 ,11
,11 2
Effect of Midgut Specific Binding Protein ABCC1 on Cry1Ac Toxicity Against Helicoverpa armigera
CHEN Lin1, WEI JiZhen2, LIU Chen1, NIU LinLin1, ZHANG CaiHong1, LIANG GeMei ,11
,11 2
通讯作者:
责任编辑: 岳梅
收稿日期:2019-04-23接受日期:2019-05-27网络出版日期:2019-10-01
| 基金资助: |
Received:2019-04-23Accepted:2019-05-27Online:2019-10-01
作者简介 About authors
陈琳,E-mail:chenlincaas@126.com。

摘要
关键词:
Abstract
Keywords:
PDF (857KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
陈琳, 魏纪珍, 刘臣, 牛琳琳, 张彩虹, 梁革梅. 棉铃虫中肠特异性结合蛋白ABCC1对Cry1Ac毒力的影响[J]. 中国农业科学, 2019, 52(19): 3337-3345 doi:10.3864/j.issn.0578-1752.2019.19.005
CHEN Lin, WEI JiZhen, LIU Chen, NIU LinLin, ZHANG CaiHong, LIANG GeMei.
0 引言
【研究意义】苏云金芽孢杆菌(Bacillus thuringiensis,Bt)产生的晶体蛋白对鳞翅目、双翅目、鞘翅目等多种昆虫和线虫具有特异性毒性,但对人畜无害[1,2,3,4]。Bt能够产生具有杀虫活性的伴孢晶体,Cry蛋白是伴孢晶体中的重要一类,目前已发现约有300多种可以分为40个不同的小类Cry蛋白,Cry1Ac蛋白是其中最早被应用于害虫防治的[1,2,3,4]。表达Bt蛋白的抗虫转基因农作物已在美国、巴西、印度、中国等多个国家广泛种植,这不仅可以有效降低相关农业害虫的危害,还可以减少由于化学农药过度使用带来的环境污染[4,5]。但是随着转Bt基因农作物种植面积的扩大,害虫对Bt蛋白的抗性[3,6-8]问题也日益严重。因此,研究昆虫对Bt蛋白产生抗性的机理对于延迟抗性产生至关重要。【前人研究进展】当昆虫取食Bt蛋白后,Bt蛋白会在昆虫中肠碱性环境中被溶解、活化,并与中肠上皮细胞上的特异性受体结合,随后造成中肠膜穿孔,细胞溶解,最终导致昆虫死亡[3]。Bt蛋白与昆虫中肠受体结合是其发挥杀虫作用的关键,主要受体蛋白表达量的变化或基因突变是引起昆虫对Bt蛋白产生抗性的重要原因。目前报道的主要受体蛋白有钙黏蛋白(cadherin,CAD)、氨肽酶N(aminopeptidase N,APN)、碱性磷酸酯酶(alkaline phosphatase,ALP)、腺苷三磷酸结合转运蛋白(ATP-binding cassette transporter,ABC)等[9,10,11,12,13,14,15]。ABC转运蛋白家族蛋白包含4个核心结构域:两个跨膜结构域(TMD),每个结构域由6个跨膜α螺旋组成;两个位于胞质侧的核苷酸结合区(NBD),与两个跨膜结构域交替。ABC转运蛋白的功能与细胞物质转运和相关物质的代谢有关[16,17,18],已有报道表明,ABC转运蛋白不仅是Bt的受体,而且ABC基因的突变或表达量的改变与昆虫对Bt产生抗性相关[14,15,16]。【本研究切入点】前期研究表明,对Bt蛋白敏感棉铃虫(Helicoverpa armigera)的HaABCC2发生突变,造成HaABCC2翻译的蛋白序列提前终止,HaABCC2蛋白功能丧失,会导致棉铃虫对Cry1Ac蛋白抗性的产生[14]。ABC转运蛋白是一个庞大的家族,且在细胞膜上以多种ABC的形式混合存在,因此研究其他ABC在Cry1Ac杀虫机制及棉铃虫对Cry1Ac抗性机制中的作用很有必要。【拟解决的关键问题】明确棉铃虫HaABCC1是否为Cry1Ac的功能性受体蛋白,HaABCC1表达量的变化是否与棉铃虫对Cry1Ac的抗性有关,进一步揭示Cry1Ac的杀虫机制及棉铃虫产生抗性的分子机制,为合理应用Bt及转基因抗虫作物、制定合理的抗性治理策略提供理论依据。1 材料与方法
试验于2017—2018年在中国农业科学院植物保护研究所植物病虫害生物学国家重点实验室完成。1.1 供试昆虫
棉铃虫新乡敏感品系(96S):1996年6月采自河南省新乡县棉田,在实验室内用人工饲料[19]饲养至今,未接触过任何杀虫剂。敏感棉铃虫品系在昆虫饲养间以温度(27±2)℃,光周期14L﹕10D,相对湿度(75±10)%的条件下饲养。成虫在产卵笼中饲养并用10%蜂蜜水补充营养。棉铃虫Cry1Ac抗性品系(BtR):室内用3.6 mg·mL-1 Cry1Ac原蛋白对96S敏感品系筛选了210代,抗性倍数为3 600倍[20]。
1.2 Cry1Ac蛋白
活化的Cry1Ac蛋白购于北京乐士宁科技有限公司(Envirologix)。生物素标记试剂盒购于武汉伊莱瑞特生物科技股份有限公司(Elabscience)。用生物素标记活化的Cry1Ac蛋白,标记过程参考试剂盒说明书。1.3 取样方法
收集敏感品系(96S)与抗性品系(BtR)棉铃虫5龄幼虫各10头,在冰上解剖,去除食物截取中肠,用4℃预冷的0.7% NaCl溶液将内含物冲洗干净,再用滤纸吸干水分,-80℃保存备用。1.4 总RNA的提取与cDNA的合成
按照Invitrogen操作说明分别提取各样品总RNA,RT-PCR和荧光定量RT-PCR所用的cDNA模板的合成参照Tiangen公司Fast QuantRT Kit说明书和SuperRealPreMix(Probe)说明书。所有样品设3次生物学重复。1.5 原核表达及Ligand blot分析
利用Primer 5.0软件分别设计ABCC1两个跨膜区(TMD)携带酶切位点EcoRV和HindⅢ的引物(表1),名称分别为TM1-EcoRV-F、TM1-HindIII-R;TM2-EcoRV-F、TM2-HindIII-R。把PCR合成的目的基因转入克隆载体,取测序正确的质粒和表达载体pET32a进行双酶切,用T4-DNA连接酶将目的片段和表达载体构建为重组质粒。将测序正确的重组质粒转入BL21(DE3)感受态细胞中并转接500 mL LB培养基中培养,加入0.8 mmol·L-1 IPTG并在25℃过夜诱导表达。高速低温离心收集菌体并超声破碎,经SDS-PAGE电泳并切取目的片段送至北京华大蛋白质研发中心进行质谱检测。并用购于生工生物工程有限公司的PAGE胶蛋白微量回收试剂盒回收目的蛋白。Table 1
