Overexpression of CsGH3.6 Enhanced Resistance to Citrus Canker Disease by Inhibiting Auxin Signaling Transduction
ZOU XiuPing, LONG JunHong, PENG AiHong, CHEN Min, LONG Qin, CHEN ShanChunNational Center for Citrus Variety Improvement, Citrus Research Institute, Southwest University/Chinese Academy of Agricultural Sciences, Chongqing 400712收稿日期:2019-05-23接受日期:2019-07-15网络出版日期:2019-11-08
| 基金资助: |
Received:2019-05-23Accepted:2019-07-15Online:2019-11-08
作者简介 About authors
邹修平(通信作者),E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (2855KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
邹修平, 龙俊宏, 彭爱红, 陈敏, 龙琴, 陈善春. 超量表达CsGH3.6通过抑制生长素信号转导 增强柑橘溃疡病抗性[J]. 中国农业科学, 2019, 52(21): 3806-3818 doi:10.3864/j.issn.0578-1752.2019.21.009
ZOU XiuPing, LONG JunHong, PENG AiHong, CHEN Min, LONG Qin, CHEN ShanChun.
0 引言
【研究意义】柑橘溃疡病(citrus bacterial canker,CBC)是一种危害极严重的世界性病害,其病原菌为柑橘黄单胞杆菌柑橘亚种(Xanthomonas citri subsp. citri,Xcc)。感染柑橘溃疡病的植株在叶、枝和果实上出现火山状病斑,严重时落叶、枯枝、落果,产量降低、果实品质变劣,一旦疫情在柑橘产区传播蔓延,对柑橘产业具有毁灭性、灾难性打击[1,2]。柑橘产业中大部分栽培品种属于溃疡病易感品种。因此,大力开展柑橘溃疡病抗(感)机理和重要基因资源挖掘的研究,对柑橘优良抗性新品种的选育和柑橘产业的健康稳定发展具有重要意义,也将进一步深化对病原菌致病机理、植物与病原互作机制的理解。【前人研究进展】植物生长素在调控柑橘溃疡病菌侵染引起的脓疱形成中起重要作用。COSTACURTA等[3]研究发现,柑橘溃疡病菌能分泌吲哚-3-乙酸(indole-3-acetic acid,IAA),而且甜橙叶片提取液能促进病原菌繁殖和IAA合成;CERNADAS等[4]研究表明,柑橘溃疡病菌侵染促进甜橙生长素合成、运输和信号转导相关基因的转录。进一步研究发现,萘乙酸(NAA)处理促进甜橙感病部位水浸状脓疱的形成,而生长素抑制剂1-N-萘基邻氨甲酰苯甲酸(NPA)抑制溃疡病症状的发展[5,6],显示生长素具有促进柑橘溃疡病症状发展的作用。目前,关于植物生长素介导寄主感病的机制研究主要集中在拟南芥、水稻等植物中[7]。病原菌通过向寄主侵染部位细胞分泌IAA或增强寄主IAA的合成,致使寄主细胞内IAA的水平急剧上升,高浓度的IAA使植物细胞壁pH下降,胞壁结构蛋白酸化,细胞壁发生重排,从而引起细胞壁松弛,细胞扩张和膨大,以利于病原菌的入侵和扩散[8,9]。而且,侵染部位高浓度的IAA抑制水杨酸(salicylic acid,SA)介导的抗病反应[7,10-11]。面对病原菌对生长素的操控,植物必然启动相应的对抗机制来维持感病部位细胞激素的动态平衡,努力消除病原菌的危害。其中,生长素响应因子GH3起着重要作用[7,8]。GH3编码蛋白具有植物激素酰胺合成酶活性,催化自由IAA与氨基酸结合,从而使其失活,当植物需要IAA时,通过IAA酰胺水解酶水解作用释放出IAA,以此调控细胞内IAA动态平衡[12,13]。另外,一些GH3也具有酰胺化SA和茉莉酸(jasmonic acid,JA)等激素的活性[14]。GH3的功能研究主要集中在拟南芥和水稻中,在调控植物抗病性反应中起重要作用[12,15]。目前认为GH3参与植物抗病反应的机制为:病原菌侵染导致寄主感病部位细胞IAA水平急剧上升,寄主通过生长素受体TIR1感知上升的IAA水平并触发生长素信号途径,迅速激活GH3表达,GH3酶将过量的IAA酰胺化,使活性IAA水平下降,进而限制植物细胞壁增生和松弛,从而正向增强寄主的抗性[9,16-17]。