 ,1, 刘迪1, 高歌1, 祝朋芳1,2, 毛洪玉
,1, 刘迪1, 高歌1, 祝朋芳1,2, 毛洪玉 ,1,2
,1,2CmWRKY15-1 Regulates Resistance of Chrysanthemum White Rust Through Salicylic Acid Signaling Pathway
BI MengMeng ,1, LIU Di1, GAO Ge1, ZHU PengFang1,2, MAO HongYu
,1, LIU Di1, GAO Ge1, ZHU PengFang1,2, MAO HongYu ,1,2
,1,2通讯作者:
责任编辑: 赵伶俐
收稿日期:2020-05-4接受日期:2020-07-13网络出版日期:2021-02-01
| 基金资助: |
Received:2020-05-4Accepted:2020-07-13Online:2021-02-01
作者简介 About authors
毕蒙蒙,E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (1421KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
毕蒙蒙, 刘迪, 高歌, 祝朋芳, 毛洪玉. CmWRKY15-1通过水杨酸信号通路调控菊花白色锈病抗性[J]. 中国农业科学, 2021, 54(3): 619-628 doi:10.3864/j.issn.0578-1752.2021.03.015
BI MengMeng, LIU Di, GAO Ge, ZHU PengFang, MAO HongYu.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】菊花(Chrysanthemum morifolium)是菊科菊属的多年生草本花卉,是我国传统名花,具有较高的观赏与经济价值。菊花白色锈病由堀氏菊柄锈菌(Puccinia horiana Henn.)引起,属冬孢菌纲、锈菌目,主寄生菌,是活体营养型病原菌,专性寄生于菊属(Chrysanthemum spp.)植物,被列为世界性检疫病害[1,2]。该病主要集中于受害植株的叶和茎等观赏部位,叶背出现坏死斑,似蟾蜍皮状,叶片褪绿、卷曲,后逐渐发展为变色、腐烂、干枯,甚至全株死亡[3]。菊花白色锈病在我国菊花产业里传播蔓延,感病品种遭受毁灭性伤害,降低了菊花产品的观赏价值与商业品质,严重影响了菊花在国际市场上的进出口生产。同时多数的生产基地由于加大了控制该病的人力与物力,而导致成本费用增加。目前基于分子手段发掘菊花WRKY类抗白色锈病相关基因的功能研究较少。因此,亟待获得有潜力的抗性基因进行抗白色锈病分子育种。【前人研究进展】WRKY转录因子作为植物抗病反应中参与信号传递途径及基因表达过程的重要调控基因,在挖掘目标基因与基因功能分析的研究领域应用广泛[4]。大量研究证明,WRKY参与了抵抗拟南芥白粉病、番茄叶卷病及灰霉病、黄瓜霜霉病、苹果轮纹病、棉花大丽轮枝菌、柑橘溃疡病、芍药细极链格孢菌[5,6,7,8,9,10,11,12]等多种植物病害。在菊花中,CmWRKY48可有效抑制蚜虫种群的增长,增强菊花对蚜虫的抗性[13],CmWRKY15增加了对黑斑病的敏感性[14]。WRKY转录因子可以调控如脱落酸(ABA)、水杨酸(SA)、茉莉酸/乙烯(JA/ET)、丝裂原活化蛋白激酶(MAPK)等多种植物生物过程信号网络[15]。水杨酸作为一种信号分子,在植物体内可转移并诱导植物抗性,植物在病原物侵染后,首先识别该应激信号,启动植物体内的防御反应,促进SA的合成,与此同时激活下游基因的表达,通过信号传递诱导植物产生病程相关蛋白(PRs蛋白)调节相关保护酶如超氧化物歧化酶(SOD)、苯丙氨酸解氨酶(PAL)等清除细胞活性氧,使植物局部获得抗性,进而阻止病原物的进一步入侵[16,17,18]。欧芹WRKY[19]、辣椒CaWRKY[20]、烟草NtWRKY12[21]、毛果杨PtrWRKY73[22]等研究中的WRKY转录因子能调节SA介导的信号通路中PRs类基因的表达,从而改变植物的抗病性,表明WRKY转录因子可参与SA途径以调节植物的防御反应。笔者课题组前期对菊花感、抗品种进行转录组测序[23],获得了差异表达基因CmWRKY15-1,菊花‘黄莺’接种白色锈病病原菌后,CmWRKY15-1的表达量上调,且该基因受SA、茉莉酸甲酯(MeJA)、乙烯(ETH)等激素诱导表达,说明该基因与菊花抗病性相关,且抗病过程受激素信号介导。通常,SA信号是针对生物活体型营养型病原体的抗性反应,例如新番茄粉孢菌(Oidium neolycopersici)和经霜霉病菌(Hyaloperonospora parasitica)等活体营养型病菌[24],而JA/ET信号则是针对坏死病原体[25]。【本研究切入点】菊花白色锈病病原菌(Puccinia horiana Henn.)是由崛氏菊柄锈菌引起的真菌,为活体营养型病菌,因此,推测菊花对抗此病原菌可能是通过SA介导的抗病通路。近年来,在研究响应菊花白色锈病侵染的转录组方面普遍开展了研究,但目前尚无报道明确指出某个基因对菊花白色锈病有调控作用,有待深入研究。【拟解决的关键问题】本研究以菊花白色锈病抗病品种‘黄莺’为试验材料,利用前期克隆获得的CmWRKY15-1序列,构建植物表达载体与干扰载体转化菊花,通过基因超表达与沉默的方法验证CmWRKY15-1在菊花抗白色锈病中的功能,为进一步研究其抗白色锈病的分子机理提供借鉴。1 材料与方法
试验于2018—2020年在沈阳农业大学林学院进行。1.1 材料与菌株
菊花白色锈病抗病品种‘黄莺’组培苗取自沈阳农业大学林学院组培实验室。超量表达载体pBI121-CmWRKY15-1、干扰载体RNAi-CmWRKY15-1由实验室前期构建。1.2 pBI121-CmWRKY15-1与RNAi-CmWRKY15-1载体的转化