表1
表1各RT-PCR、荧光定量RT-PCR及RNAi反应所用引物
Table 1
| 引物名称 Primer name | 引物序列 Primer sequence (5′- 3′) |
|---|---|
| ABCC1-F | TTAATTAACCAGTCCGGCGTCC |
| ABCC1-R | ATGTCTTACAATTCTACGCTTGA |
| GAPDH-F | CATTGAAGGTCTGATGACCACTGT |
| GAPDH-R | CAGAGGGTCCATCCACTGTCTT |
| GAPDH-Probe | CACGCCACCATTGCCACCCA |
| β-actin-F | GGCCCCGTCCACAATGA |
| β-actin-R | CCGATCCATACGGAGTACTTCCT |
| β-actin-Probe | ATCAAGATCATCGCGCCCCCAGA |
| ABCC1-F | GGCAGCGTGAAAAGAAAGAC |
| ABCC1-R | GTGATCACGGACGAGAGGAT |
| ABCC1-Probe | CGAAGCTGAGAAAACCGAGACTGGAAG |
| TMD1-EcoRV-F | GATATCGCGTTCGGCGGTCAGTTC |
| TMD1-HindIII-R | AAGCTTACGTGACATCGAGTTCATCACAATT |
| TMD2-EcoRV-F | GATATCTTGATGAGCGTCGGAATCCT |
| TMD2-HindIII-R | AAGCTTATGTTTCTACTTCTGATGTCATGCGTAC |
| siRNA-ABCC1 | GGAUGUACCUGGUGGGCAUTT |
| siRNA-EGFP | GCGUUGGGAAGUCAAGUUUTT |
| ABCC1-PmeI-F | GTTTAAACATGTCTTACAATTCTACGCTTG |
| ABCC1-StuI-R | AGGCCTTTAATTAACCAGTCCGGCGTCC |
| ABCC1-F’ | AGAAGCCTGCCTCCATACTACC |
| ABCC1-R’ | AAGAAGCGCAACTGCATAAAC |
新窗口打开|下载CSV
用Ligand blot检测原核表达得到的HaABCC1两个跨膜区片段蛋白能否与活化的Cry1Ac在体外结合。将纯化蛋白经过电泳转到PVDF膜上;加入30 mL封闭液到自封袋中并放入PVDF膜,置于摇床上80 r/min室温孵育2 h;重新加入新的30 mL脱脂牛奶再加入5 μL生物素标记的活化Cry1Ac,置于摇床上80 r/min孵育1 h;重新加入20 mL脱脂牛奶再加入2 μL二抗(HRP标记的山羊抗小鼠抗体)到自封袋中,室温孵育1 h,PBST洗膜3次,每次10 min;用ECL方法进行曝光,所用的试剂盒为EASYsee底物发光液。
1.6 RNAi及生物测定
利用RNAi干扰敏感品系(96S)棉铃虫的HaABCC1。为了提高RNAi干扰的特异性,从高特异性的HaABCC1跨膜区片段设计siRNA序列。并用加强荧光蛋白基因(EGFP)作为对照。siRNA由生工生物工程有限公司合成(表1),名称分别为siRNA-ABCC1和siRNA-EGFP。用5 μL微量注射器往3龄初棉铃虫幼虫的腹部注射2 μg·μL-1的siABCC1 1 μL。注射点用凡士林封闭,防止体液过多流失。用不被注射的棉铃虫以及注射siEGFP和DEPC水的棉铃虫作为对照。每个处理24头棉铃虫,进行3次重复。干扰后48 h利用荧光定量RT-PCR检测不同处理棉铃虫HaABCC1的表达量。
采用生物测定的方法测定Cry1Ac对干扰棉铃虫HaABCC1后死亡率的变化。往24孔板的每个孔中加入1 mL刚制作完成的棉铃虫人工饲料(液态)。待其凝固后往每个孔中加入75 μL活化的Cry1Ac蛋白使每个孔中蛋白浓度为120 μg·cm-2 。接入干扰处理48 h后的棉铃虫,每个处理24头,3次重复。以加入等量的PBS溶液为对照处理。5 d后,统计各处理的死亡虫数,计算相应的死亡率[21]。
1.7 细胞转染及细胞生物测定
利用Primer 5.0软件设计带酶切位点PmeI和StuI的HaABCC1全长引物ABCC1-PmeI-F和ABCC1-StuI- R(表1)。经PCR测序鉴定正确的质粒通过双酶切和T4-DNA连接酶连接到pAc5.1b质粒上,构建重组质粒pAc-ABCC1。质粒购于北京华越洋生物有限公司。将Sf9细胞分别平铺到12孔板中,每孔约9×105个细胞,放到28℃培养箱让细胞充分贴壁(2 h)。在Cellfectin(Invitrogen;8 μL/孔)作用下,每个孔里转染2 μg pAc5.1b(对照,空质粒)或目的基因质粒,孵育5 h。用1.5 mL Sf-900 II SFM培养基(Sf9细胞)置换转染液,然后放至28℃培养箱培养64 h。在每次独立的转染试验中,细胞被吹起,重新平铺到96孔板的3个孔中,每孔100 μL含细胞10 000个。充分贴壁2 h后,用活化的Cry1Ac处理转染细胞,活化的Cry1Ac浓度为18.5 μg·mL-1。每个质粒被重复3次转染到细胞中,每次独立的转染用活化的Cry1Ac进行3次重复的生物测定。对细胞生物测定后,Sf9细胞提取RNA并反转录为cDNA,用HaABCC1特异性引物ABCC1-F’和ABCC1-R’(表1)进行PCR验证,检验pAc-ABCC1重组质粒是否成功转入Sf9细胞中。
1.8 氨基酸序列对比分析
根据GenBank中HaABCC1的cDNA序列(KY796050),用Primer 5.0软件分别设计ABCC1-F和ABCC1-R引物(表1),以抗、感棉铃虫5龄中肠的cDNA为模板,进行PCR扩增,分别扩增出ABCC1序列。按下列程序启动PCR:94℃预变性5 min;94℃变性30 s,55℃退火30 s,72℃延伸5 min,共35个循环;72℃保温10 min。PCR反应所用的高保真聚合酶购于赛默飞世尔科技(中国)有限公司(Thermo Fisher)。扩增完成后用1%琼脂糖凝胶电泳检测,利用AxygenDNA回收试剂盒回收目的条带,然后把回收产物克隆到Peasy-T3载体上,转化入Trans1-T1感受态细胞中,挑取阳性单克隆送北京博迈德(Biomed)进行序列测定。PCR获得基因全长并经Blast序列比对确认为HaABCC1后,用DNAMAN分别对比其对应的氨基酸序列。1.9 荧光定量RT-PCR