比如超量表达OsGH3.8和OsGH3.1能增强水稻对白叶枯病和稻瘟病抗性[16,17],而OsGH3.2能赋予植物广谱抗性[9]。随着基因组、转录组、蛋白组等大规模组学技术的应用,GH3家族基因在豆类、苹果、玉米、苜蓿、番茄、棉花等作物中的功能研究已在开展,其在生物和非生物抗性、激素动态平衡、信号途径中的作用备受关注[18,19,20,21,22,23]。但在柑橘中,鲜有关于GH3家族基因参与调控植物生长发育和抗性的研究报道。【本研究切入点】前期研究发现[24],柑橘生长素早期响应基因CsGH3.6在调控生长素响应溃疡病菌侵染中起着重要作用,超量表达CsGH3.6显著降低柑橘对溃疡病的感病性,但其机制尚待解析。【拟解决的关键问题】通过分析超量表达CsGH3.6对转基因植株溃疡病抗性、激素水平、生长发育和转录组的影响,阐明CsGH3.6调控柑橘溃疡病抗性的机制,为进一步解析柑橘溃疡病菌侵染中生长素途径调控寄主抗性的机理打下基础,为柑橘抗病育种提供新思路和新材料。1 材料与方法
试验于2015—2018年在中国农业科学院柑桔研究所国家柑桔品种改良中心完成。1.1 材料
供试植物材料为CsGH3.6超量表达转基因晚锦橙(Citrus sinensis)和野生型晚锦橙 [24]。转基因植株以及野生型晚锦橙种植于中国农业科学院柑桔研究所国家柑桔品种改良中心育种圃。育种圃的温度控制在25—28℃,相对湿度控制在60%—70%,光周期L﹕D= 16 h﹕8 h。1.2 转基因植株表型观察与叶型指数分析
转基因植株种植在田间2年后,观察其表型。选取完全成熟的春稍,从顶部开始选取第3—5节间的叶片,使用直尺测量叶片的纵径和横径,利用游标卡尺测量叶片的厚度。1.3 表皮细胞观察
上午采摘完全伸展的成熟叶片,将离主脉0.5 cm处的叶片剪成0.5 cm×1 cm长方形,迅速放入3 mol·L-1的NaOH溶液中70℃水浴15 min,去除溶液,无菌水冲洗两次,再用蒸馏水冲洗4次。将叶片下表皮粘在透明胶带上,用手术刀片去尽残余叶肉组织。将粘有表皮细胞的透明胶带剪下,置于载玻片上制成临时切片,于光学显微镜下观察,并在40×物镜和10×目镜(放大倍数为400倍)条件下成像。试验重复3次,每次每个株系检测3片叶。使用ImageJ1.47软件统计气孔的数目和表皮细胞长度。气孔密度=视野范围内的气孔数/视野范围面积(个/mm2)。1.4 激素含量测定
柑橘生长素IAA、水杨酸、茉莉酸含量测定参照MARQUES等[25]的方法。采摘1 g鲜重叶片,液氮速冻,研磨成粉;加入5 mL 80%甲醇浸提过夜,然后13 000×g离心10 min。倒掉上清液,加入1mL 1%乙酸重悬沉淀。重悬液按照Oasis cartridges(Waters,美国马萨诸塞州)的方法进行纯化,纯化后的激素溶解于100 μL 10%的甲醇。提取的激素送钟鼎生物公司(南京)进行HPLC检测。1.5 转基因柑橘的抗病性评价
以转基因晚锦橙和野生型晚锦橙的成熟叶片为材料,利用针刺接种法[26]接种溃疡病菌。具体方法:采集转基因和野生型晚锦橙的成熟叶片,清水洗净,70%乙醇杀菌3—5 s,再用无菌水清洗干净。将叶片背面向上平铺在无菌培养皿中,培养皿底部铺上湿润滤纸保湿,叶柄处用湿棉花覆盖保湿,每一片叶背面针刺12个小孔,用移液枪吸取1 μL(5×104 CFU/mL)溃疡病菌悬浮液滴加到小孔处。培养皿用Parafilm封口膜密封,置于28℃光照培养箱培养。接种后第10天拍照,用软件Image J 1.47 统计病斑面积(mm2)。病斑面积的大小分为8个级别,分级方法如下:以字母R表示病斑面积,0级(R≤0.25 mm2),1级(0.25 mm2<R≤0.5 mm2),2级(0.5 mm2<R≤0.75 mm2),3级(0.75 mm2<R≤1 mm2),4级(1 mm2<R≤1.25 mm2),5级(1.25 mm2<R≤1.5 mm2),6级(1.5 mm2<R≤1.75 mm2),7级(R>1.75 mm2)。病斑面积分级后,根据以下公式统计病情指数(disease index,DI):DI=100×Σ(各级病斑数×相应级数值)/(病斑总数×最大级数)。根据病情指数,分析转基因植株抗溃疡病情况。1.6 转录组测序分析
