 ,, 陈善春
,, 陈善春 ,西南大学/中国农业科学院柑桔研究所国家柑桔品种改良中心,重庆 400712
,西南大学/中国农业科学院柑桔研究所国家柑桔品种改良中心,重庆 400712Effect of Transcription Factor CsWRKY61 on Citrus Bacterial Canker Resistance
LONG Qin, DU MeiXia, LONG JunHong, HE YongRui, ZOU XiuPing ,, CHEN ShanChun
,, CHEN ShanChun ,National Center for Citrus Variety Improvement, Citrus Research Institute, Southwest University/Chinese Academy of Agricultural Sciences, Chongqing 400712
,National Center for Citrus Variety Improvement, Citrus Research Institute, Southwest University/Chinese Academy of Agricultural Sciences, Chongqing 400712通讯作者:
责任编辑: 岳梅
收稿日期:2019-09-26接受日期:2019-11-13网络出版日期:2020-04-16
| 基金资助: |
Received:2019-09-26Accepted:2019-11-13Online:2020-04-16
作者简介 About authors
龙琴,E-mail: longlong860923@126.com。

摘要
关键词:
Abstract
Keywords:
PDF (4975KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
龙琴, 杜美霞, 龙俊宏, 何永睿, 邹修平, 陈善春. 转录因子CsWRKY61对柑橘溃疡病抗性的影响[J]. 中国农业科学, 2020, 53(8): 1556-1571 doi:10.3864/j.issn.0578-1752.2020.08.006
LONG Qin, DU MeiXia, LONG JunHong, HE YongRui, ZOU XiuPing, CHEN ShanChun.
0 引言
【研究意义】柑橘溃疡病(citrus bacterial canker,CBC)是一种柑橘检疫性细菌病害,由柑橘黄单胞杆菌柑橘亚种(Xanthomonas citri subsp. citri,Xcc)引起[1]。其病原菌几乎能够危害柑橘的所有组织,严重时会造成落叶和落果,进一步发展将会导致枝梢枯死和幼树死亡等,严重威胁世界柑橘产业的健康发展[2,3]。目前,只能通过化学农药或集中销毁苗木进行防控,但其劳动力和经济成本较高。培育抗病栽培品种是解决该问题的根本途径[4]。随着CRISPR/Cas9等现代分子育种技术的兴起,利用分子技术定向快速改良柑橘抗病性,创制新型抗性品种成为可能。然而,由于柑橘分子病理研究相对滞后,导致可供利用的抗性基因资源相对匮乏,目前柑橘抗病分子育种进展依然缓慢。因此,迫切需要挖掘柑橘源潜在的抗病基因,通过基因工程技术获得抗溃疡病的种质,为育种提供材料。【前人研究进展】WRKY转录因子是植物中最大的转录调控因子家族之一,是调控植物多方面过程的信号网络的组成部分[5],含有一个或两个WRKY结构域是其最显著的特征。WRKY结构域大约由60个氨基酸残基组成,其N端是绝对保守的,氨基酸序列为WRKYGQK,在C端则含有一个C2H2或C2HC型锌指结构。根据WRKY结构域的数量和锌指结构的类型,可将WRKY转录因子分为3个家族。Ⅰ类含两个WRKY结构域;Ⅱ类和Ⅲ类含一个WRKY结构域。其中Ⅰ类和Ⅱ类WRKY转录因子均为C2H2型锌指结构:C-X4-5-C-X22-23-H-X1-H;Ⅲ类WRKY转录因子的锌指结构基序跟前两类有所不同,为C2HC型:C-X7-C-X23-H-X1-C。此外,第Ⅱ类WRKY转录因子又可进一步分为5个亚类:Ⅱ(a)、Ⅱ(b)、Ⅱ(c)、Ⅱ(d)、Ⅱ(e)[6]。WRKY转录因子家族在拟南芥中有74个成员[7],水稻中有109个成员[8],毛白杨中有100个WRKY基因[9],在辣椒的基因组中有71个WRKY成员[10],在苹果中鉴定出了116个WRKY基因[11]。在甜橙基因组中成功注释了348个WRKY基因或片段。WRKY转录因子特异性识别并结合W-box“TTGACC/T”顺式作用元件,通过调控下游基因的表达从而调节植物的衰老、形态建成、生物和非生物胁迫响应等多方面的进程[12,13,14,15,16]。目前,已有大量关于WRKY转录因子调控寄主植物抗病方面的研究,主要集中在拟南芥和水稻中[17,18,19,20,21,22,23,24,25]。如拟南芥中WRKY31、WRKY22、WRKY50、WRKY72、WRKY70、WRKY18、WRKY40和WRKY60参与寄主多种免疫应答调节,其中WRKY70是水杨酸(SA)和茉莉酸(JA)信号途径相互拮抗的核心调节点,在植物抗病、抗虫、抗逆防御中起着重要作用[26,27,28]。