 ,, 张德权, 赵莹鑫, 摆玉蔷, 李欣, 侯成立, 郑晓春, 陈丽
,, 张德权, 赵莹鑫, 摆玉蔷, 李欣, 侯成立, 郑晓春, 陈丽 ,中国农业科学院农产品加工研究所/农业农村部农产品加工重点实验室,北京 100193
,中国农业科学院农产品加工研究所/农业农村部农产品加工重点实验室,北京 100193Water-Holding Capacity and Water Migration of Lamb Gigot During Dry Aging
WANG Xu ,, ZHANG DeQuan, ZHAO YingXin, BAI YuQiang, LI Xin, HOU ChengLi, ZHENG XiaoChun, CHEN Li
,, ZHANG DeQuan, ZHAO YingXin, BAI YuQiang, LI Xin, HOU ChengLi, ZHENG XiaoChun, CHEN Li ,Institute of Food Science and Technology, Chinese Academy of Agricultural Sciences/Key Laboratory of Agro-Products Processing, Ministry of Agriculture and Rural Affairs, Beijing 100193
,Institute of Food Science and Technology, Chinese Academy of Agricultural Sciences/Key Laboratory of Agro-Products Processing, Ministry of Agriculture and Rural Affairs, Beijing 100193通讯作者:
责任编辑: 赵伶俐
收稿日期:2020-05-4接受日期:2020-07-22网络出版日期:2021-01-01
| 基金资助: |
Received:2020-05-4Accepted:2020-07-22Online:2021-01-01
作者简介 About authors
王旭,Tel:010-62816474;E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (536KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
王旭, 张德权, 赵莹鑫, 摆玉蔷, 李欣, 侯成立, 郑晓春, 陈丽. 干法成熟过程羊腿肉持水能力与水分迁移规律[J]. 中国农业科学, 2021, 54(1): 179-189 doi:10.3864/j.issn.0578-1752.2021.01.013
WANG Xu, ZHANG DeQuan, ZHAO YingXin, BAI YuQiang, LI Xin, HOU ChengLi, ZHENG XiaoChun, CHEN Li.
开放科学(资源服务)标识码(OSID):