取转基因和野生型植株的叶片,液氮速冻,送北京百迈克科技股份有限公司进行转录组测序和信息学分析。试验设置3个生物学重复。以甜橙基因组序列(利用Nr(non-redundant protein database,非冗余蛋白数据库)、Nt(NCBI non-redundant nucleotidedatabase,非冗余核苷酸数据库)、SwissProt(SwissProt protein database,蛋白质序列数据库)、COG(Cluster of Orthologous Groups,蛋白质直系同源数据库)、Pfam(Protein families database,蛋白质家族域数据库)、GO(Gene Ontology,基因本体论数据库)、KEGG(Kyoto Encyclopedia of Genes and Genomes,东京基因与基金组百科全书)数据库对基因进行功能注释。
为了详细分析CsGH3.6调控的代谢途径和基因,对获得的转录组数据进一步进行MapMan功能注释(
1.7 数据分析
试验结果均为3次重复的平均值,用Excel 2016进行数据整理、标准偏差计算及图表的绘制,差异显著性用SPSS 20.0统计软件进行分析。2 结果
2.1 转基因植株溃疡病抗性评价
利用叶片离体接种技术对两年生CsGH3.6转基因晚锦橙植株进行溃疡病抗性评价。接种溃疡病菌10 d时,统计病斑面积和病情指数(图1)。结果显示,野生型植株病斑面积为2.67 mm2,4株(b7、b13、b19和b20)转基因株系病斑面积分别为1.52、2.48、1.21、2.22 mm2,其中,b7和b19的病斑面积显著小于野生型(图1-A)。b7、b13、b19和b20株系的病情指数分别为45.55、89.12、44.48、77.02,而野生型植株的病情指数为83.48,转基因株系b7和b19的病情指数显著低于野生型对照(图1-B)。结果表明超量表达CsGH3.6能显著提高柑橘抗溃疡病的能力。根据抗性评价结果,以下研究主要以b7和b19株系为材料开展。图1
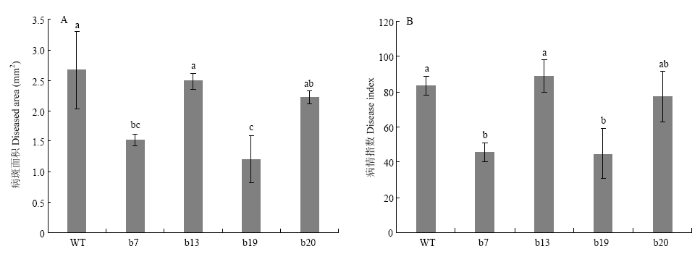 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1CsGH3.6转基因植株溃疡病抗性评价
WT:野生型Wild type;b7、b13、b19和b20:转基因植株Transgenic plant。下同 The same as below
柱上不同小写字母表示差异显著(Tukey’s检验,P<0.05)。
Fig. 1Resistance evaluation of CsGH3.6 transgenic plants to citrus bacterial canker
2.2 转基因植株自由IAA含量变化
为了分析超量表达CsGH3.6对自由IAA含量的影响,利用HPLC检测在正常情况和溃疡病诱导条件下转基因株系 b7和b19的IAA含量。结果显示,病原菌诱导前转基因植株中自由IAA浓度显著低于野生型植株(图2)。接种溃疡病菌3 d时,野生型与转基因植株中自由IAA含量均显著下降,转基因植株自由IAA含量依然显著低于野生型植株。结果表明,超量表达CsGH3.6不但抑制自由IAA的积累,而且促进病原菌侵染引起的自由IAA的下降。2.3 转基因植株SA和JA含量变化
为了分析CsGH3.6对植株体内SA和JA含量变化影响,检测了野生型与转基因植株中SA和JA的含量。结果表明,与野生型相比,转基因株系b7和b19中SA的含量显著升高,而JA的含量显著降低(图3)。图2
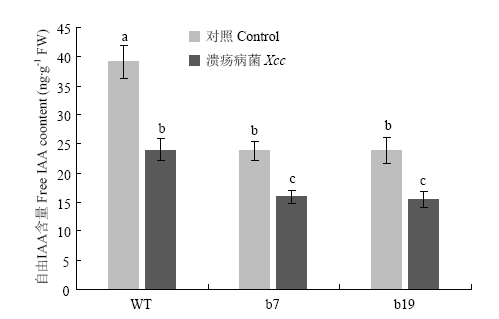 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2CsGH3.6转基因植株中自由IAA含量分析
Fig. 2Analysis of free IAA content in CsGH3.6 transgenic plants
Control:溃疡病菌侵染前自由IAA含量检测Free IAA content detection before Xcc inoculation;Xcc:溃疡病菌侵染3 d时自由IAA含量检测Free IAA content detection at 3 d after Xcc inoculation
图3
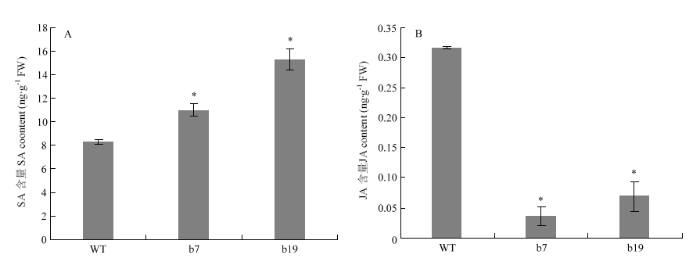 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3 CsGH3.6转基因植株SA和JA含量分析
*表示与野生型(WT)相比差异显著(Tukey’s检验,P<0.05)。
Fig. 3Analysis of SA and JA contents in CsGH3.6 transgenic plants
* represents significant difference compared with WT (Tukey’s test, P<0.05). The same as