在柑橘中,一些WRKY转录因子(WRKY22、WRKY45和WRKY31等)在调控寄主抗病反应中的可能功能已有报道,比如WRKY22可能是柑橘溃疡病效应子flg22的一个靶标基因,参与柑橘的抗病反应[29]。然而,更多的研究是关于WRKY转录因子参与激素或非生物胁迫的调节[30,31,32]。【本研究切入点】WRKY家族基因在植物抗病育种中具有广泛的应用前景,但在柑橘中鲜有研究。前期在高抗品种四季橘(Citrus madurensis)和高感品种纽荷尔甜橙(Citrus sinensis)的比较研究中发现,3个WRKY家族转录因子基因CsWRKY50、CsWRKY61和CsWRKY72与柑橘溃疡病抗性相关。在前期研究基础上,本研究以高感品种晚锦橙(Citrus sinensis)为材料,利用转基因技术探究CsWRKY50、CsWRKY61和CsWRKY72在柑橘溃疡病菌侵染中的生物学功能。【拟解决的关键问题】探明在柑橘中超量表达CsWRKY50、CsWRKY61和CsWRKY72对柑橘溃疡病抗性的影响,明确这些基因在柑橘响应溃疡病菌侵染中的生物学功能和抗病育种价值。1 材料与方法
试验于2016年7月至2018年9月在西南大学/中国农业科学院柑桔研究所国家柑桔品种改良中心完成。1.1 试验材料
柑橘遗传转化所用的材料为晚锦橙上胚轴,其种子采自中国农业科学院柑桔研究所国家柑桔品种改良中心资源圃,其无菌上胚轴的准备参考文献[33]。超量表达目的基因的植物表达载体为pLGN(本实验室改造),含NPTII抗性和GUS报告融合基因,以方便转基因植株的筛选,同时含有多克隆位点以利于外源基因的插入。植物遗传转化用农杆菌为EHA105菌株(本实验室冻存)。植物基本培养基为MS(Murashige and Skoog),购自Phyto Technology Laboratories®;常规酶制品购自大连TaKaRa公司;质粒提取和胶回收试剂盒购自Omega公司;植物总DNA和总RNA提取试剂盒购自Aidlab公司;实时荧光染料购自BIORAD公司;PGEM-T克隆载体购自Promega公司;其他常规试剂均为分析纯。
1.2 植物表达载体的构建
CsWRKY50、CsWRKY61和CsWRKY72的编码序列参见文献[34]。设计引物(表1),利用PCR在目的基因的5′和3′端分别添加合适的酶切位点,T-克隆到PGEM-T上,阳性克隆经PCR和测序验证。然后,通过酶切连接技术将CsWRKY50、CsWRKY61、CsWRKY72插入pLGN载体中CaMV 35S启动子的下游,分别构建植物表达载体p35S:CsWRKY50、p35S:CsWRKY61和p35S:CsWRKY72。将构建好的植物表达载体通过电激法转入农杆菌EHA105中用于柑橘的遗传转化。Table 1
表1
表1基因克隆和转基因植株鉴定所用引物
Table 1
| 引物名称 Primer name | 引物序列 Primer sequence (5′-3′) |
|---|---|
| WRKY50-f | ATC GGATCC ATGTCTAATATAAATCTTAT |
| WRKY50-r | CTG GTCGAC CTAAGGATTGCTTTGGTGAG |
| WRKY61-f | ACC AGATCT ATGGAGGAGAAAAGAGCTAT |
| WRKY61-r | TTA GTCGAC TCAGTCAGTGTGCTCTCTAT |
| WRKY72-f | TAG GGATCC ATGGAGGTTTTATTGAAAATG |
| WRKY72-r | CAT GTCGAC TCAACTGCTTTGATCCTTGT |
| NPTII-f | GGATTGCACGCAGGTTCTCCG |
| NPTII-r | TCAGAAGAACTCGTCAAGAAGGCG |
| s-f | TCTTCGTCAACATGGTGGAGCACGA |
| 50-r | ACTCATCAAAGGTCAAGTACTCAG |
| 61-r | TCTGGCAGTTTCAAGCTGATCATC |
| 72-r | ACAAGTTCAGACTCCATGATTTGAC |
新窗口打开|下载CSV
1.3 柑橘遗传转化
晚锦橙上胚轴遗传转化方法参照王军政[35]的方法进行。经农杆菌浸染后外植体置于共培养基(添加2 mg·L-1 BA、0.5 mg·L-1 IAA、1 mg·L-1 2,4-D、100 mg·mL-1 AS的MS培养基)培养2—3 d;然后转到筛选培养基(添加2 mg·L-1 BA、0.5 mg·L-1 IAA、500 mg·L-1 carb,50 mg·L-1 km的MS培养基)上进行抗性芽再生和筛选培养。待抗性芽长到一定程度(1 cm以上),取叶片进行GUS组织化学染色及插入基因的PCR鉴定,筛选阳性植株,将幼芽嫁接到晚锦橙实生苗上,于MS液体培养基中培养到接穗长至5 cm左右,再嫁接到枳橙实生苗上,于温室中培养。1.4 GUS组织化学染色
参照JEFFERSON等[36]的方法进行GUS组织化学染色。具体操作如下:取抗性芽的叶片,放入已加入5-溴-4-氯-3-吲哚葡糖苷酸(X-Gluc)染色液的试管中,于37℃避光保温过夜。染色完成后用95%的乙醇脱色,直至非转基因植株叶片的叶绿素完全褪去呈现白色,统计染色情况,并拍照记录。1.5 转基因植株PCR鉴定