RNAi-CmWRKY15-1载体是选择RNAi质粒pHANNIBAL中的PDK内含子,形成发夹结构,并最终构建到pBI121质粒中进行后续转化。将含有重组质粒pBI121-CmWRKY15-1与RNAi-CmWRKY15-1的农杆菌菌液从-80℃超低温冰箱中取出,进行菌株的活化,并扩大培养至OD600=0.5—0.6,作侵染液备用,并使用农杆菌介导法转化[26,27]。取苗龄为4周左右,生长良好的‘黄莺’组培苗,切成0.5 cm×0.5 cm的方形叶盘,预培养(MS+0.5 mg·L-1 6-BA+0.3 mg·L-1 NAA)2 d,菌液侵染时浓度OD600=0.5—0.6,稀释50倍,侵染7 min,共培养(MS+0.5 mg·L-1 6-BA+0.3 mg·L-1 NAA)2 d,延迟培养(MS+0.5 mg·L-1 6-BA+0.3 mg·L-1 NAA+200 mg·L-1 Cef)2 d后,转接至筛选培养基(MS +0.5 mg·L-1 6-BA+0.3 mg·L-1 NAA+200 mg·L-1 Cef+20 mg·L-1 Kan)上,每15 d更换一次培养基,直至产生抗性芽后接种于生根筛选培养基(MS+20 mg·L-1 Kan)中。
1.3 转基因植株的鉴定
取经过抗生素Kan筛选的阳性植株幼嫩叶片,提取DNA。根据pBI121载体上35S启动子特异引物nptⅡ抗性基因设计鉴定引物(表1),进行PCR扩增,检测是否能够得到目的条带。同时试剂盒提取RNA,并反转录成cDNA,作RT-PCR分析。以菊花Actin为内参基因,以已鉴定阳性条带的植株cDNA为模板,设计定量检测引物CmWRKY15-1-F/R(表1),通过qRT-PCR法测定CmWRKY15-1的表达,每个样品设有3次重复,采用2-ΔΔCT法分析结果,同时采用半定量方法加以辅证。Table 1
表1
表1定量所需引物
Table 1
| 引物Primer | 序列Sequence(5′-3′) |
|---|---|
| Npt Ⅱ F | GGCTATGACTGGGCACAACA |
| Npt Ⅱ R | GATACCGTAAAGCACGAGGAA |
| Actin-F | TCCGTTGCCCTGAGGTTCT |
| Actin-R | GATTTCCTTGCTCATCCTGTCA |
| CmWRKY15-1-F | TGGTGGCTGCATCACAT |
| CmWRKY15-1-R | GGAAGAATCAGTGCTAATACATTAA |
新窗口打开|下载CSV
1.4 白色锈病病原菌的接种
选取6—8叶期的长势良好的组培苗OE-9系、RNAi-4系及WT,无菌水中平衡2周,移栽于口径21 cm花盆中,放置组培室中培养2周。制备菌液、接种方法及接种后的植株培养条件参考文献[28]。选取植株OE-9系、RNAi-4系顶端往下第2—5位叶进行接种处理,每个株系处理15株,接种两周左右,观察RNAi-4系及WT植株发病情况,并拍照记录。分别对OE-9系、RNAi-4系及WT于接种的0、16、24、40、48、64和72 h取样,设置3个重复。
1.5 RNAi-4系植株接种白色锈病病原菌后病情统计
本研究参照ZHU等[29]和WANG等[30]对菊花白色锈病的病害分级标准与菊花白锈病抗性鉴定标准,计算RNAi-4系植株病情指数(disease severity index,DSI)以及对该植株表型进行抗性鉴定。1.6 内源SA含量测定
选择OE-9系及WT系接种0和24 h的叶片进行样本取材,选取植株顶端往下第3—4位叶,约30—100 mg。水杨酸测定试剂盒采用双抗体一步夹心法酶联免疫吸附试验(ELISA)[31]。激素结果(ng?g-1)=5*C*M/m/1000。式中,C:由标准曲线算得样品含量浓度(pmol?L-1);M:物质平均相对分子质量(g?mol-1);m:样品鲜重(g)。1.7 SA合成关键基因ICS1、PAL的表达分析
以采取的样本cDNA为模板,使用ICS1、PAL荧光定量引物(表2),对其表达量进行qRT-PCR测定。Table 2
表2
表2定量所需引物
Table 2
| 引物 Primer | 序列 Sequence(5′-3′) |
|---|---|
| ICS1-F | TCCCTACTGAAGAGGCACGG |
| ICS1-R | CCAACAGCGGGTTCACTCTC |
| PAL-F | ATGGCACCGAAGCAAGTCACAC |
| PAL-R | GATACCCGAGTAACCCTGGAGGAG |
| NPR1-F | TGTCGAGAAGGATGGAAAGCC |
| NPR1-R | GGAGGCACCCATCATCAACA |
| PR1-F | CTCAACCAAAAGGAATAGTCGG |
| PR1-R | CCCTGCCAGTTTACGCTGTA |
| PR2-F | GGCAATGGTGGTGTTGGAAC |
| PR2-R | CTTCCTCCGTCAGCAGAAGG |
| PR5-F | CCAATGGAGTTTAGCCCCGT |
| PR5-R | GTCCACAACTACCACGCTCA |
新窗口打开|下载CSV
1.8 病程相关基因NPR1、PR1、PR2、PR5等的表达分析
使用NPR1、PR1、PR2、PR5荧光定量引物(表2),对其表达量进行qRT-PCR测定。1.9 数据处理与统计分析
试验数据采用Excel 2013进行整理,使用SPSS Statistics统计分析软件进一步检验分析。2 结果
2.1 超表达植株与沉默植株的获得
经过Kan筛选分别获得18株和19株抗性植株,提取抗性苗叶片DNA,以pBI121质粒上35S启动子特异引物npt Ⅱ抗性标记基因序列设计引物,进行PCR鉴定,电泳(图1-a,1-b)结果显示,有8株转pBI121-CmWRKY15-1与RNAi-CmWRKY15-1载体的抗性植株均在678 bp处扩增出与阳性对照一致的条带,而阴性对照未见对应条带,表明质粒已基本整合到菊花‘黄莺’组培苗基因组中。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1抗性苗的PCR检测