2.4 超量表达CsGH3.6转基因晚锦橙的表型变化
两年温室表型观察发现,与野生型相比,b7转基因株系出现轻微卷叶;b19转基因株系叶片明显向上卷曲,叶片变小,颜色浅,整片叶子较野生型叶片明显下垂,茎较软易弯曲,植株呈枯萎状态(图4-A)。叶型指数统计分析表明,转基因植株叶片的纵径(图4-B)和横径(图4-C)以及叶片厚度(图4-D)均显著小于野生型。图4
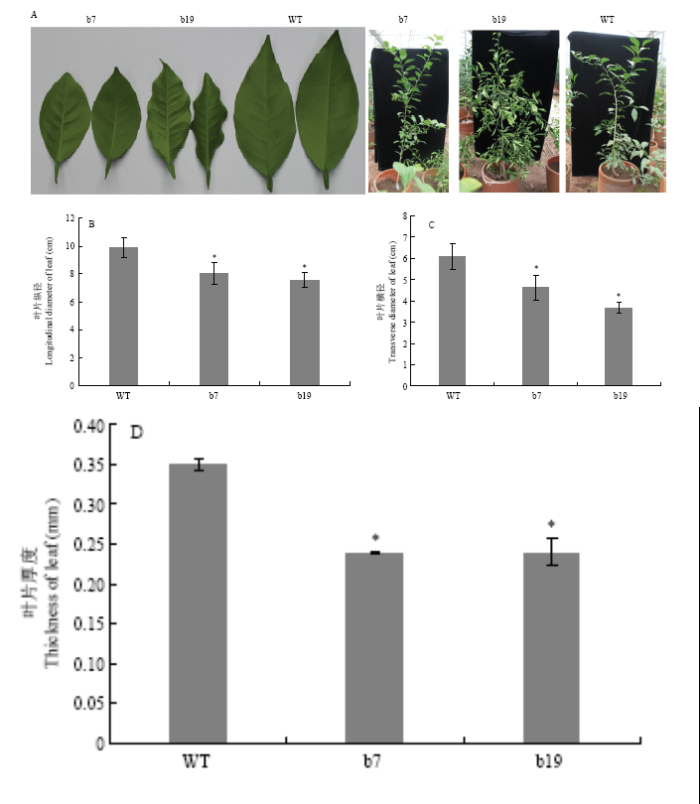 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4CsGH3.6转基因植株的表型分析
Fig. 4Phenotypes of CsGH3.6 transgenic plants
2.5 转基因植株叶片表皮细胞变化
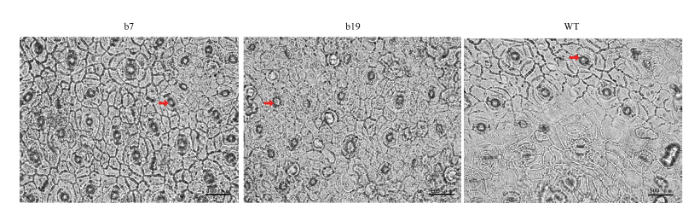
进一步分析了转基因株系b7和b19表皮细胞和气孔的变化(图5)。结果显示,转基因植株的表皮细胞长度显著降低(图6-A),气孔密度显著增加(图6-B)。
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5CsGH3.6转基因植株叶片表皮细胞显微观察
箭头指示气孔 Arrows indicate stomata
Fig. 5Microscopic observation of epidemic cells of CsGH3.6 transgenic plants
图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6CsGH3.6转基因植株表皮细胞长度(A)和气孔密度(B)统计分析
Fig. 6Statistical analysis of epidermal cell length (A) and stomatal density (B) of CsGH3.6 transgenic plants
2.6 转录组分析
为了进一步探讨CsGH3.6与柑橘溃疡病抗性的关系,对抗性水平最高的转基因株系b19进行了转录组测序分析。聚类热图分析表明,b19株系中基因表达谱与野生型相比有明显的差异(图7-A)。GO等功能注释共获得31 035个Unigene基因。与野生型相比,b19株系中有1 456个差异表达基因(DEG),940个DEG上调表达,516个DEG下调表达(图7-B)。KEGG富集分析显示(图7-C),148个DEG明显富集于20个途径和功能组,其中29个基因显著富集于植物激素信号转导途径、17个基因富集于植物-病原菌互作途径、15个基因富集于苯丙酸生物合成途径、13个基因富集于氨基糖和核苷酸的糖代谢途径。差异表达基因MapMan可视化分析进一步表明,超量表达CsGH3.6显著影响细胞壁、压力和信号传递功能组(图7-D)。特别地,超量表达CsGH3.6显著上调生物胁迫功能组,而显著下调生长素信号转导Aux/IAA 家族功能组(图7-D)。图7
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7转录组测序分析
A:基因表达量聚类热图 Clustering heat map of gene expression quantity;B:差异基因表达分析火山图Volcano map of differentially expressed genes;C:KEGG富集图 KEGG enrichment;D:MapMan可视化分析,红色表示上调,蓝色表示下调 MapMan visualization, red and blue indicate up-regulated and down-regulated, respectively
Fig. 7Transcriptome sequencing analysis
2.6.1 生长素途径相关基因 进一步利用MapMan详细调查了转录组数据库中与生长素合成和降解、生长素运输和信号转导相关的差异表达基因(表1)。在生长素合成-降解中有4个基因差异表达,其中3个上调表达,1个下调表达;生长素运输的基因有3个,均下调表达;而在生长素信号通路中差异表达的基因有19个,其中有17个基因下调表达,注释的13个AUX/IAA家族基因均下调表达。这些结果表明超量表达CsGH3.6明显抑制生长素的运输和信号转导相关基因表达。
Table 1
表1
表1b19转基因植株中生长素相关差异表达基因统计分析
Table 1
| 基因编号Gene ID | 推测的功能Putative function | 差异倍数Log2 fold change* |
