱(Amersham Phamarcia产品,1.6 cm×66 cm)以50 mmol∙L-1 Tris-HCl(pH 8.0),100 mmol∙L-1 NaCl,10%甘油缓冲液平衡。样品分别过柱、洗脱。检测活性,合并活性峰管,超滤浓缩获得纯化的BPox。调整甘油终浓度至20%,将纯化的BPox-2和BPox-3置于-40℃保存备用。
1.3 蛋白质定量测定和电泳
采用BCA微量法进行蛋白质定量测定,BCA试剂盒购于上海生工生物工程有限公司。按试剂盒说明书进行定量操作。非变性阴极凝胶电泳及酶染显色按照王蓓等[15]的方法。SDS-PAGE按GUO等[16]的方法。分子量标准采用Takara公司的Protein Molecular Weight Marker(High)。按BLUM等[17]的方法进行银盐染色。
1.4 BPox的酶学性质分析
pH在3.0—6.0采用磷酸-柠檬酸缓冲液,pH 6.0—8.0采用磷酸缓冲液,分别测定BPox的最适反应pH,重复3次。以50 mmol∙L-1磷酸缓冲液(pH 6.0)为反应缓冲系,在15—70℃条件下分别测定BPox的最适催化反应温度;测定BPox对不同酚类底物的特异性;测定不同金属离子及各抑制剂对BPox相对活性的影响;分别测定BPox催化愈创木酚和(-)-表儿茶素的Km值和Vmax。每个反应均重复3次,规定470 nm条件下每min变化0.001为1个酶活力单位(U)。
1.5 BPox的MALDI MS/MS分析鉴定
将SDS-PAGE后银盐染色显色的BPox条带用干净的刀片切出。按李开拓[18]的方法,将凝胶切成小块,脱色、干燥;Trypsin酶解;肽段经提取、干燥和溶解;之后采用AB SCIEX 5800质谱仪进行MALDI-TOF/ TOF分析。应用Mascot工具对荔枝转录组数据进行本地化检索匹配鉴定。1.6 BPox的cDNA克隆
按王蓓[19]的方法提取荔枝果皮总RNA。采用Invitrogen公司的SuperScriptTM First-Strand Synthesis System合成第一链cDNA。根据质谱的匹配结果,设计开放阅读框(ORF)扩增引物,BPx_F:5′-ATGGCTT CCACTAGTACAATCCAGT-3′,BPx_R:5′-TCAGTTG ACAGCGCTGCAAACGCT-3′;以cDNA为模板,对上述引物进行PCR扩增,产物与T载体连接,转化、鉴定并进行测序。1.7 BPox的活性变化分析
分别取不同发育时期的果皮,按王蓓等[15]的方法进行BPox的提取和活性测定,3次重复。1.8 BPox的转录水平分析
应用荧光定量PCR分析荔枝果实转色与成熟过程果皮BPox的转录水平变化。分别提取不同发育时期果皮的总RNA,采用TaKaRa公司的PrimeScript™ RT reagent Kit with gDNA Eraser进行cDNA合成;应用Primer Premier 5.0设计BPox的qPCR引物,分别为:Bpx_qF:5′-CGAGATGGAGTTGTCTTGCTTGGAG -3′和Bpx_qR:5′-TGGTTTCGTTGTAGATGCGGTTGC -3′,选择Actin为内参基因,设计一对引物,分别为:Act_qF:5′-ACTGGTGTGATGGTTGGTATGG-3′和Act_qR:5′-GTTCAATCGGGTATTTCAAGGTAAG-3′,采用Genestar公司的SYBR试剂,运用Jena公司的PCR仪QTOWER3进行扩增和溶解曲线分析。将盛花后58 d的表达量定为1,按LIVAK和SCHMITTGEN[20]提出的2-ΔΔCt法计算相对表达量并进行统计分析。1.9 数据统计分析
运用Excel 2010进行方差分析,采用LSD法进行数据的多重比较(P<0.01)。2 结果
2.1 BPox的柱层析分离纯化