利用TaqMan探针技术进行荧光定量RT-PCR,根据HaABCC1的cDNA序列设计、合成用于荧光定量RT-PCR的特异性引物和探针,以棉铃虫GAPDH(GenBank:JF417983.1)和β-Actin(GenBank:EU527017.1)作为双内参基因,合成特异性引物和探针,探针5′端采用FAM标记,3′端采用BHQ标记。引物和探针在上海Invitrogen公司合成(表1)。在20 μL荧光定量RT-PCR反应体系中含有:2× SuperReal Premix 10 μL正向引物(10 μmol·L-1)0.6 μL,反向引物(10 μmol·L-1)0.6 μL,荧光探针(10 μmol·L-1)0.4 μL,cDNA模板1 μL,50×ROX ReferenceDye 0.2 μL,RNase-Free ddH2O 7.2 μL。混匀离心后在ABI 7500荧光定量RT-PCR仪上按照如下条件反应:95℃预变性15 min,然后95℃变性3 s,60℃退火/延伸32 s,共40个循环。反应中以水作为阴性对照,每个处理3个技术重复。1.10 数据处理
对荧光定量RT-PCR结果采用2-ΔΔCt法进行计算[22]。采用SPSS软件对处理数据进行分析,处理间比较采用单因素方差分析检测,用Turkey法进行多重比较。2 结果
2.1 HaABCC1跨膜区原核表达及Ligand blot分析
通过PCR技术,经过测序验证成功克隆得到HaABCC1跨膜区TMD1(924 bp)和TMD2(882 bp),并在E. coli BL21(DE3)感受态细胞中成功原核表达,蛋白片段大小分别为34和33 kD,与预期结果相符(图1-A)。经过Ligand blot检测,HaABCC1跨膜区原核表达片段均能与活化的Cry1Ac蛋白结合(图1-B)。图1
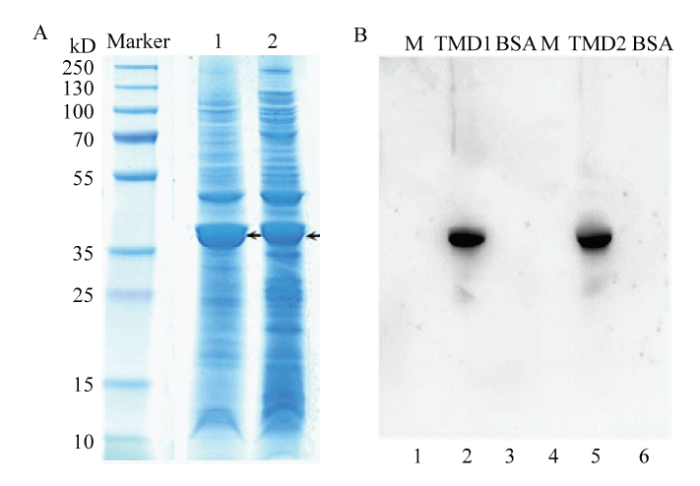 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1原核表达HaABCC1片段的SDS-PAGE电泳图及Ligand blot 检测Cry1Ac与HaABCC1的结合
A:Marker:蛋白标准品Protein marker;1:超声破碎处理后TMD1片段TMD1 fragment after ultrasound treatment;2:超声破碎处理后TMD2片段TMD2 fragment after ultrasound treatment。B:1、4:蛋白标准品Protein marker;3、6:BSA蛋白,作为对照BSA, as a control;2:TMD1片段与活化的Cry1Ac蛋白结合TMD1 fragment bound with Cry1Ac;5:TMD2片段与活化的Cry1Ac蛋白结合TMD2 fragment bound with Cry1Ac
Fig. 1SDS-PAGE of HaABCC1 fragments by prokaryotic expression and Ligand blot detection of Cry1Ac binding with HaABCC1
2.2 HaABCC1功能验证
HaABCC1被干扰后,相较于未注射的棉铃虫以及注射DEPC水和siEGFP的棉铃虫,HaABCC1的表达量分别下降了54.0%、49.4%和52.2%(P<0.05)(图2-A)。HaABCC1被干扰后的棉铃虫,除表达量明显降低外,幼虫对Cry1Ac的敏感性也发生显著改变。用活化的Cry1Ac蛋白处理HaABCC1被干扰的棉铃虫,与未注射的棉铃虫以及注射DEPC水和siEGFP的棉铃虫相比,其幼虫死亡率分别下降了65.9%、60.0%和62.2%(P<0.05)(图2-B)。图2
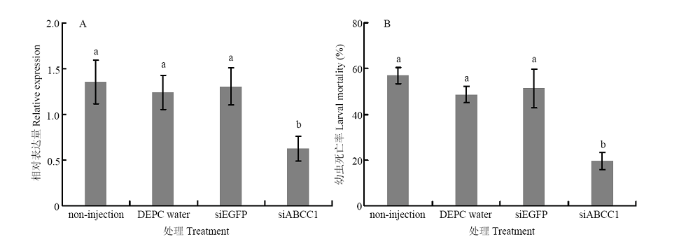 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2基因沉默后棉铃虫HaABCC1的表达量(A)及对Cry1Ac敏感性的影响(B)
柱上不同小写字母表示处理间差异显著(P<0.05)。下同
Fig. 2HaABCC1 expression after gene silencing (A) and its effect on H. armigera susceptibility to Cry1Ac (B)
Different lowercases indicate significant differences among treatments (P<0.05). The same as below
与转入pAc空质粒的Sf9细胞相比,可以看到棉铃虫pAc-ABCC1被成功导入Sf9细胞中(图3-A)。用18.5 μg·mL-1的活化Cry1Ac蛋白处理成功转入HaABCC1的Sf9细胞,结果发现细胞的死亡率显著上升(P<0.05)(图3-B),为33.8%,显著高于未转入HaABCC1的Sf9细胞的细胞死亡率(2.0%)。
图3
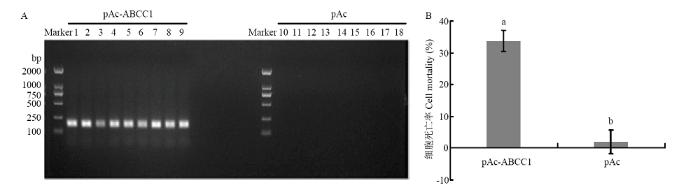 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3将 pAc-ABCC1转入Sf9细胞并用活化的Cry1Ac蛋白处理后其死亡率
A:PCR检测pAc-ABCC1质粒转入Sf9细胞Confirmation of HaABCC1 expression in cells transfected with pAc-ABCC1 by PCR。1—9:转入pAc-ABCC1质粒的Sf9细胞(3个生物学重复× 3个技术重复)pAc-ABCC1 plasmid transfected into the Sf9 cell line (3 biological replicates × 3 technical replicates);10—18:转入pAc空质粒的Sf9细胞(3个生物学重复× 3个技术重复)pAc empty plasmid transfected into the Sf9 cell line (3 biological replicates × 3 technical replicates)。B:用活化的Cry1Ac蛋白处理转入pAc-ABCC1质粒和转入pAc空质粒的Sf9细胞死亡率The mortality of Sf9 cells exposed to Cry1Ac after transfected with the pAc-ABCC1 or pAc empty plasmid