|---|---|---|
| 生长素合成-降解Auxin synthesis-degradation | ||
| Cs7g08110 | IAA β-葡萄糖基转移酶 IAA β-glucosyltransferase | 1.832 |
| Orange1.1t02388 | IAA β-D-葡萄糖基转移酶 IAA β-D-glucosyltransferase | -1.153 |
| Cs3g19760 | IAA氨基酸共轭水解酶 IAA amino acid conjugate hydrolase | 1.573 |
| Cs7g08080 | IAA氨基酸共轭水解酶 IAA amino acid conjugate hydrolase | 1.074 |
| 生长素运输Auxin transport | ||
| Orange1.1t00089 | 生长素输出载体PIN1 Auxin efflux carrier PIN1 | -1.022 |
| Cs2g16620 | 生长素输出载体PIN3 Auxin efflux carrier PIN3 | -2.790 |
| Cs3g19250 | 生长素运输相似蛋白3 Auxin transporter-like protein 3 | -1.385 |
| 生长素信号转导Auxin signal transduction | ||
| Cs5g29060 | AUX/IAA家族基因IAA11 AUX/IAA family IAA11 | -1.570 |
| Cs9g09120 | AUX/IAA家族基因IAA13 AUX/IAA family IAA13 | -1.286 |
| Cs5g30380 | AUX/IAA家族基因IAA16 AUX/IAA family IAA16 | -3.052 |
| Cs5g30390 | AUX/IAA家族基因IAA4 AUX/IAA family IAA4 | -3.905 |
| Cs7g05540 | AUX/IAA家族基因IAA29 AUX/IAA family IAA29 | -3.067 |
| Cs9g08100 | AUX/IAA家族基因IAA29 AUX/IAA family IAA29 | -4.314 |
| Cs9g08110 | AUX/IAA家族基因IAA4 AUX/IAA family IAA14 | -5.958 |
| Cs1g13970 | AUX/IAA家族基因IAA22 AUX/IAA family AUX22 | -6.028 |
| Cs4g18240 | AUX/IAA家族基因IAA29 AUX/IAA family IAA29 | -3.097 |
| cs1g13960 | AUX/IAA家族基因 AUX/IAA family | -1.775 |
| cs3g10920 | AUX/IAA家族基因IAA22 AUX/IAA family IAA22 | -1.656 |
| cs3g10930 | AUX/IAA家族基因IAA16 AUX/IAA family IAA16 | -1.831 |
| cs3g16750 | AUX/IAA家族基因IAA9 AUX/IAA family IAA9 | -1.089 |
| Orange1.1t04221 | SAUR-like相似蛋白ARG7 SAUR-like protein ARG7 | -4.445 |
| Cs8g16440 | 生长素响应因子ARF10 Auxin response factor ARF10 | -1.931 |
| cs4g04520 | 生长素响应因子ARF7 Auxin response factor ARF7 | -1.111 |
| cs4g12720 | SAUR-like相似蛋白 SAUR-like protein | 3.141 |
| orange1.1t00825 | IAA酰基化酶GH3.5 IAA-amido synthetase GH3.5 | -1.200 |
| cs4g11890 | IAA酰基化酶CsGH3.6 IAA-amido synthetase CsGH3.6 | 7.325 |
新窗口打开|下载CSV
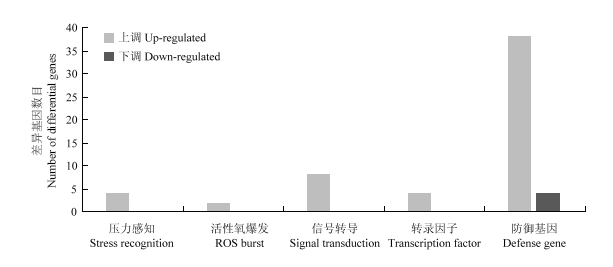
2.6.2 生物胁迫相关基因 图8显示MapMan注释的转基因株系b19中与生物胁迫相关的差异基因情况。有60个基因展现差异表达水平,这些基因涉及到压力感知、活性氧爆发、信号转导、基因转录和防御基因表达(图8)。除4个防御基因下调表达外,所有与生物胁迫相关的差异基因均上调表达,特别是42个防御基因中有38个上调表达。基因功能注释表明,所有防御基因均为病程相关蛋白PR基因。这些结果暗示,超量表达CsGH3.6激活了植株的防御反应。