将制得的荔枝果皮BPox粗酶液过Streamline Phenyl柱,采用一步洗脱,得到单一洗脱活性峰,洗脱峰具一定的拖尾特性(数据未显示)。合并活性部分共得酶液约50 mL,使BPox得以高效浓缩。膜透析浓缩后的酶液过阳离子交换纤维素CM-52柱,以0—0.3 mol∙L-1 NaCl 进行线性梯度洗脱。结果显示,荔枝果皮的BPox可进一步分辨为3个主要的活性峰;其中,第1峰活性较低且与第2峰有粘连,第2个活性峰最大,第3活性部分洗脱出峰稍宽(图1-A)。将3个活性峰管分别收集合并。非变性阴极电泳酶染结果显示,3个活性峰均表现为双谱带型,第2和第3活性峰分别为荔枝果皮BPox最主要的两个组分(图1-B)。活性弱的第1组分峰BPox-1和笔者之前对荔枝果皮BPox进行分离纯化的初步研究结果吻合[21]。将两个主要活性组分分别命名为BPox-2和BPox-3,进行进一步的柱层析纯化。
图1
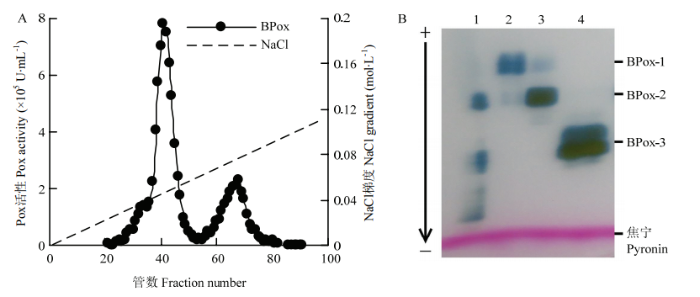 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1荔枝果皮BPox的CM-52阳离子交换柱层析分离(A)及其BPox各组分阴极凝胶电泳酶染结果(B)
1:BPox粗酶液;2:BPox-1;3:BPox-2;4:BPox-3 1: crude extract of BPox; 2: BPox-1; 3, BPox-2; 4: BPox-3
Fig. 1Cationic ion exchange chromatography profile (A), the native cathode gel electrophoresis and activity staining pattern (B) of BPox eluted from the CM-52 cellulose column
BPox-2和BPox-3在Phenyl Sepharose柱疏水层析及其之后的Superdex 200分子筛色谱均表现为单一的洗脱活性峰,这表明各自组分内的酶谱带性质极为接近而不能相互分离。分子筛色谱的结果显示BPox-2和BPox-3的分子量均为30 kD(图2)。
图2
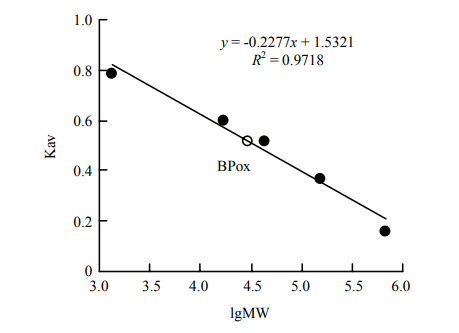 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2Superdex 200凝胶过滤层析测定BPox-2/3分子量
分子量标准由甲状腺球蛋白(670 kD)、g-球蛋白(158 kD)、卵清蛋白(44 kD)、肌红蛋白(17 kD)和维生素B12(1.36 kD)组成
Fig. 2Molecular weight estimation of BPox-2/3 by gel filtration on Superdex 200
The standard mixture (Bio-Rad) contains thyroglobulin (670 kD), g-globulin (158 kD), ovalbumin (44 kD), myoglobin (17 kD) and vitamin B12 (1.36 kD)
10%—15%的线性梯度SDS凝胶电泳后的银染结果显示,BPox-2和BPox-3获得了纯化,其中未显示可见的杂蛋白;两组分均显示双蛋白条带,对应于组分内的双酶谱带。其表观分子量极其接近,约为34 kD(图3)。说明荔枝果皮BPox的这两个组分均为单体蛋白。
图3
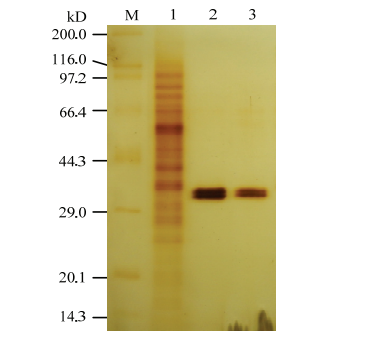 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3荔枝果皮BPox-2和BPox-3的SDS-PAGE图谱
M:Marker;1:荔枝果皮总蛋白[22];2:BPox-2;3:BPox-3
Fig. 3SDS-PAGE pattern of the BPox-2 and BPox-3 purified from litchi pericarp
M: Premixed protein marker (broad, Takara); 1: Total protein of litchi pericarp extracted according to the method described by LI et al[22]; 2, 3: Purified BPox-2 and BPox-3, respectively