0 引言
【研究意义】成熟是一种提高肉品嫩度、风味、多汁性的方法,现有的成熟方式主要有湿法成熟和干法成熟两种方式[1,2]。干法成熟通常是指将胴体或分割肉不加任何包装和保护措施,置于低温(-1—4℃)环境中自然成熟数天[3],湿法成熟则是在相同条件下将肉品进行真空包装成熟[4]。原料肉在干法成熟过程中,伴随着水分的蒸发,肉中的风味前体物含量升高,使干法成熟的肉具有很强的风味特征,肉香浓郁,嫩度也会极大提高[5,6],虽然湿法成熟也会提高原料肉嫩度,但会产生一些不良的气味,如血腥味和酸味等[7]。近年来,澳大利亚、韩国、日本以及香港等地区都对干法成熟的肉表现出极大的兴趣[8],随着人们对干法成熟肉的需求增加,干法成熟肉品将会为工业化生产和进出口销售开发新的市场。因此,研究干法成熟过程中原料肉的持水能力和内部水分的迁移规律,确定原料肉最佳的干法成熟时间,对企业生产高端产品具有重要意义。【前人研究进展】干法成熟对原料肉的嫩度、风味、多汁性有积极影响。SMITH等[9]研究表明,成熟14—35 d,干法成熟牛肉剪切力降低17%,嫩度持续改善,但由于水分蒸发,可出售总产量显著降低,然而感官多汁性评分却显著升高;LEPPER- BLILIE等[10]研究发现,随着干法成熟时间延长,大理石花纹不丰富牛肉的整体成熟风味增加,且与第14天和第21天肉的风味相比,第42天和第49天的风味最浓。原因之一是成熟过程中的水分蒸发可以使肉中的风味前体物质含量升高[11]。LI等[12]测定干/湿法成熟牛臀中肌的蒸煮损失,结果表明,干法成熟处理组的蒸煮损失显著低于湿法成熟处理组,即干法成熟牛臀中肌的持水能力优于湿法成熟。成熟过程中由于肉暴露于空气中,水分含量发生改变,内部水分也会发生迁移[13]。研究表明,以壳聚糖为包装材料的干法成熟袋中成熟的牛肉在成熟14 d时自由水含量显著降低,与水分含量的降低趋势一致[14]。郭兆斌等[15]研究青海牦牛宰后成熟过程中水分分布规律,发现宰后初期,不易流动水转化为自由水,肌肉的保水能力逐渐降低,后期恰好相反。环境湿度通过改变样品的水分含量进而影响肉品质,CHO等[16]研究发现,相对湿度65%—75%条件下干法成熟40 d的牛肉水分含量显著低于相对湿度85%的肉品,但感官评价中关于嫩度、风味、多汁性和肉品总体可接受程度的评分中前者得分较高。【本研究切入点】环境湿度是干法成熟过程中主要影响因素之一[8,13],干法成熟技术会影响肉品品质,且原料肉内部水分的变化对品质影响较大,探究羊腿肉在干法成熟过程中的持水能力及肉中水分迁移规律十分必要。【拟解决的关键问题】本试验以羊腿肉为研究对象,在湿法成熟、相对湿度80%干法成熟和相对湿度60%干法成熟条件下成熟28 d,探究不同成熟方法对羊腿肉持水能力的影响及其水分迁移规律,为生产高品质干法成熟羊肉提供科学指导。1 材料与方法
样品采集试验于2019年10—11月在内蒙古巴彦淖尔市内蒙古美洋洋食品有限公司进行;样品测定试验于2019年10—12月在中国农业科学院农产品加工研究所肉品实验室和农业农村部农产品加工重点实验室进行。1.1 样品采集
选取26只6—7月龄的小尾寒羊公羊,按伊斯兰屠宰方法进行屠宰,宰后羊胴体重量为(23.4±1.09)kg。宰后1 d,取羊后腿(带臀,去腱腿)贮存于(2±2)℃的环境中,并在52只羊后腿中随机挑选出4只羊腿作为成熟0 d的样品。其余48只羊后腿随机均分到3个试验组中:(1)湿法成熟组,真空包装(透气率为O2 9.3 mL?m-2?d-1,透水率为4.7 g?m-2?d-1);(2)相对湿度(80±5)%干法成熟组(RH80干法成熟);(3)相对湿度(60±5)%干法成熟组(RH60干法成熟),干法成熟样品均置于可移动的货架上。所有样品置于温度为(2±2)℃的低温室中成熟,每个处理组分别在成熟7、14、21和28 d取出4只羊腿,干法成熟的样品先剔除外壳,沿臀股四头肌、臀股二头肌和半腱肌之间的自然缝分离得到半膜肌、内收肌、股薄肌,将得到包含半膜肌、内收肌、股薄肌的肉块进行取样。所有样品采集后迅速用液氮冷冻,-80℃贮藏备用。1.2 主要仪器与设备
DK-S28电热恒温水浴锅,上海精宏试验设备有限公司;超低温冰箱,美国Thermo公司;NMR-2011低场核磁共振仪,上海纽迈电子科技有限公司;Vector 33傅里叶变换近红外光谱仪,德国Bruker公司;ML204/02电子天平,上海梅特勒-托利多有限公司;TD5001C电子天平,天津天马衡基仪器有限公司;DH-101烘干箱,天津中环试验仪器有限公司;FCR1000-UF-E超纯水机,青岛富勒姆科技有限公司。1.3 试验方法
1.3.1 成熟损失 宰后1 d,取下羊腿肉之后称重(湿法成熟样品包装后称重),记为m1;在成熟取样时间点时再次称取羊腿肉的质量(湿法成熟样品不打开真空包装直接称重),记为m2,计算成熟损失,成熟损失(%)=(m1-m2)/m1×100。1.3.2 水分含量 参照国标《GB 5009.3-2016 食品安全国家标准 食品中水分的测定》测定和计算成熟0、7、14、21和28 d羊腿肉中的水分含量。
1.3.3 蒸煮损失 参考HOPKINS等[17]方法并稍作修改。在成熟0、7、14、21和28 d取样时,选取长、宽约为4 cm×6 cm的肉块进行称重,记为m1,置于蒸煮袋底部,除去袋内的空气使其与肉块紧贴而后封口,将其置于71℃的水浴中加热35 min后转移到自来水中冷却30 min,冷却结束后取出肉块擦干表面水分后称重,记为m2,即蒸煮损失(%)=(m1-m2)/m1×100。
1.3.4 羊腿肉蛋白衰减全反射傅里叶变换红外线光谱(ATR-FTIR)测定 根据GANGIDI等[18]方法,取羊腿肉薄片置于ATR附件上,用压头压紧样品,使羊腿肉与晶体充分接触并开始扫描。使用OMNIC软件记录红外光谱,基线通过扣除空气背景进行校准。试验参数如下,采用积分球漫反射,分辨率8 cm-1,扫描次数64次,光谱扫描范围为4 000—600 cm-1。采用Peakfit 4.12软件分析ATR-FTIR 1 700—1 600 cm-1波段的峰,利用二阶求导和去卷曲对酰胺Ⅰ带进行分峰处理,再结合曲线拟合的方法,对蛋白质二级结构进行定量分析。
1.3.5 低场核磁检测 参照饶伟丽[19]的测定方法并稍作修改,将不同湿度、不同贮藏时间点的肉样切成4 cm×2 cm×1 cm的立方体置于核磁管中,测定氢质子低场核磁共振波谱。试验参数如下:磁场强度0.5 T;质子共振频率23 MHz;SF=23 MHz;P90=9 μs;P180=18 μs;TD=59 990;TR=3 000 ms;NS=16;Echo Count=2 000。CPMG指数衰减曲线采用Multi Exp Inv Analysis软件进行反演,得到T2值。
1.4 数据分析
本试验采用Microsoft Excel 2010 软件处理数据(平均值±标准差),利用IBM SPSS Statistics 22统计分析软件进行双因素方差分析和聚类分析,并进行Duncan多重比较,采用Microsoft Excel 2010软件制图。2 结果
2.1 干法成熟过程中羊腿肉成熟损失变化
3种成熟方法的成熟损失如图1所示。在成熟28 d内,湿法成熟组羊腿肉成熟损失差异不显著(P>0.05);干法成熟羊腿肉的成熟损失随成熟时间的延长而逐渐增大;除成熟14 d外,RH60干法成熟组羊腿肉的成熟损失显著高于RH80干法成熟组(P<0.05),且干法成熟组羊腿肉的成熟损失均显著高于湿法成熟组(P<0.05)。图1
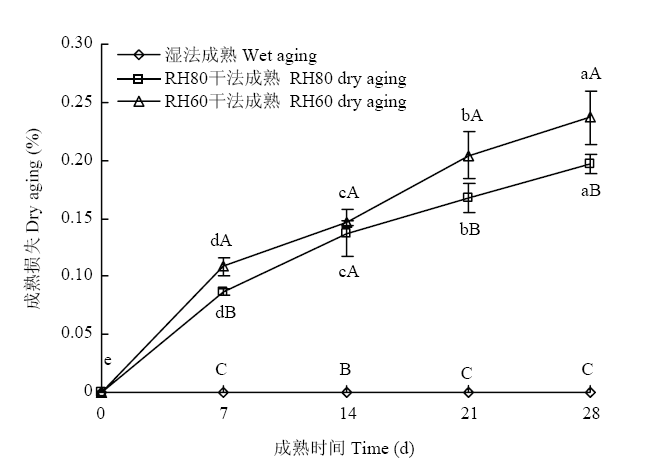 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图13种成熟方法羊腿肉的成熟损失
不同小写字母表示同种成熟方法不同成熟时间差异显著(P<0.05);不同大写字母表示相同成熟时间不同成熟方法差异显著(P<0.05)。下同
Fig. 1Drying loss of gigots with three aging groups
Different lowercase letters indicate significant differences at different aging time (P<0.05). Different capital letters indicate significant differences among different groups (P<0.05). The same as below
2.2 干法成熟过程中羊腿肉水分含量变化
3种成熟方法羊腿肉的水分含量变化如图2所示。随着成熟进行,湿法成熟和RH80干法成熟羊腿肉的水分含量呈现先上升后下降的趋势。在成熟7 d后,RH60干法成熟羊腿肉的水分含量显著低于湿法成熟(P<0.05),但RH80干法成熟组和RH60干法成熟组的羊腿肉水分含量之间无显著差异(P>0.05)。图2
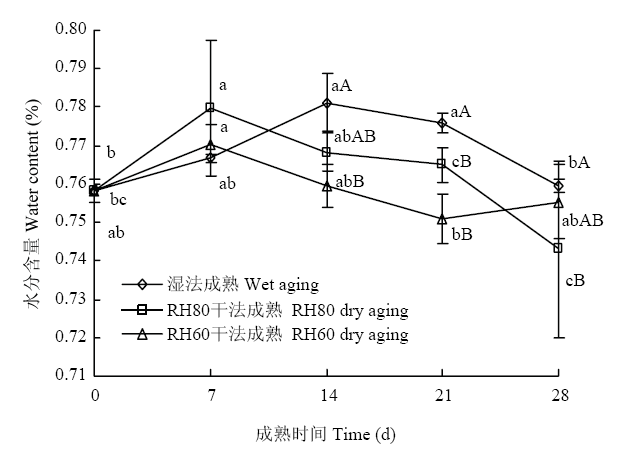 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图23种成熟方法羊腿肉的水分含量
Fig. 2Water content of gigots with three aging groups
2.3 干法成熟过程中羊腿肉蒸煮损失变化
如图3所示,3种成熟方法羊腿肉的蒸煮损失整体随成熟时间的延长而增加,表明羊腿肉的持水能力随成熟时间的延长而降低,3种成熟方法羊腿肉的蒸煮损失在21 d达到峰值,后趋于稳定。成熟21 d时,湿法成熟组、RH80干法成熟组和RH60干法成熟组羊腿肉的蒸煮损失分别为26.88%、24.52%和22.04%,比0 d时分别增加了13.33%、10.97%和8.49%。在3组之间,除成熟14 d的RH80干法成熟羊肉外,干法成熟羊腿肉蒸煮损失在成熟7、14和21 d时显著低于湿法成熟(P<0.05),且在成熟21 d时,RH60干法成熟羊腿肉蒸煮损失显著低于RH80干法成熟和湿法成熟(P<0.05)。成熟14 d时,3种成熟方法羊腿肉的蒸煮损失与0 d结果无显著差异(P>0.05)。图3
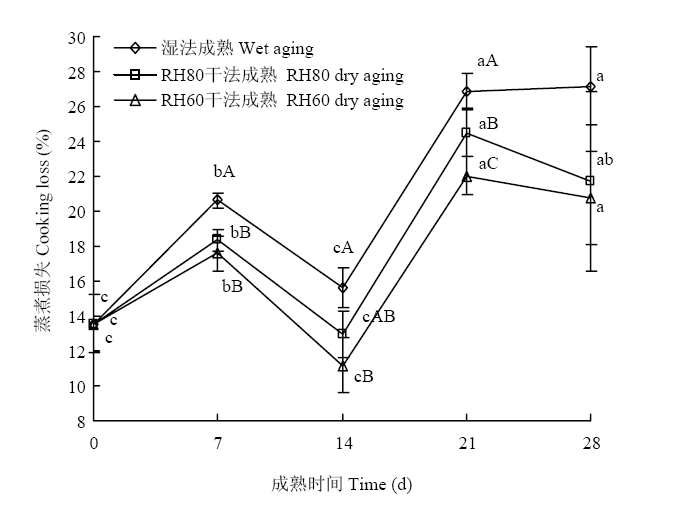 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图33种成熟方法羊腿肉的蒸煮损失
Fig. 3Cooking loss of gigots aged with three aging groups
2.4 干法成熟过程中羊腿肉蛋白质二级结构变化
羊腿肉的蛋白质二级结构结果如图4所示,在成熟过程中,3种成熟方法均会导致蛋白质二级结构中的α-螺旋和β-折叠的比例先上升后下降;成熟14 d时,3种成熟方式的α-螺旋结构的比例均上升,这与WU等[20]的研究结果一致。与成熟7 d相比,湿法成熟组、RH80干法成熟组和RH60干法成熟组在成熟14 d时无序结构分别降低9.2%、14.1%和17.26%,表明成熟14 d羊腿肉蛋白质稳定性高于成熟7 d的,持水能力较好。图4
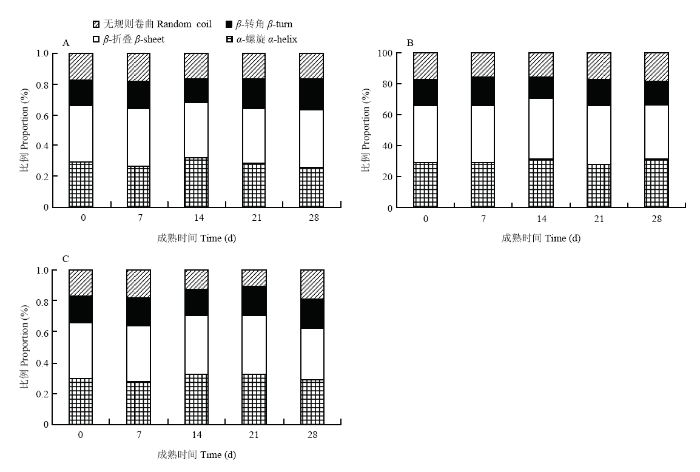 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4湿法成熟组(A)、RH80干法成熟组(B)、RH60干法成熟组(C)蛋白质二级结构相对含量变化
Fig. 4Changes of gigot protein secondary structure relative content of wet aging (A), RH80 dry aging (B), and RH60 dry aging (C)
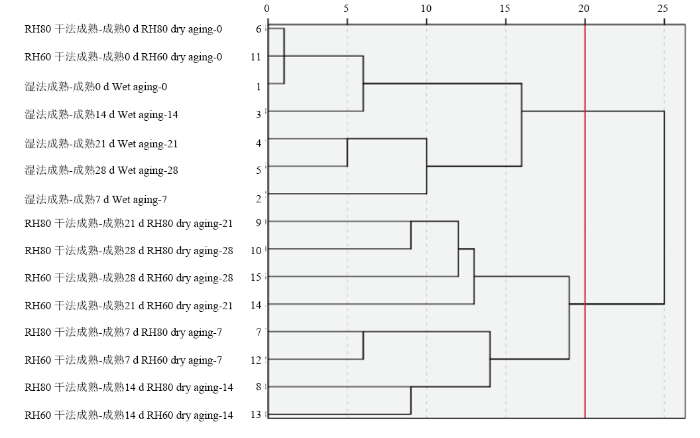
为了进一步分析不同时间处理组之间的差异,对不同处理组及不同成熟时间的成熟损失、水分含量、蒸煮损失以及蛋白质二级结构的相对含量进行聚类分析,由图5可知,欧氏距离为20时,样本被分成两大类,干法成熟处理组为一类,湿法成熟处理组为一类,可见不同成熟方式对羊腿肉持水能力有不同影响。
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5聚类分析谱系图
Fig. 5Dendrogram of HCA
2.5 干法成熟过程中羊腿肉水分分布与迁移