按照Aidlab公司DNA提取试剂盒(cat.No.DN15)方法提取GUS阳性植株的基因组DNA。为了避免植株内源目的基因的干扰,根据35S序列和目的基因序列设计目的基因表达盒的扩增(图1、表1),用于目的基因在转基因植株中整合的鉴定。PCR反应体积25 μL,反应条件:98℃ 1 min;98℃ 10 s,58℃ 10 s,72℃ 20 s,30次循环;72℃ 5 min。扩增完成后将PCR产物于琼脂糖凝胶(1.0%,W/V)中进行电泳分析。图1
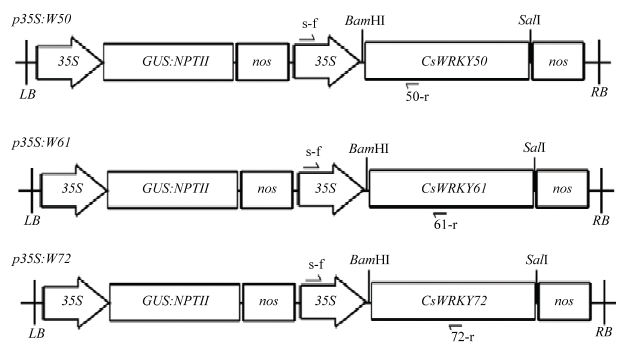 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1植物表达载体T-DNA结构示意图
35S:花椰菜花叶病毒CaMV 35S启动子Cauliflower mosaic virus 35S promoter (CaMV 35S);GUS:NPTII:β-葡萄糖醛酸酶GUS报告基因与卡那霉素NPTII抗性基因的融合基因The fusion gene of β-glucuronidase and neomycin phosphotransferase genes;nos:胭脂碱合酶基因的转录终止序列The terminator of the nopaline synthase gene。单箭头表示转基因植株鉴定用引物 Arrow indicates the primer used to determine transgenic plant
Fig. 1Structural sketch of plant expression vector T-DNAs
1.6 外源基因拷贝数的实时荧光定量PCR(qRT-PCR)分析
参照许兰珍等[37]定量PCR方法,分析转基因植株外源基因拷贝数。以柑橘内源单拷贝基因脂质转移蛋白基因LTP为内参基因[38],以前期确认的单拷贝转基因植株GA-5为对照,利用qRT-PCR分析转基因拷贝数。按照BIORAD公司的定量PCR试剂盒(cat.No.170-8882AP)说明书进行目的基因的拷贝数分析。反应体积20 μL,反应条件:95℃ 3 min;94℃ 10 s,58℃ 10 s,72℃ 10 s,40次循环;72℃ 10 min。以GA-5植株为对照,采用2-ΔΔCt法计算转基因植株中目的基因的拷贝数。1.7 目的基因表达水平的实时荧光定量PCR分析
用Aidlab公司的 EASYspin植物RNA快速提取试剂盒(cat.No.RN09)提取柑橘叶片的总RNA。严格参照BIORAD公司的iScriptTM cDNA合成试剂盒(cat.No.170-8891)说明书合成cDNA第一链。使用BIORAD公司的定量PCR试剂盒(cat.No.170- 8882AP)进行目的基因的相对定量分析。所用定量PCR引物见表2[34],使用20 μL反应体系,反应条件:95℃ 3 min;94℃ 10 s,56℃ 10 s,72℃ 10 s,共40次循环;72℃ 10 min。选用柑橘actin为内参基因,以非转基因柑橘植株为对照,采用2-ΔΔCt法计算转基因柑橘植株中目的基因的相对表达量。Table 2
表2
表2实时荧光定量PCR所用引物序列
Table 2
| 引物名称 Primer name | 引物序列 Primer sequence (5′-3′) |
|---|---|
| r-WRKY50-f r-WRKY50-r r-WRKY61-f r-WRKY61-r r-WRKY72-f r-WRKY72-r r-Actin-f r-Actin-r | ACCTGAGAGTGATTCTGCTG TCTGGACATACCCAAAAAGT GAAACAGTGAAGGGCGG TGGCAAAGAGTTAGGCG TAGCAGGAAAAATGGTG GGGCTTAGATTCGACAC CATCCCTCAGCACCTTCC CCAACCTTAGCACTTCTCC |
新窗口打开|下载CSV
1.8 转基因植株的溃疡病抗性评价
借鉴李云锋等[39]的方法分离、纯化和培养柑橘溃疡病菌。选取成熟的新叶(每个株系选取6片),洗净表面灰尘,用75%酒精消毒表面,再用无菌水冲洗3—5遍,于无菌培养皿中保湿备用。将溃疡病菌培养至OD600为0.5,再用无菌水稀释1 000倍至浓度为5×105 cfu/mL,备用。采用离体针刺法接种[40],用直径0.5 mm无菌针头沿叶片中脉两边针刺24孔,每边12孔。每孔添加1 µL溃疡病菌菌液,接种后用含有无菌水棉球覆盖叶柄保湿,石蜡带封严培养皿,于28℃,16 h·d-1光照培养10 d。每天观察发病情况并照相记录。以野生型(wild-type,WT)为对照,利用ImageJ 2.0软件计算接种10 d后病斑面积。根据病斑面积大小,将病情分为6个级别:0级(S<0.25 mm2);1级(0.25 mm2≤S<0.75 mm2);2级(0.75 mm2≤S<1.25 mm2);3级(1.25 mm2≤S<1.75 mm2);4级(1.75 mm2≤S<2.25 mm2);5级(S≥2.25 mm2)。分别对每级所包含的病斑数目进行统计,然后计算各株系的病情指数(disease index,DI),DI=100×Σ(各级病斑数×相应级数值)/(病斑总数×最大级数)。1.9 转录组测序分析
取转基因和野生型植株的叶片,液氮速冻,干冰运输送上海美吉公司进行转录组测序和信息学分析。以甜橙基因组序列(为了详细分析CsWRKY61调控的代谢途径和基因,对获得的转录组数据进一步进行MapMan功能注释(