2.2 BPox-2/3的主要酶学特性
2.2.1 最适反应pH 不同pH条件下的活性测定结果显示,BPox-2和BPox-3催化愈创木酚反应的最适pH均为6.0。两者在不同pH下的活性变化趋势十分相似,在pH 5.0—7.0内,能维持较高的活性;pH<5.5或者pH>7.0,二者的活性下降很快(图4)。图4
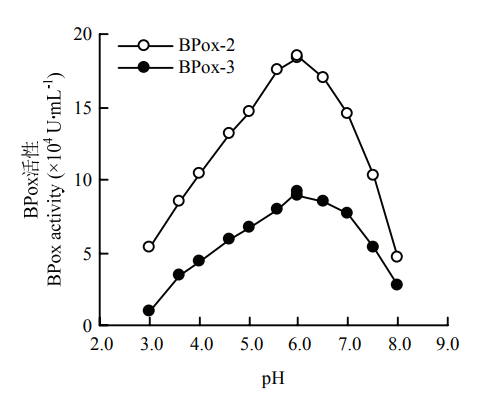 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4不同pH对BPox-2和BPox-3活性的影响
Fig. 4Effect of pH on the activities of BPox-2 and BPox-3
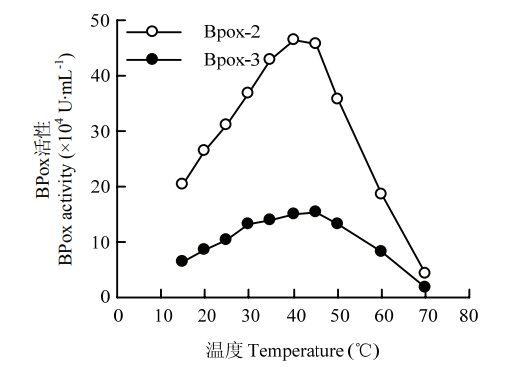
2.2.2 最适反应温度 不同温度下的活性测定结果显示,两组分对不同温度的反应趋势一致,最适温度为40℃。温度超过50℃,其活性下降明显加快(图5)。
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5不同温度对BPox-2和BPox-3活性的影响
Fig. 5Effect of temperature on the activities of BPox-2 and BPox-3
2.2.3 对不同底物的特异性 底物特异性分析结果表明,荔枝果皮BPox对不同酚类底物表现出不同的活性,但两组分对不同底物的比活力大小变化趋势基本一致,愈创木酚和(-)-表儿茶素为其最适底物,反应活性最大(表1)。BPox对其余底物的催化反应活性各有不同,其中,BPox-2对4-甲基邻苯二酚、焦性没食子酸、邻苯二酚的催化活性仅分别为愈创木酚的14.52%、10.12%和7.21%,而BPox-3分别为愈创木酚的12.53%、11.50%和10.98%;对没食子酸、绿原酸、4-甲氧基酚和对苯二酚等底物的催化反应活性为弱;对间苯二酚、藜芦醇、苯甲醇等底物未显示活性。
Table 1
表1
表1BPox-2/3对不同底物的比活力
Table 1
| 底物 Substrate | 波长 Wavelength (nm) | BPox比活力 Specific activity of BPox (×105 U∙mg-1) | |
|---|---|---|---|
| BPox-2 | BPox-3 | ||
| 愈创木酚 Guaiacol | 470 | 116.03±1.41 | 31.38±0.45 |
| (-)-表儿茶素 (-)-Epicatechin | 440 | 61.66±1.87 | 62.71±0.40 |
| 4-甲基邻苯二酚4-Methylcatechol | 420 | 16.85±0.21 | 3.93±0.07 |
| 焦性没食子酸 Pyrogallol | 420 | 11.75±0.13 | 3.61±0.17 |
| 邻苯二酚 Catechol | 420 | 8.37±0.19 | 3.45±0.34 |
| 没食子酸 Gallic acid | 420 | 3.82±0.09 | 0.88±0.16 |
| 氯原酸 Chlorogenic acid | 420 | 3.09±0.29 | 0.84±0.01 |
| 4-甲氧基酚 4-Methoxyphenol | 420 | 0.88±0.09 | 0.53±0.18 |
| 对苯二酚 Hydroquinone | 420 | 0.44±0.09 | 0.48±0.07 |
| 间苯二酚 m-Dihydroxybenzene | 420 | 0 | 0 |
| 藜芦醇 Veratryl alcohol | 420 | 0 | 0 |
| 苯甲醇 Benzylalcohol | 310 | 0 | 0 |
新窗口打开|下载CSV
2.2.4 金属离子及抑制剂的影响 以愈创木酚为底物,测定金属离子及抑制剂对BPox活性的影响。结果显示,金属离子Mn2+、Ca2+、Fe3+对BPox-2、BPox-3的抑制作用较微弱,在Cu2+的作用下,BPox表现的相对活性有所增加;螯合剂EDTA对其活性影响弱;L-半胱氨酸强烈抑制两组分的活性。终浓度为0.1 mmol∙L-1时,其相对活性只残留约20%;DTT和ASA几乎完全抑制两组分的活性(表2)。