为了探究3种成熟方法羊腿肉样品中水分的迁移和分布规律,采用低场核磁测量湿法成熟、RH80干法成熟和RH60干法成熟0、7、14、21和28 d的羊腿肉横向弛豫时间T2。如表1所示,3种成熟方法的T2弛豫时间图谱中出现4个峰,从左到右分别对应T20(0—1 ms)、T2b(2—5 ms)、T21(30—60 ms)、T22(200—350 ms)。根据前人的研究结果[21],认为T20、T2b是结合水,T21为不易流动水,T22是自由水,波谱中结合水、不易流动水和自由水所处峰的峰面积P2b、P21和P22比例能够反映3种状态水的比例。随着成熟时间延长,3个处理组中(成熟21 d的RH80干法成熟组除外)羊腿肉结合水的横向弛豫时间T2b均变化不显著(P>0.05),说明成熟方法以及成熟时间对结合水流动性影响较小。在RH60干法成熟过程中,T21在成熟14 d时显著低于成熟7 d(P<0.05),表明水分自由度降低,流动性减弱。湿法成熟和RH80干法成熟的T21在成熟14 d内均无显著差异(P>0.05),在成熟28 d显著降低(P<0.05)。T22表示细胞间自由水弛豫时间,这部分水最容易流失[22]。由表1可知,3种成熟方法的T22在成熟21 d较成熟14 d显著增加(P<0.05),表明水分自由度升高,持水能力降低,此时羊腿肉的蒸煮损失也比成熟14 d的高。在成熟7和14 d时,RH60干法成熟的T22要显著低于湿法成熟组(P<0.05),表明RH60干法成熟水分自由度低,持水能力优于湿法成熟组。Table 1
表1
表1羊腿肉弛豫时间T2随成熟时间的变化
Table 1
| 成熟方式 Aging method | 成熟时间 Aging time (d) | T20 (ms) | T2b (ms) | T21 (ms) | T22 (ms) |
|---|---|---|---|---|---|
| 湿法成熟 Wet aging | 0 | 0.631±0.074a | 4.063±0.506a | 49.77±0a | 278.868±20.541bc |
| 7 | 0.432±0.184b | 3.492±0.735ab | 48.69±2.646a | 335.875±23.616aA | |
| 14 | 0.462±0.106b | 3.028±0.579b | 49.77±0a | 265.609±0cdA | |
| 21 | 0.456±0.192b | 3.104±1.077b | 49.932±4.414a | 299.749±31.829bAB | |
| 28 | 0.437±0.063b | 2.746±0.792b | 38.123±6.359b | 254.077±17.865d | |
| RH80干法成熟 RH80 dry aging | 0 | 0.631±0.074a | 4.063±0.506a | 49.77±0ab | 278.868±20.541b |
| 7 | 0.285±0.125c | 3.15±0.421b | 49.77±0ab | 265.609±0bcB | |
| 14 | 0.481±0.081b | 3.226±0.442b | 46.529±3.55b | 248.311±18.949cAB | |
| 21 | 0.537±0.14ab | 4.022±0.81a | 51.174±5.316a | 320.63±23.616aA | |
| 28 | 0.489±0.131b | 2.816±0.476b | 35.426±5.183c | 260.707±27.683bc | |
| RH60干法成熟 RH60 dry aging | 0 | 0.631±0.074a | 4.063±0.506a | 49.77±0ab | 278.868±20.541a |
| 7 | 0.249±0.065c | 3.186±0.739b | 52.255±3.849a | 272.239±16.239aB | |
| 14 | 0.417±0.065b | 2.826±0.739b | 44.368±3.849c | 226.749±16.239bB | |
| 21 | 0.443±0.114b | 3.18±0.633b | 46.529±3.55bc | 292.127±20.541aB | |
| 28 | 0.503±0.131b | 2.829±0.608b | 37.772±3.339d | 242.545±17.865b |
新窗口打开|下载CSV
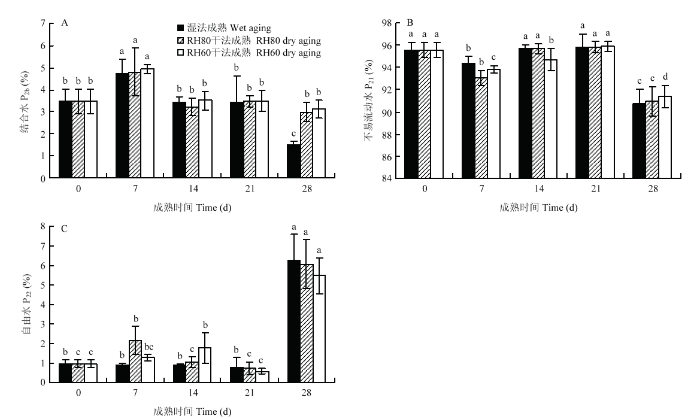
由图6可知,不管哪种成熟方法的羊腿肉中不易流动水相对含量(P21)占比最大,说明肉中水分主要以不易流动水存在,在成熟14 d前,P21先降低后升高;成熟14 d之后,P21逐渐降低。结合水(P2b)是与蛋白质等紧密结合的水分子[23],成熟前期,3种成熟方法的P2b先升高后降低,成熟后期P2b几乎不发生变化(湿法成熟28 d除外)。自由水(P22)是存在于细胞外间隙的水,主要靠毛细管凝结作用而存在于肌肉中[24],是汁液流失的主要来源,这部分水在成熟过程中很容易流失[25]。湿法成熟21 d内,羊腿肉P22未发生显著变化(P>0.05),表明真空包装对羊腿肉中自由水比例影响不显著,也表明其成熟损失没有差异。在成熟28 d时,3种成熟方法羊腿肉P22最高、P21最低,说明此时肉品持水能力最差。
图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图63种成熟方法羊腿肉中结合水P2b(A)、不易流动水P21(B)、自由水P22(C)峰面积比
Fig. 6Changes in peak area of bound water P2b (A), immobilized water P21 (B), and free water P22 (C) inside gigots with three aging groups
3 讨论
3.1 干法成熟对羊腿肉成熟损失的影响
成熟损失反映肉品在成熟过程中的重量损失率,主要受成熟过程中样品水分蒸发的影响。湿法成熟组羊腿肉由于带有真空包装袋,水分不容易蒸发,因此成熟期间肉品的成熟损失几乎无质量变化。HODGES等[26]研究表明,真空包装的样品只会造成微小的质量损失。DEGEER等[27]测定干法成熟过程中带骨和不带骨牛背最长肌的成熟损失,结果表明成熟损失随成熟时间延长而增大,KIM等[28]也证明干法成熟猪肉的成熟损失显著高于湿法成熟,这些与本研究结果一致。环境的相对湿度通过影响肉品的水分蒸发从而影响肉品的最终重量,进而影响肉品的水分含量和蒸煮损失,环境湿度相对较低,肉品表面水分蒸发速率快,成熟损失大。3.2 干法成熟对羊腿肉水分含量的影响
在成熟过程中湿法成熟和RH80干法成熟组羊腿肉水分含量先上升后下降,原因是0 d的羊腿肉处于僵直期,肉持水能力差,在取样分割过程中会损失掉水分,故水分含量低;随着时间推移,肉品进行解僵和成熟,持水能力增大,在测量切割过程中损失的水分较少,肌肉持水能力会降低,汁液流失过多,羊腿肉水分含量降低[29]。成熟7 d时,3种成熟方法的水分含量无显著差异,这与LI等[30]的研究结果一致,其试验发现成熟8 d时干法成熟与湿法成熟肉品的水分含量无显著差异。LEE等[4]试验表明,与湿法成熟样品相比,长时间的干法成熟会降低样品中的水分含量,对于形成浓郁的干法成熟牛肉风味有重要意义,这与RH60干法成熟14、21和28 d的羊腿肉水分含量显著低于湿法成熟结果一致。干法成熟过程中羊腿肉的成熟损失不断增大,这也是干法成熟羊腿肉成熟后期水分含量降低的原因之一。3.3 干法成熟对羊腿肉蒸煮损失的影响
持水能力是肉类加工的重要性能,羊腿肉蒸煮损失是衡量肌肉持水能力的重要指标之一,随着成熟时间的延长,肌肉在内源酶的作用下,破坏肌原纤维和骨架蛋白的结构,结合水的能力下降,汁液流失、蒸煮损失逐渐增大[31]。本研究中,干法成熟羊腿肉的蒸煮损失显著低于湿法成熟,表明干法成熟可以显著提高羊腿肉的持水能力。LI等[12]与本研究结果相似,结果表明干法成熟14 d牛肉蒸煮损失比湿法成熟牛肉低。LASTER等[32]也表明干法成熟牛腰肉和里脊肉的加工产量高于湿法成熟样品。成熟7 d时,3种成熟方式样品的水分含量无显著差异,但是干法成熟肉品的蒸煮损失显著低于湿法成熟肉品,持水能力优于湿法成熟肉品,但在成熟14和21 d,干法成熟羊腿肉水分含量显著低于湿法成熟,可能是样品持水能力提高,也可能是因为样品本身水分含量降低而导致蒸煮损失降低。成熟14 d羊腿肉蒸煮损失与成熟7 d的试验结果相比,蒸煮损失下降,该结果与KIM等[28]的研究结果一致。一方面可能是随着肉中蛋白质的分解,吸收K+、释放Ca2+导致离子净电荷增加,渗透压增加,持水能力升高,同时蛋白质的电荷发生改变,也导致持水能力的增加[33,34];另一方面可能是在较短的成熟时间内,细胞内蛋白降解使细胞膨胀,增加肉品的持水能力。KRISTENSEN等[35]研究表明,宰后2—7 d猪肉的持水能力下降,7 d后持水能力有所增加,进一步分析表明在细胞收缩前,骨架蛋白降解导致细胞外的水进入到细胞内,增加细胞的持水能力。DAVIS等[36]的研究也证明了猪肉第7和14天肌间线蛋白降解较多的样品持水性高。因此,干法成熟技术对提高羊腿肉持水能力有积极作用,且在成熟14 d时,羊腿肉的持水能力显著提高。3.4 干法成熟羊腿肉蛋白质二级结构分析
通常情况下,1 615—1 637 cm-1和1 682—1 700 cm-1为β-折叠,1 646—1 664 cm-1为α-螺旋,1 637—1 645 cm-1为无规则卷曲,1 664—1 681 cm-1为β-转角[37]。α-螺旋和β-折叠表征蛋白质分子的规则性,β-转角和无规卷曲通常反映出其较松散的结构[38]。在成熟过程中,3种成熟方法均会导致蛋白质二级结构中α-螺旋和β-折叠的比例先上升后下降,即表明成熟时间过长,肌纤维结构的破坏会导致蛋白质中有序结构转向无序结构,此时肌肉持水能力下降,蒸煮损失增加。α-螺旋结构的比例升高,表明蛋白构象发生了变化,也反映其内部的疏水性位点暴露程度下降,表面疏水性减少[39],预示着蛋白质的持水能力增强。在成熟过程中,无序结构降低表明成熟14 d比成熟7 d的羊腿肉蛋白质稳定性高,持水能力好;环境湿度越低,蛋白质稳定性越高,羊腿肉持水能力越好。因此,本研究结果表明干法成熟组羊腿肉成熟14 d蛋白质稳定性较好,持水能力较强。3.5 干法成熟羊腿肉水分迁移和分布分析
T2表示H质子的横向弛豫时间,其分布表示肉中存在多个水分群,T2越短,说明水与底物结合越紧密,T2越长则表示水分越自由[40]。湿法成熟组和RH80干法成熟组的T21在成熟21 d以前均无显著差异,在成熟28 d显著降低,分析原因可能是随着成熟时间的延长,蛋白质、脂肪等物质在自身酶的作用下被分解消耗,产生各种代谢物增加体系的黏度,降低水分自由度[22]。在整个成熟期间,湿法成熟组羊腿肉P21变化范围是90.765%—95.793%,P22变化范围是0.765%—6.258%;RH80干法成熟组羊腿肉P21变化范围是90.931%—95.803%,P22变化范围是0.729%—6.08%;RH60干法成熟组羊腿肉P21变化范围是91.402%—95.933%,P22变化范围是0.568%—5.474%。由此可知,3种成熟方法组羊腿肉P21变化范围大致相同,P22变化范围由小到大依次是RH60干法成熟组、RH80干法成熟组和湿法成熟组,可见干法成熟组羊腿肉的持水能力较好。低场核磁结果显示,羊腿肉在成熟0—7 d时,内部不易流动水转化为结合水;在成熟7—14 d时,结合水转化为不易流动水。成熟14 d羊腿肉的蒸煮损失显著低于成熟7 d,这是因为不易流动水对肌肉的持水能力起决定性影响[41],羊腿肉中不易流动的水增多,持水能力增强,蒸煮损失降低。在成熟21—28 d时,3组羊腿肉中不易流动水大量转化为自由水,可能是因为在成熟后期肌肉中蛋白质大量降解,肌肉结构崩塌[42],束缚水的能力下降,自由水相对含量增加。此时细胞的持水能力最弱,因而在成熟28 d时羊腿肉蒸煮损失最大。
在干法成熟过程中,肉中自由水梯度高于外部环境湿度,会通过自由扩散从肉品表面迁移到周围环境中,使其达到相对稳定的状态[22,43],湿度越低,水分迁移越多,导致羊腿肉水分含量下降,成熟损失增大。
4 结论
干法成熟羊腿肉蒸煮损失显著低于湿法成熟,表明干法成熟可以有效提高羊腿肉的持水能力。在干法成熟14 d内,不易流动水先转化为结合水再转化为不易流动水,成熟14 d羊腿肉中不易流动水增多,持水能力增强;在干法成熟21—28 d,羊腿肉中的不易流动水转化为自由水,自由水含量增多,持水能力降低。羊腿肉在较长时间的干法成熟过程中,环境湿度对样品的成熟损失和蒸煮损失影响显著,对肉品的水分含量、水分迁移和分布影响不显著。因此,干法成熟14 d是羊腿肉成熟的最佳推荐时间。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1016/j.meatsci.2013.04.039URL [本文引用: 1]