a:pBI121-CmWRKY15-1 PCR电泳,1—18:pBI121-CmWRKY15-1抗性植株。b:RNAi-CmWRKY15-1 PCR电泳,1-19:RNAi-CmWRKY15-1抗性植株。M:DNA marker;W:野生型;P:阳性对照。下同
Fig. 1PCR identification of potential transgenic plants
a: PCR of pBI121-CmWRKY15-1; 1-18: Potential transgenic plants of pBI121-CmWRKY15-1. b: PCR of RNAi-CmWRKY15-1; 1-19: Potential transgenic plants of RNAi-CmWRKY15-1. M: DNA marker; W: Negative control; P: Positive control. The same as below
将PCR鉴定出的阳性植株进行半定量检测,结果表明,CmWRKY15-1在转基因株系中均有不同程度的表达,且均高于野生型植株(图2-a)。与未转化植株WT相比,各干扰植株中CmWRKY15-1的表达水平均有不同程度的下降,下调幅度达46%—79%,表明干扰表达载体成功在干扰植株中发挥作用(图2-b)。
图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2CmWRKY15-1表达水平的半定量检测
Fig. 2Semi quantitative detection of CmWRKY15-1 gene expression level
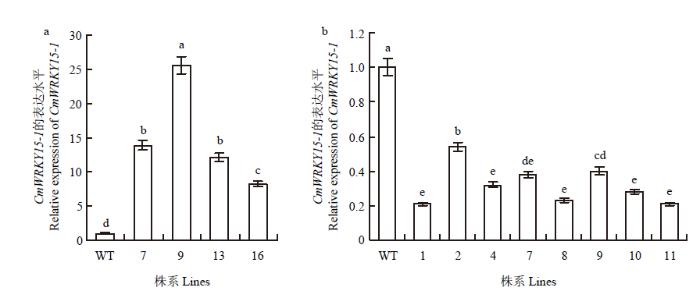
qRT-PCR结果显示,过表达植株中9号CmWRKY15-1的表达量最高,约为野生型植株的25倍,7号与13号次之,分别为13倍和12倍,与半定量结果一致(图3-a)。干扰植株中CmWRKY15-1的表达量降低幅度最大为WT的79%,最小为46%,其余集中在24—40%(图3-b)。
图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3野生型与转基因株系中CmWRKY15-1的相对表达量
不同小写字母表示差异显著(P<0.05)。下同
Fig. 3Relative expression level of CmWRKY15-1 in transgenic lines and WT
Different small letters show significant difference (P<0.05). The same as below
2.2 RNAi-4系植株接种白色锈病病原菌后表型鉴定
对RNAi-4系、WT植株各接种处理15株,每株处理4—5个叶片,分为3组。RNAi-4系及WT植株在接菌后置于培养室观察。如图4所示,接种14 d后RNAi-4系植株叶片表现为叶背的冬孢子堆明显,数量多且个体小,对应叶面病斑明显。少数叶面有少量的淡黄斑且凹陷,背部偶有不连续的冬孢子堆,数量少且大。而WT植株接种后无感病症状,即与未接种时相比无变化。对RNAi-4的病情指数(DSI)统计,发现RNAi-4系的DSI为53.67,抗性鉴定表现为感病(S)。以上结果表明,CmWRKY15-1的沉默使菊花对白色锈病的抗性减弱,敏感性增强。图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4RNAi-4系植株接种后的表型
R1和R2:RNAi-4系接种的第3位叶的叶面与叶背;W1和W2:野生型接种第3位叶的叶面与叶背
Fig. 4Phenotypic differences of plant lines RNAi-4 after inoculated
R1 and R2: The third leaves after inoculation in RNAi-4 (leaf surface and back); W1 and W2: The third leaves after inoculation in WT (leaf surface and back)
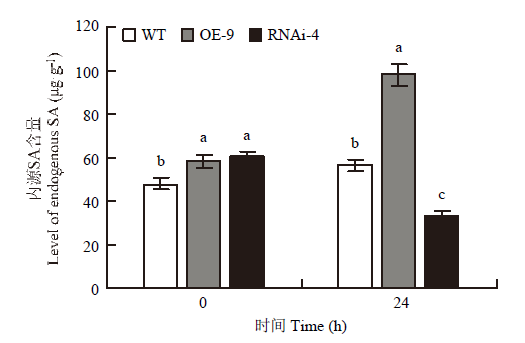
2.3 OE-9系中内源SA的含量
如图5所示,未接菌时,WT的内源SA含量为47.81 μg·g-1,OE-9系的内源SA含量比野生型WT高,为57.99 μg·g-1;OE-9系中的内源SA含量大约是WT系的1.21倍。在白色锈病病菌接种24 h后,OE-9系的内源SA含量增加,为97.78 μg·g-1,WT的内源SA也相较于未接菌时增加,为56.33 μg·g-1,约是WT的1.73倍。而RNAi-4系在接种前SA含量与OE-9系无明显差别,接种24 h后,其含量下降,为对照的59%。综上,CmWRKY15-1的过表达与沉默分别显著上调和下调SA含量,即对SA的积累有正向调节作用。图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5植株叶片的SA含量
WT:‘黄莺’野生型植株;OE-9:超表达植株;RNAi-4:沉默植株。不同小写字母表示差异显著(P<0.05)。下同
Fig. 5SA standard curve of plants
WT: Wild-type plants; OE-9: Overexpression plants; RNAi-4: Silence plants. Different small letters show significant difference (P<0.05). The same as below
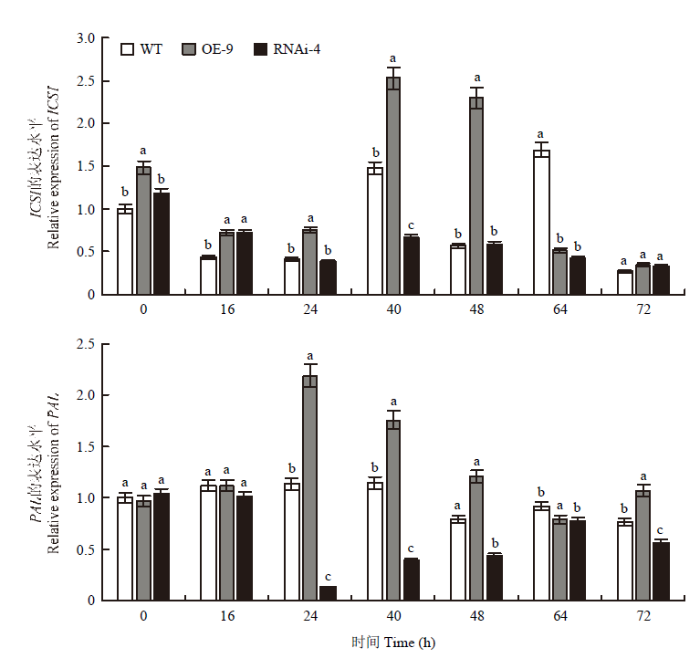
2.4 OE-9系和RNAi-4系中ICS1、PAL的表达分析