Table 2
表2
表2金属离子及抑制剂对BPox-2/3活性的影响
Table 2
| 抑制剂 Inhibitor | 终浓度 Concentration (mmol∙L-1) | BPox-2相对活性 Relative activity of BPox-2 | BPox-3相对活性 Relative activity of BPox-3 |
|---|---|---|---|
| CuSO4 | 0 | 1 | 1 |
| 0.1 | 1.05±0.016 | 1.18±0.008 | |
| 1 | 1.05±0.003 | 1.06±0.01 | |
| MnSO4 | 0.1 | 0.92±0.009 | 0.87±0.019 |
| 1 | 0.74±0.013 | 0.78±0.01 | |
| CaCl2 | 0.1 | 0.98±0.010 | 0.99±0.002 |
| 1 | 0.88±0.011 | 0.89±0.03 | |
| FeCl3 | 0.1 | 0.92±0.009 | 0.91±0.020 |
| 1 | 0.77±0.026 | 0.85±0.03 | |
| EDTA | 0.1 | 0.93±0.007 | 0.95±0.005 |
| 1 | 0.89±0.06 | 0.93±0.03 | |
| L-Cysteine | 0.1 | 0.15±0.005 | 0.22±0.001 |
| 1 | 0.019±0 | 0.058±0 | |
| DTT | 0.1 | 0.03±0.003 | 0 |
| 1 | 0 | 0 | |
| ASA | 0.1 | 0 | 0 |
| 1 | 0 | 0 |
新窗口打开|下载CSV
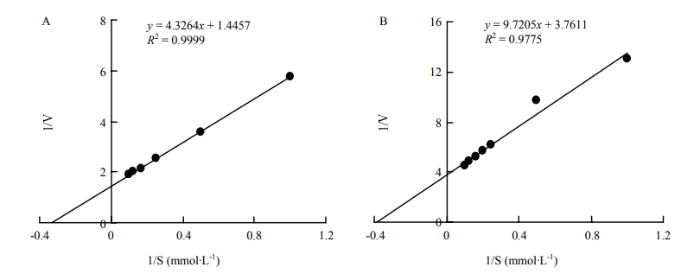
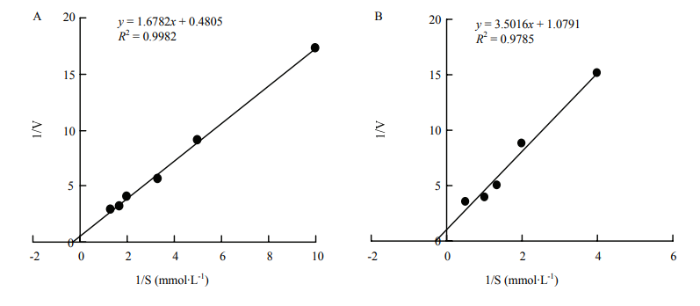
2.2.5 催化不同底物反应的Km值和Vmax 根据双倒数法进行米氏常数的测定,结果显示BPox-2和BPox-3对愈创木酚的Km值分别为2.97和2.58 mmol∙L-1,二者对愈创木酚的亲和性差异不大;但BPox-2催化愈创木酚的最大反应初速度Vmax为38.60×106 U∙mg-1,显著大于BPox-3的19.85×106 U∙mg-1(图6)。同样,BPox-2和BPox-3对(-)-表儿茶素的Km值分别为3.49和3.24 mmol∙L-1,而其Vmax分别为38.72×106和23.06×106 U∙mg-1(图7)。表明BPox-2和BPox-3对底物的亲和性基本一致,而BPox-2显示出更强的催化效率(Vmax/Km)。
图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6BPox-2(A)和 BPox-3(B)催化愈创木酚反应的双倒数曲线
Fig. 6Double reciprocal plots of guaiacol catalyzed by BPox-2(A) and BPox-3(B), respectively
图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7BPox-2(A)和 BPox-3(B)催化(-)-表儿茶素反应的双倒数曲线
Fig. 7Double reciprocal plots of (-)-epicatechin catalyzed by BPox-2(A) and BPox-3(B), respectively
2.3 BPox-2/3的质谱鉴定
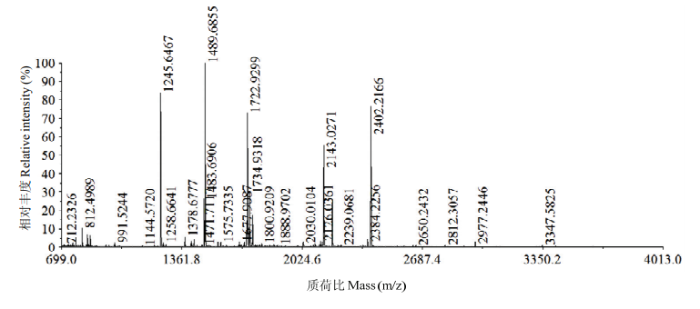
将荔枝果皮BPox-2和BPox-3两个组分内部的双谱带依SDS-PAGE分离,按从大至小,分别命名为BPox-2a、BPox-2b和BPox-3a、BPox-3b。分别对其胰酶酶解肽段进行质谱分析,结果显示,组分间的肽质量指纹(PMF)差异较大(图8、9);而组分内两个谱带的PMF高度相似,主要肽段一致,只是相对丰度存在一定的差别(数据未显示)。图8