Meat tenderness is an important quality parameter determining consumer acceptance and price. Meat tenderness is difficult to ensure in the global meat chain because the production systems are not always aiming at this purpose (ex.: cattle derived from milk production) and by the existence within the carcass of "tough" primals. Different methods can be used by the meat industry to improve meat tenderness each with its advantages and drawbacks. The application of hydrodynamic pressure or shockwaves has showed outstanding improvements by reducing the Warner Bratzler Shear Force by 25% or more. However, the technology has not penetrated into the market as first systems were based on the use of explosives and further developments seemed to lack the robustness to fulfill industrial requirements. The present paper describes the main challenges to construct a prototype for the continuous treatment of meat by shockwaves based on electrical discharges under water. Finally, improvements on the tenderness of meat by using the novel prototype are presented. (C) 2013 Elsevier Ltd.
DOI:10.5851/kosfa.2016.36.2.159URLPMID:27194923 [本文引用: 1]

Animal muscles are stored for specific period (aging) at refrigerated temperatures, during and after which the living muscles start to convert into meat and thus, attain certain superior properties in the final product. Proteolysis, lipolysis, and oxidation are the major biochemical processes involved during the postmortem aging of meat that affect the tenderness, juiciness, and flavor, as well as sometimes may introduce certain undesirable traits. This review analyzes the role of pre- and post-mortem factors that are important for aging and their effect on the chemical and physical changes in the
URLPMID:30743184 [本文引用: 1]
[本文引用: 2]
DOI:10.1111/jfds.2001.66.issue-2URL [本文引用: 1]
DOI:10.1111/jmf.1992.3.issue-2URL [本文引用: 1]
DOI:10.1016/j.meatsci.2018.04.031URLPMID:29731371 [本文引用: 1]