1.10 数据分析
试验结果均为3次重复的平均值,采用Excel 2016进行数据整理、标准偏差计算及图表绘制,差异显著性分析采用SPSS 20.0软件完成。2 结果
2.1 超量表达CsWRKY50、CsWRKY61和CsWRKY72晚锦橙植株的获得
用组成型表达的CaMV 35S启动子控制CsWRKY50、CsWRKY61和CsWRKY72的表达,分别构建植物表达载体p35S:WRKY50(W50)、p35S:WRKY61(W61)、p35S:WRKY72(W72)(图1),利用农杆菌介导法进行晚锦橙的遗传转化。首先通过GUS组织化学染色及外源NPTII的PCR扩增对卡那霉素抗性植株进行筛选(图2-A、2-B)。然后通过对目的基因的PCR扩增进一步鉴定阳性植株(图2-C)。为了避免内源目的基因的干扰,PCR扩增的上下游引物分别设计在35S启动子和目的基因上(表1、图1)。经鉴定共获得W50、W61和W72转基因植株各6、8和6株,具体见图2-A。图2
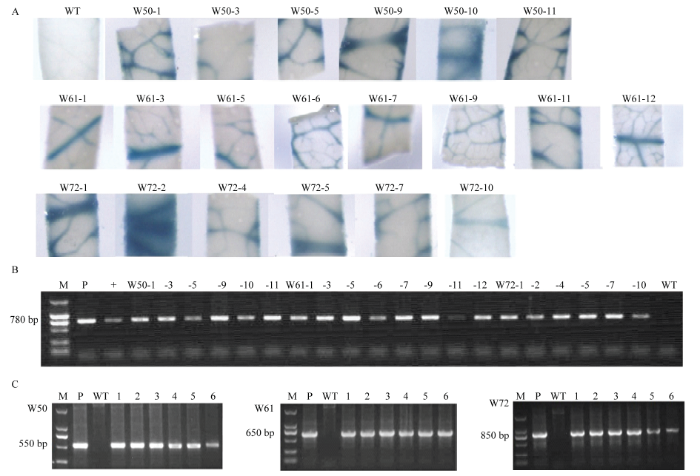 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2转基因植株鉴定
A:转基因植株的GUS组织化学染色图GUS histochemical staining of transgenic plants;B:转基因植株NPTII扩增结果Amplification results of NPTII in transgenic plants;C:部分转基因植株目的基因的PCR扩增PCR amplification of target genes in some transgenic plants。M:DNA marker;P:质粒模板Plasmid template;+:已鉴定的阳性植株对照Identified positive plant control
Fig. 2Identification of transgenic plants
2.2 转基因植株外源基因拷贝数及目的基因表达水平
以LTP为内参基因,转基因植株GA-5为对照,对GUS组织化学染色和PCR检测呈阳性的转基因植株进行qRT-PCR分析。结果表明,W50-1、W50-3,W61-1、W61-3、W61-5、W61-6、W61-7、W61-9、W61-11、W61-12和W72-5、W72-7、W72-10株系为单拷贝;W50-5和W72-1、W72-2株系为双拷贝(表3)。Table 3
表3
表3转基因植株外源基因拷贝数实时荧光定量PCR分析
Table 3
| 株系 Line | 2-ΔΔCt值2-ΔΔCt value | 预测拷贝数 Estimated copy number | |
|---|---|---|---|
| GUS | NPTII | ||
| W50-1 | 1.32 | 1.39 | 1 |
| W50-3 | 1.50 | 1.26 | 1 |
| W50-5 | 1.90 | 1.64 | 2 |
| W50-9 | 3.56 | 3.72 | 4 |
| W50-10 | 2.84 | 2.76 | 3 |
| W50-11 | 2.84 | 2.51 | 3 |
| W61-1 | 1.07 | 1.47 | 1 |
| W61-3 | 1.14 | 1.55 | 1 |
| W61-5 | 1.07 | 0.98 | 1 |
| W61-6 | 1.33 | 1.63 | 1 |
| W61-7 | 1.13 | 1.14 | 1 |
| W61-9 | 0.82 | 1.05 | 1 |
| W61-11 | 0.97 | 0.82 | 1 |
| W61-12 | 1.22 | 1.32 | 1 |
| W72-1 | 2.57 | 2.41 | 2 |
| W72-2 | 1.62 | 1.90 | 2 |
| W72-4 | 2.68 | 2.96 | 3 |
| W72-5 | 1.28 | 1.30 | 1 |
| W72-7 | 1.32 | 1.49 | 1 |
| W72-10 | 1.04 | 1.12 | 1 |
新窗口打开|下载CSV
以野生型植株为对照,利用qRT-PCR分析CsWRKY50、CsWRKY61、CsWRKY72在转基因植株中的表达水平。结果显示,所有W50转基因植株中CsWRKY50表达水平均显著高于野生型,其中W50-3和W50-5表达水平相对较高;同样,所有W61转基因植株中CsWRKY61表达水平均显著高于野生型,其中W61-5、W61-6、W61-9和W61-11的表达相对较高;5株W72转基因植株中CsWRKY72表达水平显著高于野生型,1株W72转基因植株中CsWRKY72表达低于野生型,其中W72-2和W72-5的表达相对较高(图3)。结合外源基因插入的拷贝数分析结果,选择表达水平相对较高的W50-5,W61-5、W61-9、W61-11和W72-5进行重点研究。
图3
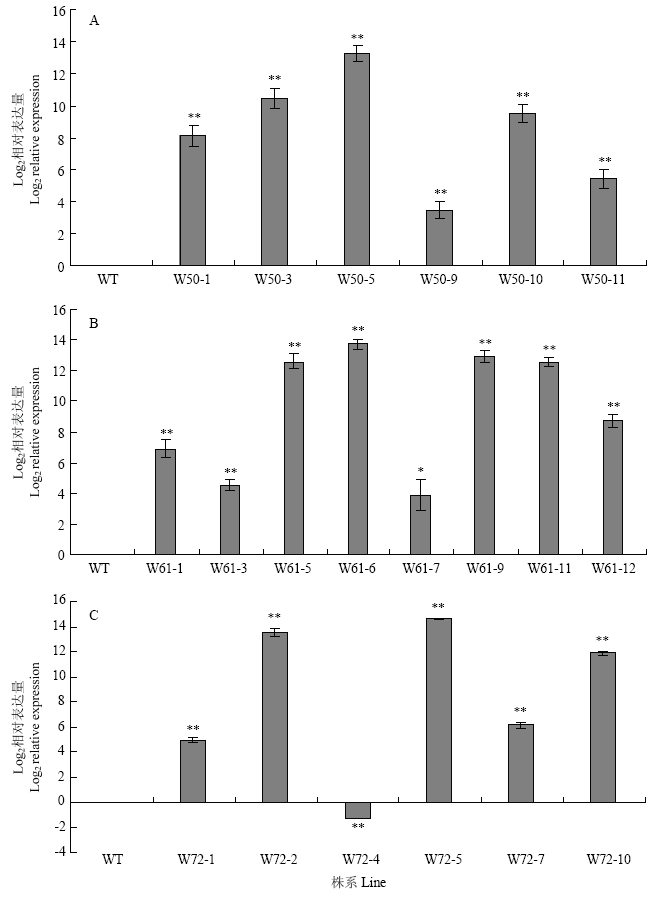 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3转基因植株中目的基因表达的实时荧光定量PCR分析
采用双尾t检验确定与WT对照相关的统计学意义(*:P<0.05;**:P<0.01) 。