Fig. 3Effects of pAc-ABCC1 plasmid transfection into Sf9 cells on the mortality after treated with activated Cry1Ac
2.3 抗、敏品系棉铃虫HaABCC1的cDNA及氨基酸序列对比分析
比较Cry1Ac抗性棉铃虫与敏感棉铃虫的HaABCC1全长,发现HaABCC1在Cry1Ac抗性品系(BtR)和敏感品系(96S)棉铃虫只有少数碱基的差异,在氨基酸水平上没有任何差异。2.4 抗、敏品系棉铃虫HaABCC1的mRNA表达量分析
进一步比较了HaABCC1在抗性品系(BtR)和敏感品系(96S)棉铃虫中表达量的差异,发现HaABCC1在抗性品系棉铃虫中的表达量显著降低(P<0.05),敏感品系的表达量是抗性品系的4.19倍(图4)。图4
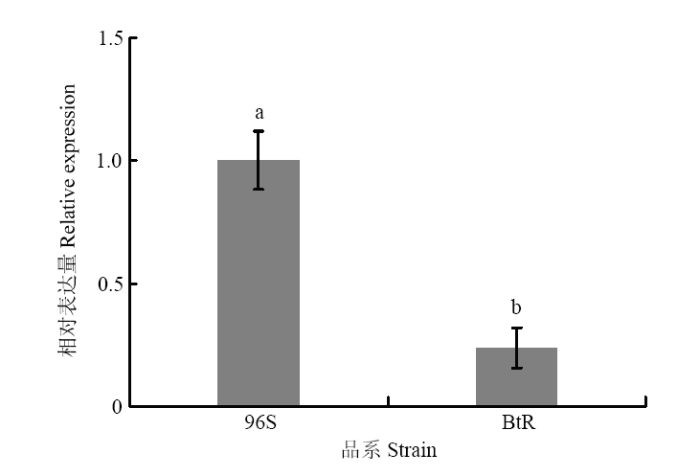 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4HaABCC1在不同品系中的表达量
Fig. 4The expression level of HaABCC1 in different strains of H. armigera
3 讨论
昆虫中肠上皮细胞刷状缘膜囊泡BBMV上的受体与活化的Bt蛋白特异性结合在Bt杀虫机制中起着关键作用[23,24]。已经有相关报道证实ABC转运蛋白是Bt蛋白的受体。例如,ZHOU等[15]通过蛋白质体外结合试验,发现Cry1Ac能与ABCC2结合;TANAKA等[25]在昆虫细胞内分别表达突变型(酪氨酸插入)和野生型家蚕的ABCC2,通过分析细胞对Bt的敏感性变化发现ABCC2参与了Cry1Ab、Cry1Ac、Cry1Fa和Cry8Ca的杀虫过程;CHEN等[26]通过在昆虫细胞系表达斜纹夜蛾(Spodoptera litura Sl-HP)ABCC3,证实其为Cry1Ac的功能受体。本研究选择在HaABCC1转运蛋白中特异性较高的两个跨膜结构域,通过在大肠杆菌中原核表达得到了两个HaABCC1跨膜结构域蛋白,通过Ligand blot分析确定这两个跨膜区片段蛋白均能与Cry1Ac在体外结合。HaABCC1被干扰后棉铃虫对Cry1Ac的敏感性显著降低,而且在Sf9细胞中表达HaABCC1能够提高Cry1Ac处理后的细胞死亡率。这些结果表明HaABCC1是Cry1Ac的特异性结合蛋白,可能是Cry1Ac的功能性受体蛋白。前期研究发现,HaABCC1在棉铃虫不同发育阶段均有表达,在4龄、5龄幼虫中表达量最高,在成虫中最低;在不同组织中均有表达,在马氏管中表达量最高,在前肠中最低。这可能与ABC转运蛋白家族的功能相关,如在哺乳类动物中ABC转运蛋白参与离子运输和毒素分泌的过程,能够将不同的外源物质、重金属等运输到体外,因此具有抵御抗体、化学治疗药物和农药的功能[27]。近年来,关于ABC转运蛋白的变化与昆虫对Bt的抗性关系也受到普遍关注。如鳞翅目昆虫的ABCB、ABCD、ABCE、ABCF之间存在一定的同源性且与昆虫对Bt的抗性有关[28];敏感棉铃虫的HaABCC2发生突变,造成其翻译的蛋白序列提前终止,HaABCC2蛋白功能丧失,这会导致棉铃虫对Cry1Ac蛋白抗性的产生[14];小菜蛾(Plutella xylostella)ABCC2突变会引起其对Cry1Ac蛋白抗性的产生[29];小菜蛾ABCH1被干扰后,会显著提高其对Cry1Ac蛋白的抗性[30];棉铃虫ABCA2的突变会引起棉铃虫对Cry2Ab蛋白的抗性[31]。本研究发现,虽然Cry1Ac抗性棉铃虫HaABCC1编码的氨基酸序列没有发生变化,但与敏感品系相比,抗性品系棉铃虫的HaABCC1表达量显著降低,推测HaABCC1表达量的降低可能与棉铃虫对Cry1Ac抗性的产生相关。
由于棉铃虫HaABCC1蛋白具有与其他ABC蛋白家族相似的结构,含有2个跨膜结构域和2个膜外区域,含有14个N-糖基化位点和16个O-糖基化位点。尤其是2个富含糖基化位点的跨膜结构域可能与Bt杀虫作用及昆虫对Bt的抗性相关。有关ABCC2在Bt杀虫机理中的作用,GAHAN等[32]推测Cry蛋白与ABCC2结合可能是形成寡聚体的基础,并把穿孔前的结构拉近到跨膜区以便于穿孔;TANAKA等[25]研究发现,在细胞内共表达钙黏蛋白(CaLP)和ABCC2,共表达后的细胞比仅表达ABCC2时更大;类似地,在Sf9细胞中共表达HevABCC2(Heliothis virescens)和HevCaLP,对细胞有更高的毒性;而TAY等推测ABCA2可以独立参与Cry2Ab的作用模型,来完成结合和穿孔[31];真核表达抗、感家蚕ABCC2,结果表明该基因协助穿孔[33];ABCC2的修饰和敲除可能阻碍了Bt蛋白作用机制的最后一步[24]。基于不同ABC转运蛋白之间相似的结构,棉铃虫ABCC1也可能与其他ABC转运蛋白一样,两个跨膜结构域的变化可能与昆虫对Bt的抗性相关。但本研究并没有发现HaABCC1两个跨膜结构域的氨基酸序列发生改变,HaABCC1全长氨基酸序列也没有变化,仅HaABCC1表达量发生了改变。由于ABC蛋白是一个庞大的家族,且在细胞膜上也混合存在,有可能是很多种蛋白共同起作用。因此,笔者推测ABCC1可能和其他ABC家族蛋白或与其他受体蛋白共同在Bt杀虫机制及抗性机制中起作用。
TAY等[31]利用EPIC(exon-primedinton-crossing)技术和目的基因的测序技术,通过双向遗传连锁分析了抗Cry2Ab棉铃虫,发现其抗性的产生是由于ATP结合盒转运体基因ABCA上的3个与抗性相关的INDEL的突变而引起,且HaABCA1和HaABCA2的突变与棉铃虫对Cry2Ab的抗性相关;XIAO等[14]也报道了HaABCC2的突变引起棉铃虫对Cry2Ab的抗性。前期研究发现HaABCC1被干扰后,棉铃虫对Cry2Ab的敏感性显著降低,推测ABCC1也是Cry2Ab的功能性受体蛋白[34]。上述结果表明,ABC转运蛋白可能不仅是Cry1A的受体蛋白,也是Cry2A的受体,这些共同的受体是否与Cry1A和Cry2A类蛋白间的交互抗性相关,有待进一步研究。