Postmortem aging is a value-adding process and has been extensively practiced by the global meat industry for years. The rate and extent of aging impacts on meat quality characteristics are greatly affected by various biochemical/physiological changes occurring during the pre-rigor phase through post-rigor aging processes. This should also mean that the positive aging impacts on eating quality attributes can be further maximized through establishing specific post-harvest aging strategies. In this review, we propose the smart-aging concept, which is to develop innovative template strategies through identifying optimal aging regimes to maximize positive aging impacts on meat quality and value. The concept requires a good understanding of the physical, biochemical and post-harvest factors that affect the aging of beef. This knowledge coupled with the ability to non-invasively determine muscle composition early postmortem will create opportunities to tailor the process of muscle conversion to meat and the subsequent aging processes to deliver meat with consistent and improved eating qualities and functionality.
DOI:10.1186/s40781-016-0101-9URL [本文引用: 2]
DOI:10.1016/j.meatsci.2007.10.028URL [本文引用: 1]

Abstract
Paired beef short loins from US Choice (n = 48) and US Select (n = 48) carcasses were assigned to be dry or wet aged for 14, 21, 28 or 35 d. After aging, short loins were processed to determine retail yields and processing times. Upon completion of cutting tests, steaks were served to consumers to assess palatability characteristics. Retail cutting tests showed that dry-aged short loins had reduced yields and increased cutting times when compared to wet-aged short loins. Consumers were unable to determine differences between dry- and wet-aged steaks and for aging periods; however, USDA quality grade had a significant impact on consumer perception of palatability attributes.DOI:10.1016/j.meatsci.2015.10.017URLPMID:26551359 [本文引用: 1]

The study objective was to evaluate the effect of post-mortem aging period (14 to 49days), dry vs. wet (D vs W) type of aging on the palatability of bone-in (BI) beef short loins (n=96) and boneless (BL) strip loins (n=96) possessing United States Department of Agriculture marbling scores between Slight and Small. Warner-Bratzler shear force (WBSF) scores decreased linearly over time (P=0.0001). WBSF was not influenced by aging method or loin type. Aged flavor was higher for DBL than for DBI with WBL and WBI intermediate. Dry aging strip loins increase aged flavor yet did not improve beefy flavor compared to wet aging. Based on objective data and panelist's scores for tenderness, juiciness and aged flavor, a boneless, 28days wet aged strip steak, cooked to 71 degrees C would provide the best combination of eating satisfaction and value.
DOI:10.1016/j.meatsci.2015.09.008URLPMID:26437054 [本文引用: 1]

The objectives of this study were to evaluate different dry-aging regimes and their impacts on quality attributes and metabolite profiles of beef loins. Thirty loins (M. longissimus lumborum) from 15 beef carcasses at 2 days post-mortem were obtained. Each loin was cut in half yielding 60 sections, which were randomly assigned to six treatments including 4 dry-aging (2 temperatures (1 or 3 degrees C) x 2 air-velocities (0.2 or 0.5 m/s)) and 2 wet-aging regimes for 3 weeks; n=10/treatment. The sensory panel found that dry-aged loins had better flavour and overall liking (P<0.05), but there were no differences in tenderness and juiciness. No differences in drip/cook-loss and colour were observed. Metabolite analysis showed that 7 metabolites, including several flavour precursors, were more abundant in the dry-aged beef compared to the wet-aged beef, which may contribute to the enhanced flavours of the dry-aged beef. Overall, dry-aging loins at 3 degrees C with 0.2m/s resulted in the greatest improvement in beef palatability.
DOI:10.1016/j.meatsci.2013.05.009URL [本文引用: 2]

This study investigated meat quality and consumer preference after ageing beef gluteus medius in a water vapour-permeable dry-ageing bag or in vacuum for 14 days. Higher ageing and trim losses but lower thawing loss, cooking loss and water content were found in samples aged in dry ageing bags compared to those aged in vacuum. Samples aged in dry ageing bags had higher total bacteria and yeast counts but lower lactic acid bacteria counts than those aged in vacuum, both before and after trimming. Meat aged in dry ageing bag was more tender and juicier and overall preferred by consumers compared with samples aged in vacuum. Female participants outperformed the males in detecting differences in palatability. No differences were found in pH, smell, shear force, colour, Enterobacteriaceae, and mould counts. Thus, by using a dry ageing bag, it is possible to produce dry-aged meat in a more controlled condition without negative effects on sensory or other quality attributes. (c) 2013 Published by Elsevier Ltd.
DOI:10.1016/j.ijgfs.2011.11.005URL [本文引用: 2]
DOI:10.1016/j.foodchem.2015.03.088URLPMID:25872440 [本文引用: 1]

The effects of using electrospun chitosan fibres as a wrapping material for dry-ageing beef was studied and compared to traditional dry-ageing and wet-ageing of beef for up to 21 days. The chitosan treatment showed improved results in terms of yield, reduction of microbial counts, yeasts and moulds, and lighter appearance compared to traditional dry-ageing. Weight and trimming losses were minimal in the wet-ageing beef. However, significant growth of lactic acid bacteria was observed in this group. Transverse relaxation times indicated a lower degree of muscle denaturation during ageing in the chitosan dry-ageing beef compared to the traditional dry-ageing meat. A principal component analysis furthermore indicated that 60.6% of the variation between samples and ageing treatments could be described by differences in the water content and distribution in the muscle. The study showed that electrospun chitosan fibre mats have potential as a wrapping material for improved quality during dry-ageing of beef.
URL [本文引用: 1]
URL [本文引用: 1]
URLPMID:30675105 [本文引用: 1]
DOI:10.1071/AN09162URL [本文引用: 1]
DOI:10.1111/jfds.2003.68.issue-1URL [本文引用: 1]
[D].
URL [本文引用: 1]
[D].
URL [本文引用: 1]
DOI:10.1021/jf061576wURLPMID:17061838 [本文引用: 1]