Fig. 3qRT-PCR analysis of target gene expression in transgenic plants
Statistical significance related to the WT control was determined by two-tailed Student’s t-test (*: P<0.05; **: P<0.01). The same as
2.3 转基因植株溃疡病抗性评价
对目的基因表达水平显著提高的转基因株系进行溃疡病抗性评价。结果表明,在所获得的转基因植株中,只有超量表达CsWRKY61的转基因植株表现明显的抗性增强,其病斑明显小于野生型植株,而超量表达CsWRKY50和CsWRKY72的转基因植株病斑大小与野生型相比无明显差异(图4-A)。图4
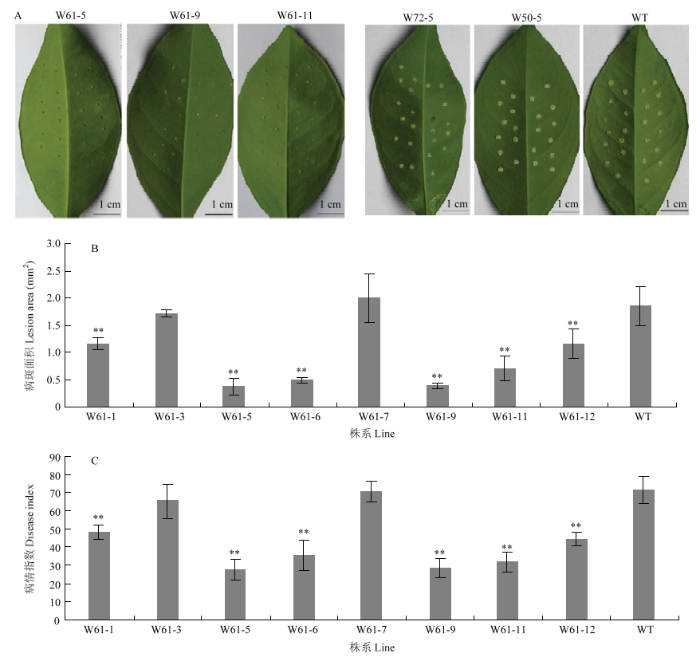 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4转基因植株的溃疡病抗性评价
Fig. 4Resistance evaluation of transgenic plants
为了对超量表达CsWRKY61转基因植株的抗性水平进行定量比较,进一步对其病斑面积和病情指数进行了统计分析。结果显示,针刺接种10 d,W61-3和W61-7株系的病斑面积与野生型无明显差异,其余株系的病斑面积均显著小于野生型,其中W61-5、W61-6、W61-9和W61-11株系的病斑面积相对较小(图4-B)。此外,病情指数的统计结果显示,W61-3和W61-7株系的病情指数与野生型无明显差异,而其余株系的病情指数均显著降低(图4-C)。结果表明,与野生型相比CsWRKY61转基因植株对溃疡病的抗性显著提高,其中W61-5、W61-6、W61-9和W61-11株系的抗性水平相对较高。
2.4 超量表达CsWRKY61对植株表达谱的影响
对抗性水平最高的转基因株系W61-5和W61-9进行了转录组测序分析。聚类热图分析表明,W61-5和W61-9株系中基因表达谱与野生型相比有明显的差异(图5-A)。此外,与野生型相比,W61-5和W61-9株系分别有1 671和2 933个差异表达基因。在W61-5中有1 116个基因上调表达,555个基因下调表达,在W61-9中有2 010个基因上调表达,923个下调表达,其中有1 469个差异表达基因在两株转基因植株中具有相似的表达谱(图5-B、5-C)。MapMan pathway富集分析显示,两株转基因植株中生物胁迫和信号转导相关途径均被显著激活,以W61-9株系变化更加明显(图5-D)。结果表明,超量表达CsWRKY61正调控植物应答生物胁迫和信号转导途径。图5
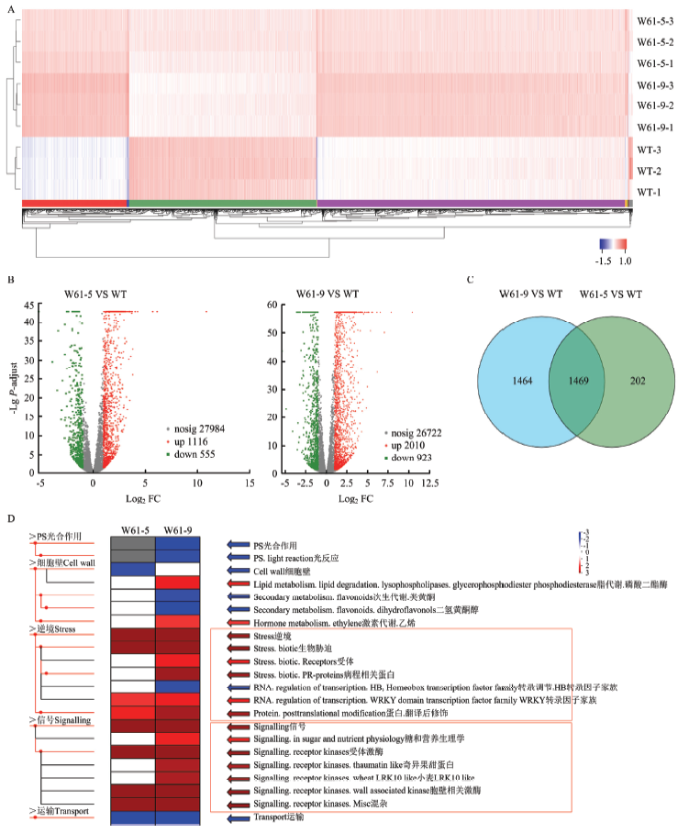 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5转录组结果总概括
A:差异基因表达分析聚类热图。红色表示上调,蓝色表示下调Cluster heat map for differential gene expression analysis. Red and blue indicate up-regulated and down-regulated, respectively。B:差异基因表达分析火山图。红色表示上调,绿色表示下调Volcano map of differential gene expression analysis. Red and green indicate up-regulated and down-regulated, respectively。C:差异基因表达分析维恩图。中间重叠部分代表共有的差异基因Venn diagram of differential gene expression analysis. The middle overlap represents the shared genes。D:差异表达基因的MapMan可视化分析。红色表示上调,蓝色表示下调MapMan visual analysis of differential genes. Red and blue indicate up-regulated and down-regulated, respectively
Fig. 5Overview of the transcriptome results
进一步分析W61-9株系中与生物胁迫相关的差异基因情况。结果显示,有85个差异基因直接与生物胁迫相关,且有75个基因显著上调表达。这些基因包括病原入侵的感知、活性氧爆发、信号转导、转录因子和防御基因。另外,许多与胁迫相关的激素信号、细胞壁和次生代谢等基因也显著上调表达(图6、表4)。
图6
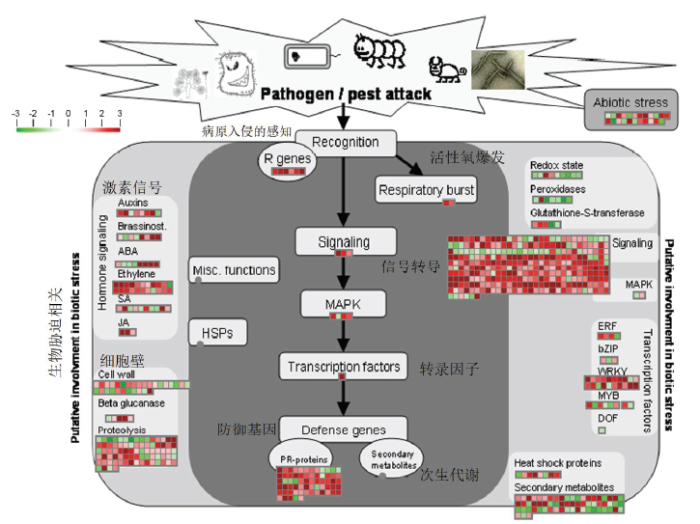 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6生物胁迫相关的差异基因情况