为了进一步验证CmWRKY15-1是通过SA介导的信号途径来响应菊花白色锈病的猜测,对OE-9系、RNAi-4系和WT在接菌处理前后不同时间点的SA合成基因表达情况进行分析。如图6所示,处理后,WT中ICS1的表达在40和64 h的表达水平较高,OE-9株系中,ICS1的表达呈先增加后降低的趋势,在40 h时其表达量为WT的1.67倍,48 h时为3.8倍。RNAi-4株系中ICS1的表达在接种后出现降低且一直保持在较低水平。OE-9株系中PAL的表达呈先上升后下降的趋势,处理24 h后其表达保持较高水平,其中24 h时的表达量为对照的2.3倍。RNAi-4株系中PAL的表达整体呈下降趋势,一直低于WT,与SA含量测定结果基本一致。图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6ICS1、PAL的表达分析
Fig. 6Relative expression levels of ICS1 and PAL
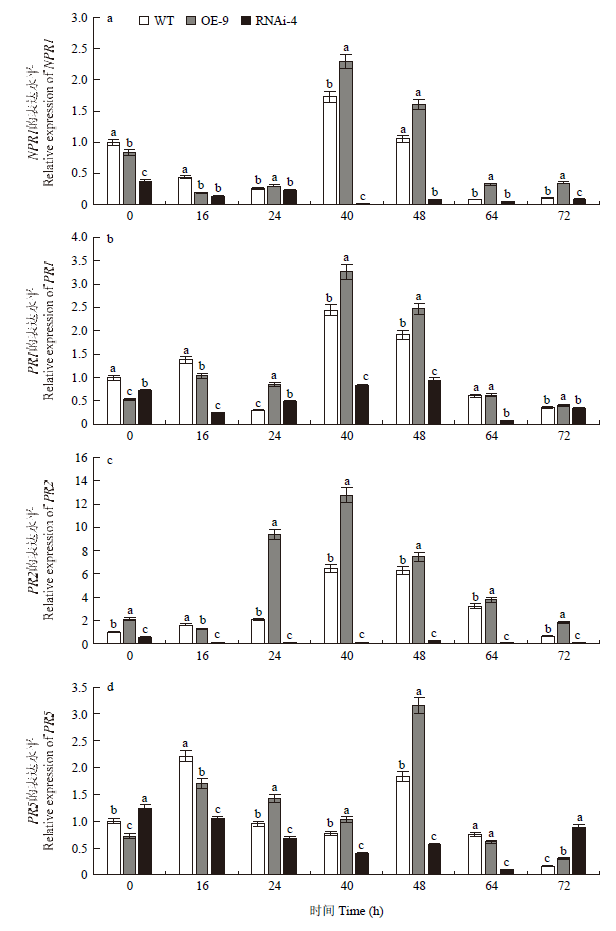
2.5 OE-9系和RNAi-4系中NPR1、PR1、PR2、PR5的表达分析
在接种前,响应SA信号的病程相关基因在转基因株系和野生型株系中的表达量相差不大。随着白色锈病病原菌处理时间的延长,在OE-9株系中响应SA信号的这些基因分别在不同时间出现峰值,NPR1、PR1、PR2均在40 h时达到峰值(图7-a和图7-b),而PR5相对延后,在48 h时出现峰值。相比WT,这4个基因均有不同程度的增加(图7-c和图7-d)。在RNAi-4株系中除PR5外,其他基因的表达量均显著低于WT、OE-9中的表达量,且NPR1、PR2的表达随着病菌处理时间的延长呈现下降的趋势,处理40 h时,NPR1表达量仅为对照的1.04 %,PR2表达量约为对照的1.73 %(图7-a和图7-c)。图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7NPR1、PR1、PR2、PR5的表达分析
Fig. 7Relative expression levels of NPR1, PR1, PR2 and PR5
3 讨论
在整个RNAi过程中,dsRNA的选择和设计至关重要,可直接影响RNAi技术的高效性及特异性[32]。根据已有研究中报道的通过人为设计dsRNA或自我互补的发夹RNA(iphRNA)导入植物,可诱导启动植物体内的RNA沉默[33,34],本研究选择CmWRKY15-1长度为300 bp的特异性片段作为干扰片段,RNAi载体pHANNIBAL中的PDK内含子构建发夹结构,进行CmWRKY15-1的干扰。为保证表达载体在细胞中可以持续产生siRNA以达到长久抑制靶细胞mRNA的目的,将iphRNA最终构建到植物常用且高效的表达载体pBI121中进行后续转化。在载体的构建过程中,综合考虑质粒pHANNIBAL、pBI121的多克隆酶切位点,以及发夹结构形成,在设计干扰片段引物时引入酶切位点Sma I,在反义片段中引入酶切位点Cla I,并在测序时,保证酶切位点未发生碱基突变。qRT-PCR测定干扰植株中CmWRKY15-1的相对表达量主要集中在对照(WT)的0.2—0.4倍,本试验选择RNAi-4进行扩繁,作为CmWRKY15-1后期功能验证材料。植物的防御体系是一个复杂的信号调节网络,植物激素在这个信号网络中起着重要的作用,例如水杨酸(SA)信号通路,茉莉酸/乙烯(JA-ET)信号通路[35,36]。WRKY-TFs是SA、JA和ET信号通路中重要的调控节点,通过这些信号通路参与植物防御[37]。本研究中,RNAi-4株系CmWRKY15-1的沉默导致抗病品种‘黄莺’对白色锈病的抵抗力降低,表现为感病,表明CmWRKY15-1对菊花抗白色锈病具有正调控作用。前期的研究发现,SA、MeJA、ETH 3种外源激素均能诱导菊花叶片中CmWRKY15-1的表达,而ABA处理后,CmWRKY15-1的表达没有明显改变。由此推测,CmWRKY15-1可能参与SA、MeJA、ETH 3种激素介导的抗病途径。在SA介导的信号通路中,由NPR1正向调控其下游的PR1来获得抗性[38],植物防御基因PDF1.2在JA-ET所介导的信号通路起重要作用,是植物抗病的标记基因[39]。利用qPCR技术分析转基因植株中JA-ET信号途径中抗病标志基因PDF1/2的表达情况,结果表明,无论是超表达植株还是沉默植株中PDF1/2的表达与对照相比均无明显变化。与此同时,在接菌处理后,OE-9系中的SA含量增加,RNAi-4系中SA含量降低也验证了该猜测。另外,CmWRKY15-1的超表达也促进了SA合成途径关键基因的表达,而CmWRKY15-1的沉默则表现相反结果,这与SA含量的变化一致。由此证明CmWRKY15-1通过SA信号转导途径参与菊花抗病。另外,在病菌侵染后,SA信号途径的病程相关基因PRs在OE-9系中均表达上调,特别是PR2、PR5,而在RNAi-4植株中表达下调。CHEN等[40]研究的过表达拟南芥AtWRKY18在丁香假单胞菌侵染后,激活了PR表达从而提高了转基因拟南芥对丁香假单胞菌的抗性,表明AtWRKY18在植物抗丁香假单胞菌侵染调节中起正调控作用。TANG等[41]通过酵母单杂分析发现MaWRKY1转录因子能够与PR1-1、PR2等基因结合,提高部分PRs的表达,增强植物对炭疽病的抵御能力。GhWRKY22的沉默使其相关抗病标志基因PR1、PR3、PR4、PR5、PAL和PDF1. 2的表达下调,增强了转基因植株对大丽花黄萎病的敏感性,揭示GhWRKY22正调控棉花的抗性[42]。本研究与上述结果一致,CmWRKY15-1在参与菊花白色锈病病菌抗性调节中起正调控作用,且通过SA信号通路调控,但CmWRKY15-1在SA信号通路中与PR直接作用还是作用于其他靶基因,仍需进一步研究。
4 结论