Fourier transform infrared (FT-IR) microspectroscopy and low-field (LF) proton NMR transverse relaxation measurements were used to study the changes in protein secondary structure and water distribution as a consequence of aging (1 day and 14 days) followed by salting (3%, 6%, and 9% NaCl) and cooking (65 degrees C). An enhanced water uptake and increased proton NMR relaxation times after salting were observed in aged meat (14 days) compared with nonaged meat (1 day). FT-IR bands revealed that salting induced an increase in native beta-sheet structure while aging triggered an increase in native alpha-helical structure before cooking, which could explain the effects of aging and salting on water distribution and water uptake. Moreover, the decrease in T2 relaxation times and loss of water upon cooking were attributed to an increase in aggregated beta-sheet structures and a simultaneous decrease in native protein structures. Finally, aging increased the cooking loss and subsequently decreased the final yield, which corresponded to a further decrease in T2 relaxation times in aged meat upon cooking. However, salting weakened the effect of aging on the final yield, which is consistent with the increased T2 relaxation times upon salting for aged meat after cooking and the weaker effect of aging on protein secondary structural changes for samples treated with high salt concentration. The present study reveals that changes in water distribution during aging, salting, and cooking are not only due to the accepted causal connection, i.e., proteolytic degradation of myofibrillar structures, change in electrostatic repulsion, and dissolution and denaturation of proteins, but also dynamic changes in specific protein secondary structures.
URL [本文引用: 1]
URL [本文引用: 1]
URL [本文引用: 3]
URL [本文引用: 3]
DOI:10.1002/mrc.3825URLPMID:22674672 [本文引用: 1]

The goal of this review is to give an overview of general trends in the application of the NMR related to fish processing and quality and to provide some viewpoints on the current situation. Three novel examples of the application of the methodologies magnetic resonance spectroscopy, magnetic resonance imaging, and low-field NMR are also presented. The capability of these techniques to be utilized as a tool to optimize fish processing, and thereby improving product quality, as well as to confirm labelling information, are demonstrated.
DOI:10.1016/j.meatsci.2005.04.022URLPMID:22064064 [本文引用: 1]

Unacceptable water-holding capacity costs the meat industry millions of dollars annually. However, limited progress has been made toward understanding the mechanisms that underlie the development of drip or purge. It is clear that early postmortem events including rate and extent of pH decline, proteolysis and even protein oxidation are key in influencing the ability of meat to retain moisture. Much of the water in the muscle is entrapped in structures of the cell, including the intra- and extramyofibrillar spaces; therefore, key changes in the intracellular architecture of the cell influence the ability of muscle cells to retain water. As rigor progresses, the space for water to be held in the myofibrils is reduced and fluid can be forced into the extramyofibrillar spaces where it is more easily lost as drip. Lateral shrinkage of the myofibrils occurring during rigor can be transmitted to the entire cell if proteins that link myofibrils together and myofibrils to the cell membrane (such as desmin) are not degraded. Limited degradation of cytoskeletal proteins may result in increased shrinking of the overall muscle cell, which is ultimately translated into drip loss. Recent evidence suggests that degradation of key cytoskeletal proteins by calpain proteinases has a role to play in determining water-holding capacity. This review will focus on key events in muscle that influence structural changes that are associated with water-holding capacity.
URL [本文引用: 1]
URL [本文引用: 1]
DOI:10.1111/jfds.1974.39.issue-1URL [本文引用: 1]
DOI:10.1016/j.meatsci.2009.08.017URL [本文引用: 1]

Abstract
This experiment investigated the combined effects of two dry-aging methods (unpackaged and in a bag), two loin-cut styles (bone-in shell loins and boneless strip loins), and two aging times (21 and 28 days) on the physical, chemical, sensory, and microbial properties of dry-aged beef. Sections from shell and strip loin were assigned randomly to be aged unpackaged or aged packaged in a bag with high moisture permeability. Weight losses increased with aging time. Shell loins lost more (P < 0.05) weight during aging compared with strip loins; dry aging in a bag had less (P < 0.05) weight loss than unpackaged aging. There were no differences (P > 0.05) in any of the sensory traits between shell and strip loins or dry aging using a traditional method or in a bag. Dry aging in a bag creates positive effects on yields, no negative effects on product quality, and adds flexibility and control of the aging environment.DOI:10.1007/s10068-018-0418-xURLPMID:30483418 [本文引用: 2]

This study investigated the effects of aging methods (wet aging and dry aging) and aging times (7 and 14 days) on the physicochemical and sensory characteristics of meat quality using pork loin. Dry-aged loin (DA) had significantly lower moisture content and higher crude fat protein content than wet-aged loin (WA). The pH of DA was significantly higher than that of WA and it increased with the aging time. DA showed lower cooking loss and higher aging loss than WA (p < 0.001). Lipid oxidation and metmyoglobin content of DA were higher than those of WA (p < 0.001). Shear force in DA was lower than that in WA (p < 0.001) and myofibril protein index (MFI) increased in DA. In addition, DA recorded higher scores of roast color, flavor and overall acceptability compared to WA. These results suggested that the application of dry-aging on pork improved physicochemical, textural and sensory characteristics.
[D].
URL [本文引用: 1]
[D].
URL [本文引用: 1]
DOI:10.1016/j.meatsci.2014.03.014URL [本文引用: 1]

The objective of this study was to investigate beef quality of longissimus muscle after ageing in dry ageing bags, traditional dry ageing or vacuum for 8 or 19 days. Lower ageing weight loss, odour score and microbial growth were found in meat aged in dry ageing bags than after traditional dry ageing. The sensory panel detected no differences for most of the sensory attributes between samples using the two dry ageing methods, except for the odour of the cutting surface. The dry-aged steaks had more umami and butter fried meat taste compared with vacuum-aged steaks. Ageing time affected most of the sensory traits in this study, which improved as ageing time increased from 8 to 19 days. In a consumer test, meat aged for 21 days in dry ageing bags was preferred than the samples aged in vacuum. This may be due to the higher tenderness and juiciness obtained during storage in dry ageing bags than meat aged in vacuum. (C) 2014 Elsevier Ltd.
DOI:10.1111/jmf.2001.12.issue-2URL [本文引用: 1]
DOI:10.1016/j.meatsci.2008.03.024URL [本文引用: 1]

Abstract
Top Choice (n = 48) and Select (n = 48) paired bone-in ribeye rolls, bone-in strip loins, and boneless top sirloin butts were assigned randomly to one of two aging treatments, dry or wet, and were aged for 14, 21, 28 or 35 d. Cutting tests, performed to determine retail yields and processing times, showed dry-aged subprimals had lower total saleable yield percentages and increased processing times compared to wet-aged subprimals. Sensory and Warner–Bratzler shear evaluation was conducted to determine palatability characteristics. For the most part, aging treatment and aging period did not affect consumer sensory attributes. However, ribeye and top loin steaks from the Top Choice quality grade group received higher sensory ratings than their Select counterparts. For top sirloin steaks, no consumer sensory attributes were affected by aging treatment, aging period, or quality grade group.DOI:10.1111/jfpp.12499URL [本文引用: 1]
DOI:10.1016/j.meatsci.2006.08.005URL [本文引用: 1]

Abstract
Exercise has been shown previously to reduce the water holding capacity (WHC) of meat in lamb. The consequence of changes in the distribution of ions pre- and post-rigor and proteolysis on WHC is relatively unknown. Twelve crossbred lambs were used to investigate the effect of exercise on the meat quality traits of the Longissimus thoracis et lumborum (LTL) muscle. There were no treatment effects on Warner–Bratzler shear force (WBSF), myofibril and sarcoplasmic protein solubility, denaturation or sarcomere length. With exercise the initial pH of the muscle was lower and the rate of pH fall to rigor was faster compared to controls. Exercise caused increased purge and meat fluid had a lower osmolarity, magnesium, potassium and sodium concentration. Proteolysis of desmin occurred after day 3 and vinculin on day 7 of ageing with exercise. It was concluded that exercise caused changes in the distribution of ions and the proteolysis of muscle proteins that reduced the ability of the muscle to bind or hold water.DOI:10.1016/s0309-1740(00)00125-xURLPMID:22061914 [本文引用: 1]