图8
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8生物胁迫相关差异基因统计分析
Fig. 8Statistical analysis of differentially expressed genes related to biological stress
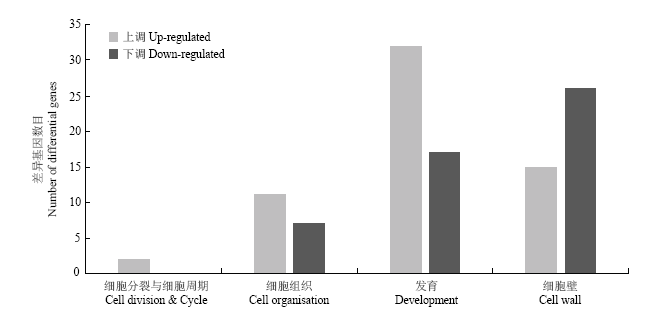
2.6.3 植物生长发育相关基因 MapMan分析显示,与植物生长发育相关的差异基因富集于细胞分裂与细胞周期、细胞组织、发育功能组和细胞壁。上调表达基因数目(60个)明显多于下调表达基因数目(50个)。细胞壁在植物生长发育中起着重要作用,转录组数据显示15个细胞壁相关基因上调表达,26个基因下调表达(图9)。
图9
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图9生长发育相关差异基因统计分析
Fig. 9Statistical analysis of differentially expressed genes related to growth and development
表2展示了差异倍数≥2的细胞壁相关基因功能注释。参与细胞壁合成的两个重要基因纤维素合成酶基因和细胞壁蛋白基因差异表达水平在5倍以上。3个参与细胞壁修饰的木葡聚糖内糖基转移酶基因也展现出较高的表达水平。角质合成相关基因LCAC和蜡质合成相关基因KCS1表达水平上调,而与细胞壁疏松相关的延展蛋白基因EXLB1下调表达。
Table 2
表2
表2b19转基因植株中部分细胞壁相关的差异表达基因
Table 2
| 基因编号Gene ID | 推测的功能Putative function | 差异倍数Log2 fold change |
|---|---|---|
| 纤维素合成Cellulose synthesis | ||
| Cs4g02000 | 木质素特异纤维素合酶 Xylem-specific cellulose synthase | 7.304 |
| 细胞壁蛋白Cell wall protein | ||
| Cs8g16830 | 成束蛋白样阿拉伯半乳聚糖蛋白12 FASCICLIN-like arabinogalactan-protein 12 FLA12 | 5.439 |
| 细胞壁修饰Cell wall modification | ||
| Cs4g03060 | 木葡聚糖内糖基转移酶相关蛋白 Xyloglucan endotransglycosylase-related protein (XTR6) | 2.591 |
| Cs4g03130 | 2.031 | |
| Cs4g03140 | 2.227 | |
| Cs5g28330 | 角质素合成酶相关基因LCAC Cutin synthesis-related gene LCAC | 1.576 |
| Orange1.1t00556 | 植物蜡质合成相关基因KCS1 Wax biosynthesis-related gene KCS1 | 1.408 |
| Cs5g07854 | 延展蛋白EXLB1 Expansin EXLB1 | -1.393 |
新窗口打开|下载CSV
3 讨论
生长素响应因子GH3在调控植物的生长发育、激素动态平衡、生物和非生物胁迫相关抗性中起着重要作用。本研究中,超量表达CsGH3.6转基因植株中自由IAA水平显著降低,植株表型出现GH3过表达导致自由IAA缺失引起的典型表型变化[16],证明CsGH3.6具有酰基化IAA的生物学功能。另外,转基因植株JA含量也显著下降,暗示CsGH3.6也可能具有酰基化JA的功能[28]。溃疡病抗性评价表明,超量表达CsGH3.6显著增强植株对溃疡病的抗性[24]。而且,本研究发现,超量表达CsGH3.6不仅抑制活性IAA的积累,且促进病原菌侵染后活性IAA水平的显著下降。生长素IAA被认为是一种致病因子,具有促进柑橘溃疡病菌致病的作用[5,24]。这些结果暗示,CsGH3.6通过酰基化侵染部位活性IAA来正向调控柑橘对溃疡病的抗性。病原菌侵染中脓疱形成是柑橘溃疡病症状发展的关键[29]。病原菌通过分泌IAA或促进寄主IAA的合成,调控寄主细胞的膨大和增生,进而促进脓疱的形成[5,24],以利于病原菌突出寄主表面向周围非侵染组织扩散。因此,植物表皮细胞在柑橘调控溃疡病抗性中起着重要作用[30]。