CmWRKY15-1的过表达增加了内源SA含量并促进了SA合成途径关键基因的表达,而CmWRKY15-1的沉默与之相反。响应SA抗病信号的基因表达分析显示,OE-9系中PRs表达量高于同样病菌处理的WT,特别是PR2、PR5;而PRs在RNAi-4系中的表达量低于WT。即CmWRKY15-1对菊花抗白色锈病具有正调控作用,且通过SA介导的信号通路在菊花抵抗白色锈病病菌侵染过程中发挥作用。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.bbagrm.2011.09.002URLPMID:21964328 [本文引用: 1]

The WRKY gene family has been suggested to play important roles in the regulation of transcriptional reprogramming associated with plant stress responses. Modification of the expression patterns of WRKY genes and/or changes in their activity contribute to the elaboration of various signaling pathways and regulatory networks. Furthermore, a single WRKY gene often responds to several stress factors, and then their proteins may participate in the regulation of several seemingly disparate processes as negative or positive regulators. WRKY proteins also function via protein-protein interaction and autoregulation or cross-regulation is extensively recorded among WRKY genes, which help us understand the complex mechanisms of signaling and transcriptional reprogramming controlled by WRKY proteins. Here, we review recent progress made in starting to reveal the role of WRKY transcription factors in plant abiotic stresses. This article is part of a Special Issue entitled: Plant gene regulation in response to abiotic stress.
URLPMID:30107882 [本文引用: 1]
[本文引用: 1]
URLPMID:26706073 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.3864/j.issn.0578-1752.2016.07.006URL [本文引用: 1]

【目的】从前期转录组测序结果中筛选获得一个在霜霉威(propamocarb)胁迫条件下差异上调表达的基因CsWRKY30,对其进行克隆并分析其在霜霉威胁迫下的功能,了解黄瓜低霜霉威残留的分子机制。【方法】通过PCR技术扩增CsWRKY30全长,利用NCBI和PlantCARE在线工具分别进行该基因编码蛋白的保守结构域分析和启动子序列分析;利用实时荧光定量PCR分析CsWRKY30在霜霉威胁迫及其他胁迫条件下的相关表达模式;通过与GFP蛋白融合对CsWRKY30蛋白进行亚细胞定位;通过花序侵染法将CsWRKY30构建的植物过表达载体转化到哥伦比亚野生型拟南芥中,对获得的纯合转基因株系在霜霉威胁迫条件下的功能进行鉴定。【结果】CsWRKY30的CDS序列全长为1 014 bp,其编码的337个氨基酸中包含1个由60个氨基酸组成的WRKY结构域。CsWRKY30表达模式分析结果显示,黄瓜遭受霜霉威胁迫时,CsWRKY30在低霜霉威残留品系D0351中表达量显著上调,而在高霜霉威残留品系D9320中表达量并没有发生改变;在霜霉威胁迫的0.5—9 h间,该基因在D0351中的表达量一直明显高于对照,然而在24 h以后,该基因的表达不再显著上调。组织特异性表达分析表明,CsWRKY30主要在黄瓜果实中表达。蛋白亚细胞定位结果表明,CsWRKY30定位于细胞核。对CsWRKY30转基因拟南芥进行霜霉威胁迫发现,在未处理条件下,CsWRKY30 转基因拟南芥与野生型拟南芥表型上无明显差异;在2 mmol?L-1霜霉威处理条件下,CsWRKY30转基因拟南芥萌发率及主根长均明显高于野生型拟南芥。在其他逆境作用下,CsWRKY30对霜霉威和多主棒孢霉菌条件积极响应,对干旱和高盐没有作用,同时受到脱落酸(ABA)信号诱导。【结论】黄瓜CsWRKY30在霜霉威胁迫条件下发挥重要作用,过量表达CsWRKY30可显著提高转基因拟南芥对霜霉威胁迫的抵抗能力。
DOI:10.3864/j.issn.0578-1752.2016.07.006URL [本文引用: 1]