该图为MapMan注释的转基因株系W61-9中与生物胁迫相关的差异基因情况。每个方块表示一个基因,红色表示上调表达,绿色表示下调表达The figure shows the differential genes involved in biotic stress of W61-9 transgenic line by MapMan. Each square represents one gene. Red and green indicate up-regulated expression and down-regulated expression, respectively
Fig. 6Differential genes related to biotic stress
Table 4
表4
表4转基因植株中生物胁迫相关的差异基因情况
Table 4
| 通路Pathway | 基因编号Gene ID | 描述Description | Log2 fold change |
|---|---|---|---|
| 感知Recognition | cs7g19210 | 抗病蛋白家族Disease resistance protein (TIR-NBS-LRR class) family | 2.464 |
| orange1.1t04440 | 抗病蛋白家族Disease resistance protein (TIR-NBS-LRR class) family | 2.839 | |
| orange1.1t05036 | Toll-Interleukin-Resistance (TIR) domain family protein | 3.153 | |
| cs5g18230 | Toll-Interleukin-Resistance (TIR) domain family protein | 3.639 | |
| 活性氧爆发Respiratory burst | cs4g06920 | NADPH/respiratory burst oxidase protein D (RbohD) | 1.962 |
| cs8g12000 | 核黄素合成酶样超家族蛋白Riboflavin synthase-like superfamily protein | 2.631 | |
| 信号转导 Signaling transduction | orange1.1t03802 | alpha/beta-Hydrolases superfamily protein | 3.683 |
| cs5g04790 | alpha/beta-Hydrolases superfamily protein | 2.423 | |
| cs6g07420 | PAR1 protein | 3.644 | |
| cs7g07000 | 假定的配体门控离子通道亚家族Putative ligand-gated ion channel subunit family | 3.786 | |
| cs1g16140 | 富亮氨酸重复I Leucine rich repeat I | 4.144 | |
| orange1.1t03347 | 富亮氨酸重复II Leucine rich repeat II | 3.177 | |
| orange1.1t04450 | 富亮氨酸重复VII Leucine rich repeat VII | 3.279 | |
| cs2g29910 | 富亮氨酸重复XII Leucine rich repeat XII | 4.256 | |
| orange1.1t04450 | 富亮氨酸重复XI Leucine rich repeat XI | 3.279 | |
| cs2g29890 | 富亮氨酸重复XIII Leucine rich repeat XIII | 2.459 | |
| cs2g13280 | 奇异果甜蛋白受体激酶Thaumatin like receptor kinases | 3.153 | |
| cs2g13360 | 奇异果甜蛋白受体激酶Thaumatin like receptor kinases | 3.199 | |
| cs1g11930 | DUF 26受体激酶DUF 26 receptor kinases | 3.791 | |
| cs1g11960 | DUF 26受体激酶DUF 26 receptor kinases | 3.052 | |
| cs8g12370 | 豆科凝集素Legume-lectin | 5.351 | |
| cs2g13360 | Wheat LRK10 like | 3.199 | |
| cs1g11930 | S位点糖蛋白S-locus glycoprotein like | 3.791 | |
| cs1g11960 | S位点糖蛋白S-locus glycoprotein like | 3.052 | |
| cs8g01090 | 豆科凝集素Legume-lectin | 2.194 | |
| cs8g14010 | 胞壁相关激酶Wall associated kinase | 3.464 | |
| cs9g12300 | 胞壁相关激酶Wall associated kinase | 3.013 | |
| cs9g14490 | 胞壁相关激酶Wall associated kinase | 3.918 | |
| cs9g08050 | 赖氨酸基序Lysine motif | 1.079 | |
| cs2g02680 | 赖氨酸基序Lysine motif | 2.853 | |