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8BPox-2a的MALDI-TOF/MS的肽质量指纹图谱
Fig. 8Peptide mass fingerprinting of BPox-2a from MALDI-TOF MS
图9
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图9BPox-3a的MALDI-TOF/MS的肽质量指纹图谱
Fig. 9Peptide mass fingerprinting of BPox-3a from MALDI-TOF/MS
对4个蛋白组分的部分肽段进行进一步的MS/ MS分析,并结合PMF数据,对荔枝转录组数据[23]进行检索匹配。结果显示,1个m/z为2 402.2的肽段为BPox-2和BPox-3所共有,其MS/MS的分析结果表明其为转录组中Unigene 0021422所编码(编码产物GenBank ID:696949335),而MS/MS解析的另外肽段同样匹配于该转录本(表3)。上述的质谱分析结果表明,荔枝果皮BPox-2和BPox-3为同一基因(后命名为BPox2)的编码产物因翻译后加工修饰的显著不同而形成明显差异的同工酶组分,而一些翻译后修饰的细微区别,导致组分内形成不同的谱带。
Table 3
表3
表3MALDI MS/MS解析BPox-2/3的肽段序列
Table 3
| BPox | 质荷比 m/z | 序列号 Protein ID | 肽段序列 Peptide fragment sequence |
|---|---|---|---|
| BPox-2a | 964.3965 | gi|696949335 | FDNSYYR |
| 1575.7296 | MGNISPLTGTNGEIR (Oxidation M) | ||
| 2402.1899 | TASLSAANSDLPSPFADLATLIAR | ||
| BPox-2b | 964.4012 | gi|696949335 | FDNSYYR |
| 2402.2012 | TASLSAANSDLPSPFADLATLIAR | ||
| BPox-3a | 2402.2175 | gi|696949335 | TASLSAANSDLPSPFADLATLIAR |
| BPox-3b | 1575.7279 | gi|696949335 | MGNISPLTGTNGEIR (Oxidation M) |
| 2402.2183 | TASLSAANSDLPSPFADLATLIAR |
新窗口打开|下载CSV
2.4 BPox2的cDNA克隆
BPox-2/3的MS/MS结果显示,其匹配的cDNA包含一个完整的开放阅读框(ORF)。基于此,设计扩增BPox2的ORF引物,提取荔枝果皮总RNA,以其逆转录的cDNA为模板,通过PCR扩增获得1个大小约1 000 bp的特异产物。克隆、测序的结果表明,该ORF大小为960 bp,共编码319个氨基酸,与BPox-2/3串联质谱解析的结果完全吻合(图10)。应用SignalP-5.0(
图10
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图10荔枝果皮BPox的cDNA及其编码的氨基酸序列
划线部分表示MALDI TOF MS/MS鉴定的BPox组分的共有肽段序列;标记为绿色和蓝色的部分分别表示为编码的信号肽和潜在的糖基化位点
Fig. 10cDNA sequence and its deduced amino acid sequence of BPox in litchi pericarp
The underlined indicates the peptide fragment digested from litchi BPox isomers of which the sequence was identified by MALDI TOF MS/MS. A Putative signal peptide (green) and a potential glycosylation site (blue) were signed as well, respectively