4 结论
原核表达的两个棉铃虫HaABCC1跨膜区片段蛋白均能与Cry1Ac在体外结合;HaABCC1被干扰后,能显著降低Cry1Ac对棉铃虫的毒力;将HaABCC1导入Sf9细胞后能显著提高Cry1Ac处理后的死亡率;与敏感品系相比,Cry1Ac抗性品系HaABCC1表达量显著降低。因此,HaABCC1可能是Cry1Ac的功能受体蛋白,并可能参与对Cry1Ac的抗性机制。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 2]
[本文引用: 2]
[本文引用: 4]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 5]
[本文引用: 3]
[本文引用: 2]
[本文引用: 1]
Magsci [本文引用: 1]

腺苷三磷酸结合盒转运蛋白(ATP-binding cassette transporter),简称ABC转运蛋白(ABC transporter),是继细胞色素P450单加氧酶、羧酸酯酶、谷胱甘肽S转移酶之后又一类重要的解毒酶系,因其在杀虫剂解毒等方面起着非常重要的作用,近年来逐渐受到广泛关注。ABC转运蛋白是一大类跨膜蛋白,其核心结构通常由4个结构域组成,包括2个高度疏水的跨膜结构域(transmembrane domains , TMD)和2个核苷酸结合域(nucleotide binding domains, NBD)。根据序列相似性和保守结构域,可以把ABC转运蛋白家族分为8个亚家族,每个亚家族的成员数及功能不同。这类蛋白在各种生物体内均有分布,其主要功能包括转运物质、信号传导、细胞表面受体及参与细胞内DNA修复,转录及调节基因的表达过程等。此外,近年来的研究表明,ABC转运蛋白的突变或过表达不仅与节肢动物对化学农药的抗药性密切相关,而且在抗Bt毒素方面也起着非常重要的作用,对转Bt作物造成严重威胁。本文综述了节肢动物ABC转运蛋白的结构,ATP水解介导的作用机制,亚家族的分类、结构及生理功能,以及由ABC转运蛋白介导的抗药性研究进展,旨在深入了解ABC转运蛋白的研究现状及其在节肢动物抗药性方面的作用,为阐明节肢动物抗药性机制提供新的理论依据,对改进农业害虫的抗性监测和治理策略也具有一定的指导意义。
Magsci [本文引用: 1]

腺苷三磷酸结合盒转运蛋白(ATP-binding cassette transporter),简称ABC转运蛋白(ABC transporter),是继细胞色素P450单加氧酶、羧酸酯酶、谷胱甘肽S转移酶之后又一类重要的解毒酶系,因其在杀虫剂解毒等方面起着非常重要的作用,近年来逐渐受到广泛关注。ABC转运蛋白是一大类跨膜蛋白,其核心结构通常由4个结构域组成,包括2个高度疏水的跨膜结构域(transmembrane domains , TMD)和2个核苷酸结合域(nucleotide binding domains, NBD)。根据序列相似性和保守结构域,可以把ABC转运蛋白家族分为8个亚家族,每个亚家族的成员数及功能不同。这类蛋白在各种生物体内均有分布,其主要功能包括转运物质、信号传导、细胞表面受体及参与细胞内DNA修复,转录及调节基因的表达过程等。此外,近年来的研究表明,ABC转运蛋白的突变或过表达不仅与节肢动物对化学农药的抗药性密切相关,而且在抗Bt毒素方面也起着非常重要的作用,对转Bt作物造成严重威胁。本文综述了节肢动物ABC转运蛋白的结构,ATP水解介导的作用机制,亚家族的分类、结构及生理功能,以及由ABC转运蛋白介导的抗药性研究进展,旨在深入了解ABC转运蛋白的研究现状及其在节肢动物抗药性方面的作用,为阐明节肢动物抗药性机制提供新的理论依据,对改进农业害虫的抗性监测和治理策略也具有一定的指导意义。
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
Magsci [本文引用: 1]

<P class=MsoNormal ><FONT size=3><SPAN >氨肽酶</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">N</FONT></SPAN><SPAN >(</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">aminopeptidase N, APN</FONT></SPAN><SPAN >)和钙粘蛋白(</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">cadherin</FONT></SPAN><SPAN >)是存在于鳞翅目昆虫中肠刷状缘膜囊(</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">brush border membrane vesicles, BBMV</FONT></SPAN><SPAN >)上</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Bt</FONT></SPAN><SPAN >毒素</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Cry<?xml:namespace prefix = st1 /><st1:chmetcnv UnitName="a" SourceValue="1" HasSpace="False" Negative="False" NumberType="1" TCSC="0" w:st="on">1A</st1:chmetcnv></FONT></SPAN><SPAN >的受体。