| cs7g31060 | 褶皱样Crinkly like | 2.283 | |
| cs5g17510 | 褶皱样Crinkly like | 1.589 | |
| cs9g12040 | Ralf-like 32 (RALFL32) | 5.157 | |
| cs9g12160 | Ralf-like 32 (RALFL32) | 3.365 | |
| cs7g27120 | 钙调蛋白结合蛋白Calmodulin binding protein-like | 3.590 | |
| cs9g02980 | 钙调蛋白结合蛋白Calmodulin binding protein-like | 3.336 | |
| cs2g21150 | 蛋白激酶激酶Mitogen-activated protein kinase kinase | 2.077 | |
| cs3g27320 | 受体样胞浆激酶VII Receptor like cytoplasmatic kinase VII | 3.278 | |
| cs9g15620 | 受体样胞浆激酶VII Receptor like cytoplasmatic kinase VII | 4.530 | |
| cs6g20470 | AAA ATPase 1 | 5.065 | |
| cs5g18300 | 天冬氨酸蛋白酶Aspartate protease | 4.689 | |
| orange1.1t03718 | 半胱氨酸蛋白酶Cysteine protease | 3.775 | |
| cs2g19970 | F-box和相关的相互作用域包含蛋白质 F-box and associated interaction domains-containing protein | 4.369 | |
| cs2g31260 | F-box和相关的相互作用域包含蛋白质 F-box and associated interaction domains-containing protein | 7.072 | |
| 转录因子 Transcription factor | orange1.1t02158 | 富含亮氨酸的重复单位Leucine-rich repeat | 1.487 |
| cs5g24240 | 抗病蛋白Disease resistance protein (TIR-NBS-LRR class) | 3.080 | |
| cs5g04160 | WRKY61 DNA结合蛋白WRKY DNA-binding protein 61 | 10.442 | |
| cs1g03870 | WRKY51 DNA结合蛋白WRKY DNA-binding protein 51 | 7.885 | |
| cs7g06330 | WRKY40 DNA结合蛋白WRKY DNA-binding protein 40 | 3.707 | |
| cs4g07780 | R2R3 MYB 转录因子基因家族R2R3 MYB transcription factor gene family | 6.169 | |
| cs8g12680 | 转录因子RLTR1 Transcription factor RLTR1 | 2.614 | |
| 防御基因 Defense gene | orange1.1t04673 | 抗病蛋白Disease resistance protein | 3.487 |
| orange1.1t04706 | 抗病蛋白Disease resistance protein (TIR-NBS-LRR class) | 2.892 | |
| orange1.1t04750 | Toll-Interleukin receptor | 3.454 | |
| orange1.1t05036 | 抗病蛋白Disease resistance protein (TIR-NBS-LRR class) | 3.153 | |
| orange1.1t05250 | Toll-Interleukin receptor | 3.423 | |
| cs2g10790 | NPR1-like protein 3 | 1.251 |
新窗口打开|下载CSV
对W61-9株系中与信号转导途径相关的差异基因情况进一步分析。结果显示,信号转导途径中主要是激酶受体基因受到影响,其中有10类激酶受体基因受到显著影响,且绝大部分都是上调表达,包括富亮氨酸重复、奇异果甜蛋白、未知功能域(DUF 26)、植物凝集素、叶锈病LRK10 like、S-基因座、胞壁相关激酶、赖氨酸基序、褶皱样和受体样胞浆激酶(图7-A、表4)。此外,与翻译后修饰及蛋白质降解相关的基因也绝大部分上调表达(图7-B、表4)。
图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7受体样激酶和蛋白质代谢相关基因的表达情况