2.5 荔枝转色过程中BPox的活性变化及其基因表达
盛花后58 d,‘乌叶’幼果果皮BPox的活性表现弱,为0.2167×104 U∙g-1 FW。至盛花后76 d,果实开始转色(图11-a),其果皮BPox活性显著上升,达9.95×104 U∙g-1 FW(P<0.01);之后,果皮BPox的活性一直保持快速上升趋势,直至花后90 d果实成熟(图11-b)。而qPCR的结果显示,果实转色前,BPox2的转录水平很低;伴随果实着色开始,其表达水平达到高峰,为盛花后69 d的60.56倍,之后回落;盛花后80—90 d,果皮BPox2的表达仍呈显著上升趋势(图11-c)。图11
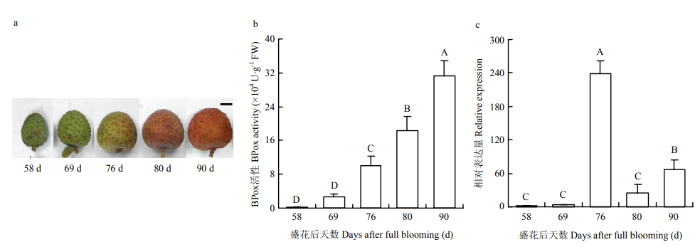 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图11荔枝果实着色和成熟过程中外观(a)、BPox活性(b)和BPox2表达(c)的变化
不同大写字母表示极显著差异(P<0.01) Different capital letters indicate significant difference at P<0.01
Fig. 11Changes of appearance (a), BPox activity (b) and BPox2 gene expression (c) in litchi pericarp during the fruit coloration and maturation
3 讨论
通过提取和多步骤的柱层析分离,纯化获得荔枝果皮BPox-2和BPox-3。对二者的生化特性分析表明,其最适pH、最适反应温度、底物特异性等总体表现一致,其特性和荔枝可溶性Pox(SPox)组分以及其他来源的Pox表现比较接近[10,19,24]。对比发现,荔枝果皮BPox对愈创木酚和(-)-表儿茶素的催化效率显著高于SPox组分[19]。(-)-表儿茶素是荔枝果皮重要的多酚类物质,是采后果皮PPO酶促褐变的直接底物[25,26]。初步研究表明,采后BPox活性变化显著,与荔枝果皮褐变关系紧密[21]。BPox与荔枝采后果皮内源多酚类物质代谢的关系,有待进一步的研究揭示。一般而言,植物Pox为糖基化蛋白[6]。本研究应用MALDI串联质谱对BPox-2和BPox-3进行蛋白鉴定。以此为基础,克隆获得了荔枝BPox2的cDNA。分析显示,cDNA编码的多肽仅含1个潜在的糖基化位点,其大小与纯化得到的酶蛋白分子很接近。这说明,荔枝BPox肽链上的糖基及其他非氨基酸成分所占比重低,侧链修饰程度轻。对比笔者之前的研究发现,荔枝SPox cDNA编码的氨基酸序列(GenBank ID:205326621)则多达9个潜在的糖基化位点,而理论编码产物与纯化得到的2个酶组分大小的比值分别为0.802和0.712,显示其翻译后的糖基化及其他修饰程度大大高于前者[19]。
此外,根据C端液泡分选信号(vacuolar sorting signals,VSS)序列的有无,植物分泌型Pox分为液泡型和胞外型[27]。研究显示,只有液泡型Pox具C端延伸(C-terminal extension,CE)结构[1,2];尽管保守性不高,CE具VSS序列[27,28,29]。通过与定位于液泡的辣根Pox C1a、大豆Pox等以及与荔枝SPox cDNA编码的多肽进行序列比对,显示BPox2 cDNA编码产物短缩的C端缺乏VSS序列,预示果皮BPox-2/3定位于胞外。这和荔枝BPox呈离子结合态,可能结合于细胞壁或质膜系统相吻合。关于其结构特点及与其功能的关联性如何有待进一步的研究确认。