本实验将棉铃虫</SPAN><I ><SPAN lang=EN-US><FONT face="Times New Roman">Helicoverpa armigera</FONT></SPAN></I><SPAN >氨肽酶</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">N1</FONT></SPAN><SPAN >基因</SPAN><I ><SPAN lang=EN-US><FONT face="Times New Roman">Haapn1</FONT></SPAN></I><SPAN >和钙粘蛋白基因</SPAN><I ><SPAN lang=EN-US><FONT face="Times New Roman">Ha_BtR</FONT></SPAN></I><SPAN >双链</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">RNA</FONT></SPAN><SPAN >(</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">dsRNA</FONT></SPAN><SPAN >)注入棉铃虫</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">4</FONT></SPAN><SPAN >龄幼虫体内</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >以研究这两种受体基因沉默后对</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Cry<st1:chmetcnv UnitName="ac" SourceValue="1" HasSpace="False" Negative="False" NumberType="1" TCSC="0" w:st="on">1Ac</st1:chmetcnv></FONT></SPAN><SPAN >毒力的影响。结果表明</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">: </FONT></SPAN><SPAN >注射</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">dsRNA</FONT></SPAN><SPAN >(</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">1 </FONT></SPAN><SPAN >μ</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">g/</FONT></SPAN><SPAN >头)进行基因沉默后</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, <I >Haapn1</I> mRNA</FONT></SPAN><SPAN >表达量比注射缓冲液(</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">elution solution, ES</FONT></SPAN><SPAN >)的对照下降了</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">30%~49%, <I >Ha_BtR</I> mRNA</FONT></SPAN><SPAN >表达量下降了</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">30%~37%</FONT></SPAN><SPAN >。注射</SPAN><FONT face="Times New Roman"><I ><SPAN lang=EN-US>Haapn1</SPAN></I><SPAN lang=EN-US> dsRNA</SPAN></FONT><SPAN >的幼虫在</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">40</FONT></SPAN><SPAN >和</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">70 μg/cm<SUP>2</SUP> Cry<st1:chmetcnv UnitName="ac" SourceValue="1" HasSpace="False" Negative="False" NumberType="1" TCSC="0" w:st="on">1Ac</st1:chmetcnv></FONT></SPAN><SPAN >活化毒素下的死亡率显著低于注射</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">ES</FONT></SPAN><SPAN >的幼虫</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >而在</SPAN><SPAN lang=EN-US><FONT face="Times New Roman"> 100 </FONT></SPAN><SPAN >和</SPAN><SPAN lang=EN-US><FONT face="Times New Roman"> 170 μg/cm<SUP>2</SUP> Cry<st1:chmetcnv UnitName="ac" SourceValue="1" HasSpace="False" Negative="False" NumberType="1" TCSC="0" w:st="on">1Ac</st1:chmetcnv></FONT></SPAN><SPAN >原毒素处理下两者死亡率无显著差异</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">; Cry<st1:chmetcnv UnitName="ac" SourceValue="1" HasSpace="False" Negative="False" NumberType="1" TCSC="0" w:st="on">1Ac</st1:chmetcnv></FONT></SPAN><SPAN >活化毒素以及原毒素对注射</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Ha_BtR dsRNA</FONT></SPAN><SPAN >幼虫与注射</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">ES</FONT></SPAN><SPAN >幼虫的毒力均无显著差异。