本研究对转基因植株表皮细胞分析发现,表皮细胞变短。表皮细胞变短暗示活性IAA的下调抑制了细胞的正常生长,这种细胞生长的钝化可能不利于病原菌侵染中脓疱的形成和扩散。细胞壁是植物防御病原菌侵染的第一道物理屏障。转录组数据显示,细胞壁合成相关基因表达水平明显升高,特别是与增强细胞壁强度相关的角质和蜡质合成基因LCAC和KCS1表达水平上调;相反,与细胞壁疏松相关的延展蛋白基因EXLB1表达水平下调。结果暗示转基因植株细胞壁强度增强。细胞壁的这种变化同样有利于抑制脓疱的形成,增强植株的抗性[29]。综上所述,超量表达CsGH3.6抑制侵染部位活性IAA的积累,IAA含量的下降改变了细胞壁重构相关基因的表达或蛋白质的活性,进而抑制寄主细胞壁松弛和细胞膨大,阻碍病原菌的入侵和扩散,这可能是CsGH3.6调控侵染部位脓疱形成的内在机制。另外,本研究发现转基因植株气孔密度显著增大。IAA负调控气孔发育[31],抑制IAA的积累和极性运输会诱发气孔簇生,比如拟南芥PIN1和PIN3突变体展现气孔簇生状[32,33]。转录组测序表明,转基因植株中PIN1和PIN3表达水平显著下降,与转基因植株气孔密度增大相关。气孔是溃疡病菌入侵柑橘的自然入口,有研究表明气孔密度与柑橘品种溃疡病抗性呈一定的负相关性,即气孔密度越大,品种的抗病性越低[34,35]。因此,气孔密度的增大可能会削弱CsGH3.6超量表达对脓疱形成的抑制作用,影响转基因植株抗性水平的进一步提升。CsGH3.6是如何从细胞发育水平影响病原菌侵染中脓疱的形成有待进一步深入研究。
本研究显示,超量表达CsGH3.6增强了柑橘的抗病反应。首先,激素分析表明,转基因植株SA含量显著增加,暗示SA介导的抗性反应增强。在温室和田间中,外施SA及其类似物能有效增强柑橘对溃疡病的抗性,已广泛用于柑橘溃疡病防治中[36]。同样,超量表达SA信号下游的中心调节子基因AtNPR1显著增强转基因柑橘对溃疡病的抗性,这种抗性增强与PR基因的表达增强紧密相关[37]。因此,转基因植株中大量PR基因的上调表达暗示超量表达CsGH3.6增强了SA介导的抗病反应。其次,JA通常拮抗SA介导的抗病反应[38],因此,超量表达CsGH3.6转基因植株JA含量降低有利于增强植物抗病性。有研究表明,侵染部位高浓度的IAA能抑制SA介导的抗病反应[7,10-11],说明转基因植株中IAA积累的下降能促进SA介导的抗病反应。综上,超量表达CsGH3.6可能通过削弱或解除IAA和JA对SA抗病途径的抑制,进而增强植株的防御反应。
超量表达CsGH3.6对植株的形态发育造成了严重影响。转基因植株呈现叶片向上卷曲,分枝增多,植株萎蔫,丧失顶端优势的表型,与拟南芥超量表达GH3.5及其获得性突变体植株表型相似[39]。生长素是顶端优势形成的重要调控物质。当生长素运输受阻或其信号通路受抑制,生长素对侧芽活动的抑制解除,进而导致植株顶端优势丧失,分枝增多,叶小而卷曲[40]。转录组测序显示,转基因植株中减少的自由IAA显著抑制生长素的运输和信号转导途径相关基因表达,特别是所有注释的AUX/IAA家族基因均显著下调表达。AUX/IAA是生长素早期响应因子,通过泛素化途径调控转录因子ARF的表达,进而影响细胞大小和植株生长发育[41]。当细胞中IAA降低,AUX/IAA抑制ARF激活下游基因AUX/IAA、GH3、SAUR的表达,最终抑制IAA的信号转导,影响植株的正常生长[40]。这种抑制作用会进一步抑制AUX/IAA转录[41]。而PIN1和PIN3的表达下调说明生长素在植物体内的分配受阻。另外,超量表达CsGH3.6导致的自由IAA减少进一步上调IAA糖基化和酰基化基因表达,这会进一步抑制自由IAA的积累。这些结果清楚说明,超量表达CsGH3.6显著抑制了IAA的积累和信号转导,进而影响许多与细胞、组织和个体发育相关基因的表达,改变植株的形态特征。
由于超量表达CsGH3.6转基因植株生长发育受到影响,尽管已经获得了溃疡病抗性提高的转基因株系,但在柑橘溃疡病抗性育种方面依然受到限制。一种解决策略是利用病原菌诱导型启动子驱动CsGH3.6的表达,当植物感染病原病菌时才会启动CsGH3.6表达,从而有利于获得表型正常且具有溃疡病抗性的转基因柑橘[42]。
4 结论
超量表达CsGH3.6通过酰基化自由IAA来抑制生长素信号转导,改变植物细胞的形态建成以利于寄主抗性的提升,同时,解除IAA和JA对SA的拮抗,促进SA介导的抗病反应,增强柑橘对溃疡病的抗性,研究结果为利用生长素信号转导改良柑橘溃疡病抗性提供了依据。(责任编辑 岳梅)
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
.