动态观测的结果表明,荔枝果实在转色前,其果皮BPox很不活跃,而伴随着果皮转色,BPox的活性迅速启动升高直至果实成熟,这和笔者前期的研究结果一致[15]。而qPCR的结果也显示,在果皮转色期开始,BPox2表达水平大幅上升形成峰值,和BPox的活性变化趋势一致;但之后,其表达水平回落;伴随果实成熟进程,其表达水平又显著上升,与活性变化一致。综上,果皮BPox的变化受转录水平调控,其变化与果实成熟进程关系密切。在草莓中发现一个编码碱性Pox的基因FaPRX27,研究显示该基因主要参与果实成熟进程中木质素的生物合成,FaPRX27的高表达会竞争多酚类物质的代谢前体,改变其代谢流的走向,从而抑制果实花色苷的生物合成和累积[4]。而其作为果实木质素合成的关键基因,对于提高果实质地具有重要作用[5]。在离体培养的李愈伤组织中也发现了1个与花色苷累积特异相关的结合态Pox组分[30]。花色苷是荔枝果实成熟果皮着色最重要的色素类物质[13,31-32],关于BPox的亚细胞定位,及其与果皮木质素代谢、花色苷积累的关系等,是进一步需要研究的重点问题。
4 结论
从成熟荔枝果皮分离纯化获得离子结合态过氧化物酶最主要的2个组分BPox-2和BPox-3。对其分析结果显示,其最适反应pH、最适反应温度、底物特异性等酶学特性与荔枝果皮可溶性Pox(SPox)组分相似,但BPox对愈创木酚和(-)-表儿茶素的催化效率显著高于SPox。MALDI串联质谱分析结果表明,BPox-2与BPox-3应同为BPox2所编码,因翻译后修饰差异而形成同工酶。cDNA克隆和序列分析发现,其编码的多肽链大小与纯化得到的BPox接近,结果表明,相较于SPox,果皮BPox的翻译后修饰程度较低。对果皮BPox活性和基因转录水平的动态变化分析显示,BPox2参与荔枝果皮的着色和成熟进程,其活性受转录水平调控。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1093/pcp/pce061URL [本文引用: 2]
DOI:10.1093/jxb/ern318URL [本文引用: 2]
DOI:10.1016/j.plantsci.2012.09.018URL [本文引用: 1]
DOI:10.1104/pp.113.222778URL [本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.foodchem.2004.02.004URL [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
DOI:10.1016/j.foodchem.2004.03.023URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/s11947-011-0762-9URL [本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 5]
[本文引用: 5]
DOI:10.1016/j.plaphy.2012.05.021URL [本文引用: 1]
DOI:10.1002/(ISSN)1522-2683URL [本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 4]
[D].
[本文引用: 4]
DOI:10.1006/meth.2001.1262URL [本文引用: 1]
[D].
[本文引用: 2]
[D].
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
DOI:10.1186/s12864-015-1433-4URL [本文引用: 1]
DOI:10.1016/j.foodchem.2003.07.013URL [本文引用: 1]
DOI:10.1016/j.foodres.2006.05.001URL [本文引用: 1]
DOI:10.1021/jf070964aURL [本文引用: 1]
DOI:10.1093/pcp/pcq205URL [本文引用: 2]
DOI:10.1007/s00253-003-1273-zURL [本文引用: 1]
DOI:10.1007/s00299-010-0884-yURL [本文引用: 1]
DOI:10.1007/s00299-002-0527-zURL [本文引用: 1]
DOI:10.1021/jf104432rURL [本文引用: 1]
[本文引用: 1]