A:W61-9中受体样激酶相关基因的表达情况Differential expression of receptor-like kinase-related genes of W61-9 line;B:W61-9中蛋白质代谢相关基因的表达情况Differential expression of genes related to protein metabolism of W61-9 line该结果通过MapMan分析。每个方块表示一个基因,红色表示上调表达,绿色表示下调表达The results were analyzed by MapMan. Each square represents one gene. Red and green indicate up-regulated expression and down-regulated expression, respectively
Fig. 7Expression of receptor-like kinase genes and protein metabolism related genes
3 讨论
WRKY转录因子家族成员众多,参与调节植物对生物、非生物胁迫的响应以及生长发育等多方面进程[12]。在柑橘中,AYADI等发现CsWRKY12和CsWRKY14在所有非生物胁迫条件下都可能上调,但CsWRKY16是唯一被病原菌诱导上调表达的基因,表明其在柑橘抗病性方面有潜在价值;此外还发现CsWRKY14可能与黄龙病的防御反应相关[31]。在水稻中,WRKY89的过表达增强了水稻对紫外线的耐受性和抗病性[42];过表达OsWRKY62和OsWRKY76增加了水稻对稻瘟病和白叶枯的敏感性,而抑制表达能够增强其抗病性[21]。在拟南芥中,超量表达WRKY33能够提高对灰霉病的抗性,敲除该基因增加了对灰霉菌的敏感性[43];异源表达来自葡萄的VqWRKY52提高了转基因植株对白粉病的抗性[44]。在杨树中,转PtWRKY89的毛白杨对黑斑病的耐受性增强;过表达PtrWRKY89的毛果杨对溃疡病的抗性提高[45]。而在本研究中,过表达CsWRKY61增强了转基因柑橘对溃疡病的抗性,表明WRKY转录因子在植物抗病性方面有很大的应用价值。本研究发现超量表达CsWRKY61通过激活转基因植株中生物胁迫和信号转导相关途径来提高溃疡病抗性。这些基因主要涉及病原入侵的感知、活性氧爆发、转录因子和防御基因,与胁迫相关的激素信号、细胞壁和次生代谢等基因,以及受体样激酶基因。有研究表明,病原菌侵染后感病部位会出现活性氧的急剧上升,从而诱导植物发生细胞程序性死亡,这种“活性氧爆发”被称作是细胞水平上寄主植物对病原菌侵染的最早应答之一,对寄主抗病性有积极作用[46,47]。大量研究显示转录因子、激素信号、细胞壁和次生代谢相关基因在植物抗病性中发挥重要作用[48,49,50,51,52]。GAO等研究表明AtWRKY61对萝卜皱纹病毒有负调控作用,可能参与植物免疫信号通路[53]。在水稻中,WRKY61被鉴定为稻瘟病抗性基因[54]。此外,有研究发现AtWRKY61对多种胁迫都有响应,且这种响应很可能通过其抑制相关基因表达而实现;同时AtWRKY61分别与AtWRKY9和AtWRKY72存在蛋白互作,这种互作可能对其参与调节多种胁迫的应答反应有重要作用[55]。本研究中转录组测序揭示,有20个WRKY转录因子受到CsWRKY61超量表达的显著影响,这些WRKY转录因子与CsWRKY61的互作关系有待深入研究。此外,病程相关蛋白(PR蛋白)是寄主植物在受到病原物侵染后诱导表达丰度最高的一类蛋白,是系统获得性抗性(SAR)的分子标记。QIU等[16]研究表明,WRKY转录因子与PR蛋白存在相互调节关系。课题组前期研究发现,CsWRKY与CsPR-1相互影响,CsWRKY22和CsWRKY50的转录水平在过表达CsPR-1的转基因柑橘中明显上调[34]。在本研究中,转录组测序结果显示超量表达CsWRKY61的转基因植株中绝大部分PR蛋白的表达显著提高。这些结果与超量表达CsWRKY61显著提高了转基因植株对溃疡病的抗性相吻合,但其具体的调节机制有待进一步研究。
GAO等[56]研究表明,AtWRKY50可能通过正调控水杨酸信号途径,负调控茉莉酸信号途径从而增强对灰霉病的抗性;周鹏飞[34]研究表明,受溃疡病菌诱导后,CsWRKY50的转录水平在抗病品种四季橘中大幅度提高,而在感病品种纽荷尔脐橙中变化不明显,表明CsWRKY50可能对溃疡病抗性有积极作用;此外,BHATTARAI等[57]发现WRKY72型转录因子参与了番茄和拟南芥的基础免疫以及番茄R基因Mi-1介导的基因对基因的抗性;XU等[27]研究表明,接种溃疡病菌后,随着接种时间的增加CsWRKY72在感病品种纽荷尔脐橙中的表达量逐渐下降,而在抗病品种四季橘中无明显变化,推测溃疡病菌侵染通过下调CsWRKY72的表达,可能对Mi-1介导的抗病过程有抑制作用。然而,本研究中抗病性评价结果显示超量表达CsWRKY50和CsWRKY72的植株病斑面积与野生型对照无明显差异,推测可能是转基因植株太少未筛选到抗性株系,或者转基因植株通过其他途径抵消了CsWRKY50和CsWRKY72发挥的作用。
4 结论
CsWRKY61能够激活与生物胁迫和信号转导相关的途径,是柑橘抗病育种中有潜在应用价值的抗性基因。研究结果为柑橘溃疡病抗性遗传改良提供了重要基因资源。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[D].
[本文引用: 4]
[D].
[本文引用: 4]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[D].
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