当同时注射</SPAN><I ><SPAN lang=EN-US><FONT face="Times New Roman">Haapn1</FONT></SPAN></I><SPAN >及</SPAN><FONT face="Times New Roman"><I ><SPAN lang=EN-US>Ha_BtR</SPAN></I><SPAN lang=EN-US> dsRNA</SPAN></FONT><SPAN >后</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >干扰后的幼虫对</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Cry<st1:chmetcnv UnitName="ac" SourceValue="1" HasSpace="False" Negative="False" NumberType="1" TCSC="0" w:st="on">1Ac</st1:chmetcnv></FONT></SPAN><SPAN >活化毒素和原毒素的敏感性均显著下降。本研究进一步证明了棉铃虫</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Haapn1</FONT></SPAN><SPAN >和</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Ha_BtR</FONT></SPAN><SPAN >均是</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Bt</FONT></SPAN><SPAN >毒素</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Cry<st1:chmetcnv UnitName="ac" SourceValue="1" HasSpace="False" Negative="False" NumberType="1" TCSC="0" w:st="on">1Ac</st1:chmetcnv></FONT></SPAN><SPAN >的功能受体</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >这两种受体蛋白共同参与</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Cry<st1:chmetcnv UnitName="ac" SourceValue="1" HasSpace="False" Negative="False" NumberType="1" TCSC="0" w:st="on">1Ac</st1:chmetcnv></FONT></SPAN><SPAN >的毒杀作用过程。该结果也提示</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, <I >Haapn1</I></FONT></SPAN><SPAN >或</SPAN><I ><SPAN lang=EN-US><FONT face="Times New Roman">Ha_BtR</FONT></SPAN></I><SPAN >基因产生突变都可能导致棉铃虫对</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Cry<st1:chmetcnv UnitName="ac" SourceValue="1" HasSpace="False" Negative="False" NumberType="1" TCSC="0" w:st="on">1Ac</st1:chmetcnv></FONT></SPAN><SPAN >产生抗性。</SPAN></FONT></P>
Magsci [本文引用: 1]

<P class=MsoNormal ><FONT size=3><SPAN >氨肽酶</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">N</FONT></SPAN><SPAN >(</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">aminopeptidase N, APN</FONT></SPAN><SPAN >)和钙粘蛋白(</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">cadherin</FONT></SPAN><SPAN >)是存在于鳞翅目昆虫中肠刷状缘膜囊(</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">brush border membrane vesicles, BBMV</FONT></SPAN><SPAN >)上</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Bt</FONT></SPAN><SPAN >毒素</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Cry<?xml:namespace prefix = st1 /><st1:chmetcnv UnitName="a" SourceValue="1" HasSpace="False" Negative="False" NumberType="1" TCSC="0" w:st="on">1A</st1:chmetcnv></FONT></SPAN><SPAN >的受体。