【目的】从前期转录组测序结果中筛选获得一个在霜霉威(propamocarb)胁迫条件下差异上调表达的基因CsWRKY30,对其进行克隆并分析其在霜霉威胁迫下的功能,了解黄瓜低霜霉威残留的分子机制。【方法】通过PCR技术扩增CsWRKY30全长,利用NCBI和PlantCARE在线工具分别进行该基因编码蛋白的保守结构域分析和启动子序列分析;利用实时荧光定量PCR分析CsWRKY30在霜霉威胁迫及其他胁迫条件下的相关表达模式;通过与GFP蛋白融合对CsWRKY30蛋白进行亚细胞定位;通过花序侵染法将CsWRKY30构建的植物过表达载体转化到哥伦比亚野生型拟南芥中,对获得的纯合转基因株系在霜霉威胁迫条件下的功能进行鉴定。【结果】CsWRKY30的CDS序列全长为1 014 bp,其编码的337个氨基酸中包含1个由60个氨基酸组成的WRKY结构域。CsWRKY30表达模式分析结果显示,黄瓜遭受霜霉威胁迫时,CsWRKY30在低霜霉威残留品系D0351中表达量显著上调,而在高霜霉威残留品系D9320中表达量并没有发生改变;在霜霉威胁迫的0.5—9 h间,该基因在D0351中的表达量一直明显高于对照,然而在24 h以后,该基因的表达不再显著上调。组织特异性表达分析表明,CsWRKY30主要在黄瓜果实中表达。蛋白亚细胞定位结果表明,CsWRKY30定位于细胞核。对CsWRKY30转基因拟南芥进行霜霉威胁迫发现,在未处理条件下,CsWRKY30 转基因拟南芥与野生型拟南芥表型上无明显差异;在2 mmol?L-1霜霉威处理条件下,CsWRKY30转基因拟南芥萌发率及主根长均明显高于野生型拟南芥。在其他逆境作用下,CsWRKY30对霜霉威和多主棒孢霉菌条件积极响应,对干旱和高盐没有作用,同时受到脱落酸(ABA)信号诱导。【结论】黄瓜CsWRKY30在霜霉威胁迫条件下发挥重要作用,过量表达CsWRKY30可显著提高转基因拟南芥对霜霉威胁迫的抵抗能力。
DOI:10.1111/ppl.12251URLPMID:25132131 [本文引用: 1]

Grapevine (Vitis vinifera ssp. vinifera) is one of the most important fruit species; however, it is highly susceptible to various pathogens, which can cause severe crop losses in viticulture. It has been shown that several WRKY class transcription factors (TFs) are part of the signal transduction cascade, which leads to the activation of plant defense reactions against various pathogens. In the present investigation, a full-length cDNA was isolated from V. vinifera leaf tissue encoding a predicted protein, designated VvWRKY33, which shows the characteristics of group I WRKY protein family. VvWRKY33 induction correlates with the expression of VvPR10.1 (pathogenesis-related 10.1) gene in the leaves of the resistant cultivar 'Regent' after infection with Plasmopara viticola, whereas in the susceptible cultivar 'Lemberger' VvWRKY33 and VvPR10.1 are not induced. Corresponding expression of the TF and VvPR10.1 was even obtained in uninfected ripening berries. In planta, analysis of VvWRKY33 has been performed by ectopic expression of VvWRKY33 in grapevine leaves of greenhouse plants mediated via Agrobacterium tumefaciens transformation. In consequence, VvWRKY33 strongly increases resistance to P. viticola in the susceptible cultivar 'Shiraz' and reduces pathogen sporulation of about 50-70%, indicating a functional role for resistance in grapevine. Complementation of the resistance-deficient Arabidopsis thaliana Columbia-0 (Col-0) mutant line wrky33-1 by constitutive expression of VvWRKY33 restores resistance against Botrytis cinerea to wild-type level and in some complemented mutant lines even exceeds the resistance level of the parental line Col-0. Our results support the involvement of VvWRKY33 in the defense reaction of grapevine against different pathogens.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:26184088 [本文引用: 1]
DOI:10.1371/journal.pone.0143349URLPMID:26600125 [本文引用: 1]