The water-holding capacity (WHC) of pork decreases post-mortem but has been shown to increase during subsequent ageing. In order to test a hypothesis that water-holding capacity increases during ageing due to degradation of the cytoskeleton, WHC was followed 10 days post-mortem and related to the extent of proteolysis of cytoskeletal proteins. A fast method for measuring WHC in small meat samples was developed by the use of centrifugation. The WHC of fresh pork decreases in the first part of post-mortem storage after which it increases to the level of 1 day PM. No changes in total water content of the meat were observed which could explain changes in WHC during ageing. Vinculin and desmin degrade gradually during ageing while talin degrades rapidly. These observations are consistent with the hypothesis that degradation of the cytoskeleton slowly removes the linkage between lateral shrinkage of myofibrils and shrinkage of entire muscle fibres, so removing the force that causes flow into the extracellular space. Inflow of previously expelled water is then possible, so increasing WHC as observed in later periods of storage.
DOI:10.1016/S0309-1740(03)00154-2URL [本文引用: 1]

Abstract
The effects of preinjection aging time on pork loins injected with a salt/phosphate/lactate solution were investigated. Ninety-six fresh pork loins, in two replicates, were divided into two treatments. Loins in the first treatment were injected to 13% of loin weight at 1 day post-slaughter with a brine containing 2.17% salt/3.04% phosphate/20.8% lactate. The second group was injected with the same brine 4 days after slaughter. Color and Warner-Bratzler shear (WBS) force were measured immediately, 7 and 14 days after injection. Purge was measured 8 and 15 days postmortem. Western blots to measure troponin-T degradation were performed on samples from the two loins that resulted in the lowest shear force, and from the two loins that resulted in the highest shear force, as measured by Warner-Bratzler shear. The two loins with the most purge loss and the two loins with the least purge loss were also analyzed by Western blots for desmin degradation. The L∗ and b∗ color values were higher (P<0.05) and purge was greater (P<0.05) for loins injected 1 day postmortem than for loins injected 4 days postmortem. Western blots demonstrated that injection did not affect protein degradation. Therefore, differences between individual animals that affect protein degradation remain important regardless of injection treatment.DOI:10.3864/j.issn.0578-1752.2017.05.013URL [本文引用: 1]

【Objective】Hot-pressure extraction was utilized to prepare chicken bone protein (CBP) to study the effect of extraction time on gelatin properties. The study was expected to provide a reference for the preparation technology of edible gelatin by using chicken bone by-products. 【Method】After being adjusted to the same concentration of protein, CBP at different extraction times were incubated at 4℃ to form gelatin. The content of total soluble substance (TSS), crude protein and hydroxyproline (Hyp), secondary structure of protein and distribution of molecular weight (MW), as well as color of gelatin and gel strength were studied. Furthermore, correlation analysis between indexes of CBP was also performed to establish possible linkages between physicochemical properties and gelatin properties of CBP.【Result】The results showed that hot-pressure extraction time had a significant effect on the yield of CBP (P<0.05), leading to the variable structure of protein and consequently affected the properties of gelatin made by CBP. The contents of TSS, crude protein and Hyp were increased significantly during extraction (0-120 min) (P<0.05). The result of MW distribution suggested that protein experienced dramatic degradation from 40 to 120 min (P<0.05). The proportion of MW between 10 and 30 kDa decreased from 59.82% (40 min) to 13.99% (120 min) while the ratio of MW <10 kDa increased from 35.46% (40 min) to 86.01% (120 min). In addition, the extraction time caused the denaturation of protein and thus leading to the change of secondary structure. The CBP was composed of 100 percent of α-helix (0-40 min), and then β-sheet emerged at 60 and 90 min (9.9% and 17.6%, respectively) but disappeared at 120 min, which indicated that the secondary structure of protein experienced transformation during extraction. Results of CBP gelatin properties suggested that CBP at 0 min could not form a gelatin and thus had no gelatin properties. Gel strength at 20, 40 and 60 min was at same level and better than CBP gelatins of 90 and 120 min. In addition, transparency of CBE solutions was significantly impacted by extraction time, which increased sharply during hot-pressure extraction (20-120 min) (P<0.05). Meanwhile, extraction time significantly affected the color of gelatins (P<0.05) and gelatin at 40 and 60 min met the requirements of high lightness, low yellowness and high whiteness. However, water holding capacity was insensitive to the extraction time (P>0.05). Correlation analysis showed that the content of Hyp, degradation of protein were considerably related to gelatin properties (P<0.05).【Conclusion】In conclusion, extraction time could significantly affect the gelatin properties prepared by CBP at different extraction times. Given yield of protein, color of gelatin, gel strength, etc., CBP extracted at 40 min and 60 min were better proper for preparation of gelatin.
DOI:10.3864/j.issn.0578-1752.2017.05.013URL [本文引用: 1]

【Objective】Hot-pressure extraction was utilized to prepare chicken bone protein (CBP) to study the effect of extraction time on gelatin properties. The study was expected to provide a reference for the preparation technology of edible gelatin by using chicken bone by-products. 【Method】After being adjusted to the same concentration of protein, CBP at different extraction times were incubated at 4℃ to form gelatin. The content of total soluble substance (TSS), crude protein and hydroxyproline (Hyp), secondary structure of protein and distribution of molecular weight (MW), as well as color of gelatin and gel strength were studied. Furthermore, correlation analysis between indexes of CBP was also performed to establish possible linkages between physicochemical properties and gelatin properties of CBP.【Result】The results showed that hot-pressure extraction time had a significant effect on the yield of CBP (P<0.05), leading to the variable structure of protein and consequently affected the properties of gelatin made by CBP. The contents of TSS, crude protein and Hyp were increased significantly during extraction (0-120 min) (P<0.05). The result of MW distribution suggested that protein experienced dramatic degradation from 40 to 120 min (P<0.05). The proportion of MW between 10 and 30 kDa decreased from 59.82% (40 min) to 13.99% (120 min) while the ratio of MW <10 kDa increased from 35.46% (40 min) to 86.01% (120 min). In addition, the extraction time caused the denaturation of protein and thus leading to the change of secondary structure. The CBP was composed of 100 percent of α-helix (0-40 min), and then β-sheet emerged at 60 and 90 min (9.9% and 17.6%, respectively) but disappeared at 120 min, which indicated that the secondary structure of protein experienced transformation during extraction. Results of CBP gelatin properties suggested that CBP at 0 min could not form a gelatin and thus had no gelatin properties. Gel strength at 20, 40 and 60 min was at same level and better than CBP gelatins of 90 and 120 min. In addition, transparency of CBE solutions was significantly impacted by extraction time, which increased sharply during hot-pressure extraction (20-120 min) (P<0.05). Meanwhile, extraction time significantly affected the color of gelatins (P<0.05) and gelatin at 40 and 60 min met the requirements of high lightness, low yellowness and high whiteness. However, water holding capacity was insensitive to the extraction time (P>0.05). Correlation analysis showed that the content of Hyp, degradation of protein were considerably related to gelatin properties (P<0.05).【Conclusion】In conclusion, extraction time could significantly affect the gelatin properties prepared by CBP at different extraction times. Given yield of protein, color of gelatin, gel strength, etc., CBP extracted at 40 min and 60 min were better proper for preparation of gelatin.
URLPMID:30198667 [本文引用: 1]