[本文引用: 1]
[本文引用: 1]
.
[本文引用: 1]
[本文引用: 3]
[本文引用: 1]
[本文引用: 4]
[D].
[本文引用: 2]
[D].
[本文引用: 2]
.
[本文引用: 3]
.
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
.
[本文引用: 1]
.
[本文引用: 1]
.
DOI:10.1093/jxb/ers300Magsci [本文引用: 1]

Plant responses to abiotic stresses are coordinated by arrays of growth and developmental processes. Indole-3-acetic acid (IAA) and abscisic acid (ABA) play critical roles in developmental programmes and environmental responses, respectively, through complex signalling and metabolism networks. However, crosstalk between the two phytohormones in the stress responses remains largely unknown. Here, it is reported that a GH3 family gene, OsGH3-2, encoding an enzyme catalysing IAA conjugation to amino acids, is involved in the modulation of ABA level and stress tolerance. Expression of OsGH3-2 was induced by drought but was suppressed by cold. Overexpression of OsGH3-2 in rice caused significant morphological aberrations related to IAA deficiency, such as dwarfism, smaller leaves, and fewer crown roots and root hairs. The overexpressing line showed significantly reduced carotene, ABA, and free IAA levels, greater stomata aperture, and faster water loss, and was hypersensitive to drought stress. However, the overexpressing line showed increased cold tolerance, which was due to the combined effects of reduced free IAA content, alleviated oxidative damage, and decreased membrane penetrability. Furthermore, expression levels of some ABA synthesis- and stress-related genes were significantly changed in the overexpression line. It was conclude that OsGH3-2 modulates both endogenous free IAA and ABA homeostasis and differentially affects drought and cold tolerance in rice.
.
[本文引用: 3]
.
[本文引用: 2]
.
[本文引用: 1]
.
[本文引用: 1]
.
[本文引用: 1]
.
[本文引用: 1]
.
[本文引用: 1]
.
[本文引用: 1]
[D].
[本文引用: 5]
[D].
[本文引用: 5]
[本文引用: 1]
[本文引用: 1]
.
[本文引用: 1]
.
[本文引用: 1]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
.
DOI:10.1007/s10658-008-9415-xMagsci [本文引用: 1]

<a name="Abs1"></a>Soil application of the systemic insecticide imidacloprid (Admire?, Bayer Crop Science) produced season-long control of citrus canker caused by <i>Xanthomonas citri</i> sbsp. <i>citri</i>. Imidacloprid is a neo-nicotinoid that breaks down <i>in planta</i> into 6-chloronicotinic acid, a compound closely related to the systemic acquired resistance (SAR) inducer isonicotinic acid. Potted Swingle citrumelo seedlings (<i>Citrus paradisi</i> × <i>Poncirus trifoliata</i>) were treated with imidacloprid and the SAR inducers, isonicotinic acid, and acibenzolar-s-methyl as soil drenches or with acibenzolar-s-methyl as a foliar spray 1week prior to inoculation of immature leaves with <i>X. citri</i> sbsp. <i>citri</i>. Seedlings were re-inoculated four times over a 24-week period. SAR induction was confirmed by expression of the <i>PR-2</i> gene (β-1,3 glucanase). Soil drenches of imidacloprid, isonicotinic acid, and acibenzolar-s-methyl induced a high and persistent up-regulation of <i>PR-2</i> gene expression and reduced the number of canker lesions for up to 24 weeks compared to 4 weeks for foliar acibenzolar-s-methyl. Soil applied inducers of SAR reduced canker lesions up to 70% compared with the untreated inoculated plants. Lesions on leaves were small, necrotic, and flat compared to pustular lesions on inoculated untreated plants. Populations of <i>X. citri</i> sbsp. <i>citri</i> per leaf were reduced 1–3 log units in soil-treated plants compared to inoculated untreated plants.
.
[本文引用: 1]
.
[本文引用: 1]
.
[本文引用: 1]
.
[本文引用: 2]
.
[本文引用: 2]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