Abscisic acid (ABA) has an important role in the responses of plants to pathogens due to its ability to induce stomatal closure and interact with salicylic acid (SA) and jasmonic acid (JA). WRKY transcription factors serve as antagonistic or synergistic regulators in the response of plants to a variety of pathogens. Here, we demonstrated that CmWRKY15, a group IIa WRKY family member, was not transcriptionally activated in yeast cells. Subcellular localization experiments in which onion epidermal cells were transiently transfected with CmWRKY15 indicated that CmWRKY15 localized to the nucleus in vivo. The expression of CmWRKY15 could be markedly induced by the presence of Alternaria tenuissima inoculum in chrysanthemum. Furthermore, the disease severity index (DSI) data of CmWRKY15-overexpressing plants indicated that CmWRKY15 overexpression enhanced the susceptibility of chrysanthemum to A. tenuissima infection compared to controls. To illustrate the mechanisms by which CmWRKY15 regulates the response to A. tenuissima inoculation, the expression levels of ABA-responsive and ABA signaling genes, such as ABF4, ABI4, ABI5, MYB2, RAB18, DREB1A, DREB2A, PYL2, PP2C, RCAR1, SnRK2.2, SnRK2.3, NCED3A, NCED3B, GTG1, AKT1, AKT2, KAT1, KAT2, and KC1were compared between transgenic plants and controls. In summary, our data suggest that CmWRKY15 might facilitate A. tenuissima infection by antagonistically regulating the expression of ABA-responsive genes and genes involved in ABA signaling, either directly or indirectly.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:8896462 [本文引用: 1]
[本文引用: 1]
DOI:10.3389/fpls.2011.00032URLPMID:22639590 [本文引用: 1]

The promoter of the salicylic acid-inducible PR-1a gene of Nicotiana tabacum contains binding sites for transcription factor NtWRKY12 (WK-box at position -564) and TGA factors (as-1-like element at position -592). Transactivation experiments in Arabidopsis protoplasts derived from wild type, npr1-1, tga256, and tga2356 mutant plants revealed that NtWRKY12 alone was able to induce a PR-1a::beta-glucuronidase (GUS) reporter gene to high levels, independent of co-expressed tobacco NtNPR1, TGA2.1, TGA2.2, or endogenous Arabidopsis NPR1, TGA2/3/5/6. By in vitro pull-down assays with GST and Strep fusion proteins and by Fluorescence Resonance Energy Transfer assays with protein-CFP and protein-YFP fusions in transfected protoplasts, it was shown that NtWRKY12 and TGA2.2 could interact in vitro and in vivo. Interaction of NtWRKY12 with TGA1a or TGA2.1 was not detectable by these techniques. A possible mechanism for the role of NtWRKY12 and TGA2.2 in PR-1a gene expression is discussed.