In order to further clarify the influence mechanism of different freezing temperature on meat quality in meat industry. The effects of freezing at -18, -23 and -38 on the stability of protein secondary structures of beef were studied. The attenuated total reflectance Fourier transfer infrared spectroscopy(ATR-FTIR)technique and automatic deconvolution, curve fitting and other calculation and analysis methods were used to analyze the changes of beef myofibrillar protein infrared spectra and secondary structures during -18, -23 and -38 freezing-thawing processes. ATR-FTIR results showed that the peak high and peak area of infrared spectra of beef myofibrillar protein in the freezing-thawing processes were changed, and the red shift or blue shift of wavenumbers occurred. The intensities of the absorption peak of 3 500~3 300 cm-1 in the infrared spectra of the frozen-thawed beef were reduced or even disappeared. This indicated that the intramolecular and intermolecular hydrogen bonding interactions, which formed by the bound water O-H group and the amino acid CO group, in thawed beef myofibrillar protein were broken. In other words, freezing can result in the destruction of beef myofibrillar protein secondary structures and protein advanced structures unfolded. Once the beef is thawed, the unfolded protein would reaggregation, and protein renaturation. Freezing could affect the stability of beef myofibrillar protein, the relative content of alpha-helix, beta-sheet, and beta-turn of beef myofibrillar protein were decreased, and the alpha-helix and ordered structures changed to the randon coil and disordered structures. After thawing, the increase of beta-sheet relative content of beef myofibrillar protein at -38 was greater than that of -23 and -18 . The stability of -38 frozen beef myofibrillar protein was the best, and the protein renaturation was also the best after thawing. That is, the lower the freezing temperature, the lower the measure of freezing denaturation of beef myofibrillar protein, and the better the secondary structures stability of beef myofibrillar protein. The experimental study based on the actual production condition of the meat industry. And the effect of freezing temperatures on beef protein denaturation and the possible mechanism were revealed at the micro-aspect. It can be seen that the ATR-FTIR technology can reflect the changes of protein secondary structures in the process of freezing-thawing of beef, and reveal the regularity of beef protein denaturation, which can be used to identify and evaluate the quality of frozen meat. The experimental results provide a reference for the freezing preservation process and a method for the quality evaluation of meat.
URLPMID:30198667 [本文引用: 1]

In order to further clarify the influence mechanism of different freezing temperature on meat quality in meat industry. The effects of freezing at -18, -23 and -38 on the stability of protein secondary structures of beef were studied. The attenuated total reflectance Fourier transfer infrared spectroscopy(ATR-FTIR)technique and automatic deconvolution, curve fitting and other calculation and analysis methods were used to analyze the changes of beef myofibrillar protein infrared spectra and secondary structures during -18, -23 and -38 freezing-thawing processes. ATR-FTIR results showed that the peak high and peak area of infrared spectra of beef myofibrillar protein in the freezing-thawing processes were changed, and the red shift or blue shift of wavenumbers occurred. The intensities of the absorption peak of 3 500~3 300 cm-1 in the infrared spectra of the frozen-thawed beef were reduced or even disappeared. This indicated that the intramolecular and intermolecular hydrogen bonding interactions, which formed by the bound water O-H group and the amino acid CO group, in thawed beef myofibrillar protein were broken. In other words, freezing can result in the destruction of beef myofibrillar protein secondary structures and protein advanced structures unfolded. Once the beef is thawed, the unfolded protein would reaggregation, and protein renaturation. Freezing could affect the stability of beef myofibrillar protein, the relative content of alpha-helix, beta-sheet, and beta-turn of beef myofibrillar protein were decreased, and the alpha-helix and ordered structures changed to the randon coil and disordered structures. After thawing, the increase of beta-sheet relative content of beef myofibrillar protein at -38 was greater than that of -23 and -18 . The stability of -38 frozen beef myofibrillar protein was the best, and the protein renaturation was also the best after thawing. That is, the lower the freezing temperature, the lower the measure of freezing denaturation of beef myofibrillar protein, and the better the secondary structures stability of beef myofibrillar protein. The experimental study based on the actual production condition of the meat industry. And the effect of freezing temperatures on beef protein denaturation and the possible mechanism were revealed at the micro-aspect. It can be seen that the ATR-FTIR technology can reflect the changes of protein secondary structures in the process of freezing-thawing of beef, and reveal the regularity of beef protein denaturation, which can be used to identify and evaluate the quality of frozen meat. The experimental results provide a reference for the freezing preservation process and a method for the quality evaluation of meat.
DOI:10.1016/j.meatsci.2008.02.014URL [本文引用: 1]

Abstract
Secondary structures, gelation properties and their relationships in porcine myosin were studied by circular dichroism, dynamic rheological measurement and scanning electron microscopy. Gelling of porcine myosin involved a change in myosin conformation with protein–protein and protein–water interactions. The gelation properties were strongly pH and temperature dependent. Near the pI (pH 5.5 and 6.0), porcine myosin could spontaneously coagulate at 15 °C resulting partially from the presence of more β-sheets. Myosin at pH 6.5–9.0 began to form a gel at temperatures greater than 38 °C. Heating caused α-helices to partially turn into β-sheets and random coils. Subsequently, myosin aggregated and formed a gel network. The gel strength decreased and the water-holding capacity (WHC) increased with increasing pH. Correlation analysis indicated that both the unfolding of α-helices and the formation of β-sheets favored the gelation of porcine myosin. A high β-sheet fraction prior to heating resulted in a low WHC of resultant gel. A compact and uniform gel was also obtained at pH 6.5.URL [本文引用: 1]

Low-field nuclear magnetic resonance, with the advantages of rapid and non-destructive determination and small volume samples, has been exploited and applied in the field of meat science. The principle of low-field nuclear magnetic resonance is introduced in this paper. Moreover, recent advances in the application of low-field nuclear magnetic resonance in determining water contents and other related quality characteristics of meat and meat products is outlined.
URL [本文引用: 1]

Low-field nuclear magnetic resonance, with the advantages of rapid and non-destructive determination and small volume samples, has been exploited and applied in the field of meat science. The principle of low-field nuclear magnetic resonance is introduced in this paper. Moreover, recent advances in the application of low-field nuclear magnetic resonance in determining water contents and other related quality characteristics of meat and meat products is outlined.
DOI:10.2527/1996.745993xURLPMID:8726731 [本文引用: 1]

Postmortem (PM) and mu-calpain-induced degradation of specific skeletal muscle proteins was monitored by SDS-PAGE and Western blotting. Samples were removed from bovine longissimus thoracis (LT) at approximately 45 min PM for the preparation of at-death (0-d) myofibrils (MF). The LT was excised at 1 d PM, vacuum-packaged, and stored at 2 degrees C. Samples were removed for Warner-Bratzler shear force analysis and biochemical analysis at 1, 3, 7, 14, 28, and 56 d PM. The protease mu-calpain was purified from bovine skeletal muscle and used to digest at-death MF at pH 5.6, 4 degrees C, 100 microM CaCl2. Degradation of the proteins titin, nebulin, filamin, desmin, and troponin-T was monitored in the PM and mu-calpain-digested samples by using SDS-PAGE and Western blotting. The PM samples that had significantly lower shear force (LSF) values (P < .05) at 1 d PM exhibited faster degradation of these five proteins than the higher shear force (HSF) samples. In LSF samples, the intact titin band (T1) was absent by 7 d PM and nebulin was absent by 3 d PM. In LSF samples, some filamin was degraded by 3 d PM, but in HSF samples degradation was not apparent until 14 d PM. In LSF samples, desmin was degraded more rapidly PM than in HSF samples. Troponin-T was broken down PM to yield two major polypeptides of approximately 28 and 30 kDa; these polypeptides appeared earlier PM in LSF samples. Degradation products, similar to those observed PM, for all five proteins also were detected in Western blots of mu-calpain-digested MF, suggesting the calpain system plays a key role in PM protein degradation.
[D].
URL [本文引用: 1]
[D].
URL [本文引用: 1]
DOI:10.1016/0309-1740(92)90056-AURLPMID:22059627 [本文引用: 1]

The water condition of cured and uncured pork, having different ageing times, was studied in the process of heating to 80 degrees C, using nuclear-magnetic resonance methods. In the transverse magnetization decay functions five components could be distinguished, differing from each other by their relative weights and relaxation times. The greatest relative weight (60-70%) was characteristic of the component having relaxation time 35-45 ms ('d'), which could be related to immobilized water. The change of the component's 'd' relative weight while heating was significantly dependent on the ageing time of meat and the factor of curing or not curing before heating. It was shown that the greatest amount of immobilized water and its strongest bonding were observed in uncured pork during the first 6-7 h and after 48 h post mortem. Cured meat possesses the highest amount of immobilized water at room temperature after 48-96 h post mortem, however, its bonding at heating is higher in the case of meat which is cured during the first hours and after 96 h post mortem.