[本文引用: 1]
[本文引用: 1]
URLPMID:25096754 [本文引用: 1]
DOI:10.1016/s0952-7915(00)00183-7URLPMID:11154919 [本文引用: 1]

Not more than 10 years ago it was generally accepted that pathogen-inducible defense mechanisms in plants are triggered through a central signaling cascade that regulates a multicomponent defense response. Now we know that the plant defense system is regulated through a complex network of various signaling cascades.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1038/s41438-020-0267-7URLPMID:32284869 [本文引用: 1]

In this study, the disease resistance gene PlWRKY65 was isolated from the leaves of Paeonia lactiflora and analyzed by bioinformatics methods, and the localization of the encoded protein was explored. Quantitative real-time PCR (qRT-PCR) was also used to explore the response of this gene to Alternaria tenuissima. The results showed that the gene sequence contained multiple cis-acting elements involved in the response to hormone signaling molecules belonging to the IIe subgroup of the WRKY family, and the encoded proteins were located in the nucleus. The PlWRKY65 gene has a positive regulatory effect on A. tenuissima infection. After silencing the PlWRKY65 gene via virus-induced gene silencing (VIGS), it was found that the gene-silenced plants were more sensitive to A. tenuissima infection than the wild plants, exhibiting more severe infection symptoms and different degrees of changes in the expression of the pathogenesis-related (PR) genes. In addition, we showed that the endogenous jasmonic acid (JA) content of P. lactiflora was increased in response to A. tenuissima infection, whereas the salicylic acid (SA) content decreased. After PlWRKY65 gene silencing, the levels of the two hormones changed accordingly, indicating that PlWRKY65, acting as a disease resistance-related transcriptional activator, exerts a regulatory effect on JA and SA signals. This study lays the foundation for functional research on WRKY genes in P. lactiflora and for the discovery of candidate disease resistance genes.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:17938933 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1046/j.1365-313x.2003.01717.xURLPMID:12694596 [本文引用: 1]

The signal transduction network controlling plant responses to pathogens includes pathways requiring the signal molecules salicylic acid (SA), jasmonic acid (JA), and ethylene (ET). The network topology was explored using global expression phenotyping of wild-type and signaling-defective mutant plants, including eds3, eds4, eds5, eds8, pad1, pad2, pad4, NahG, npr1, sid2, ein2, and coi1. Hierarchical clustering was used to define groups of mutations with similar effects on gene expression and groups of similarly regulated genes. Mutations affecting SA signaling formed two groups: one comprised of eds4, eds5, sid2, and npr1-3 affecting only SA signaling; and the other comprised of pad2, eds3, npr1-1, pad4, and NahG affecting SA signaling as well as another unknown process. Major differences between the expression patterns in NahG and the SA biosynthetic mutant sid2 suggest that NahG has pleiotropic effects beyond elimination of SA. A third group of mutants comprised of eds8, pad1, ein2, and coi1 affected ethylene and jasmonate signaling. Expression patterns of some genes revealed mutual inhibition between SA- and JA-dependent signaling, while other genes required JA and ET signaling as well as the unknown signaling process for full expression. Global expression phenotype similarities among mutants suggested, and experiments confirmed, that EDS3 affects SA signaling while EDS8 and PAD1 affect JA signaling. This work allowed modeling of network topology, definition of co-regulated genes, and placement of previously uncharacterized regulatory genes in the network.
[本文引用: 1]
[本文引用: 1]
DOI:10.3389/fpls.2015.00170URLPMID:25859250 [本文引用: 1]

Transcriptional regulation is a central process in plant immunity. The induction or repression of defense genes is orchestrated by signaling networks that are directed by plant hormones of which salicylic acid (SA) and jasmonic acid (JA) are the major players. Extensive cross-communication between the hormone signaling pathways allows for fine tuning of transcriptional programs, determining resistance to invaders and trade-offs with plant development. Here, we give an overview of how SA can control transcriptional reprogramming of JA-induced genes in Arabidopsis thaliana. SA can influence activity and/or localization of transcriptional regulators by post-translational modifications of transcription factors and co-regulators. SA-induced redox changes, mediated by thioredoxins and glutaredoxins, modify transcriptional regulators that are involved in suppression of JA-dependent genes, such as NPR1 and TGA transcription factors, which affects their localization or DNA binding activity. Furthermore, SA can mediate sequestering of JA-responsive transcription factors away from their target genes by stalling them in the cytosol or in complexes with repressor proteins in the nucleus. SA also affects JA-induced transcription by inducing degradation of transcription factors with an activating role in JA signaling, as was shown for the ERF transcription factor ORA59. Additionally, SA can induce negative regulators, among which WRKY transcription factors, that can directly or indirectly inhibit JA-responsive gene expression. Finally, at the DNA level, modification of histones by SA-dependent factors can result in repression of JA-responsive genes. These diverse and complex regulatory mechanisms affect important signaling hubs in the integration of hormone signaling networks. Some pathogens have evolved effectors that highjack hormone crosstalk mechanisms for their own good, which are described in this review as well.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