本实验将棉铃虫</SPAN><I ><SPAN lang=EN-US><FONT face="Times New Roman">Helicoverpa armigera</FONT></SPAN></I><SPAN >氨肽酶</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">N1</FONT></SPAN><SPAN >基因</SPAN><I ><SPAN lang=EN-US><FONT face="Times New Roman">Haapn1</FONT></SPAN></I><SPAN >和钙粘蛋白基因</SPAN><I ><SPAN lang=EN-US><FONT face="Times New Roman">Ha_BtR</FONT></SPAN></I><SPAN >双链</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">RNA</FONT></SPAN><SPAN >(</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">dsRNA</FONT></SPAN><SPAN >)注入棉铃虫</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">4</FONT></SPAN><SPAN >龄幼虫体内</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >以研究这两种受体基因沉默后对</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Cry<st1:chmetcnv UnitName="ac" SourceValue="1" HasSpace="False" Negative="False" NumberType="1" TCSC="0" w:st="on">1Ac</st1:chmetcnv></FONT></SPAN><SPAN >毒力的影响。结果表明</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">: </FONT></SPAN><SPAN >注射</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">dsRNA</FONT></SPAN><SPAN >(</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">1 </FONT></SPAN><SPAN >μ</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">g/</FONT></SPAN><SPAN >头)进行基因沉默后</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, <I >Haapn1</I> mRNA</FONT></SPAN><SPAN >表达量比注射缓冲液(</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">elution solution, ES</FONT></SPAN><SPAN >)的对照下降了</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">30%~49%, <I >Ha_BtR</I> mRNA</FONT></SPAN><SPAN >表达量下降了</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">30%~37%</FONT></SPAN><SPAN >。注射</SPAN><FONT face="Times New Roman"><I ><SPAN lang=EN-US>Haapn1</SPAN></I><SPAN lang=EN-US> dsRNA</SPAN></FONT><SPAN >的幼虫在</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">40</FONT></SPAN><SPAN >和</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">70 μg/cm<SUP>2</SUP> Cry<st1:chmetcnv UnitName="ac" SourceValue="1" HasSpace="False" Negative="False" NumberType="1" TCSC="0" w:st="on">1Ac</st1:chmetcnv></FONT></SPAN><SPAN >活化毒素下的死亡率显著低于注射</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">ES</FONT></SPAN><SPAN >的幼虫</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >而在</SPAN><SPAN lang=EN-US><FONT face="Times New Roman"> 100 </FONT></SPAN><SPAN >和</SPAN><SPAN lang=EN-US><FONT face="Times New Roman"> 170 μg/cm<SUP>2</SUP> Cry<st1:chmetcnv UnitName="ac" SourceValue="1" HasSpace="False" Negative="False" NumberType="1" TCSC="0" w:st="on">1Ac</st1:chmetcnv></FONT></SPAN><SPAN >原毒素处理下两者死亡率无显著差异</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">; Cry<st1:chmetcnv UnitName="ac" SourceValue="1" HasSpace="False" Negative="False" NumberType="1" TCSC="0" w:st="on">1Ac</st1:chmetcnv></FONT></SPAN><SPAN >活化毒素以及原毒素对注射</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Ha_BtR dsRNA</FONT></SPAN><SPAN >幼虫与注射</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">ES</FONT></SPAN><SPAN >幼虫的毒力均无显著差异。当同时注射</SPAN><I ><SPAN lang=EN-US><FONT face="Times New Roman">Haapn1</FONT></SPAN></I><SPAN >及</SPAN><FONT face="Times New Roman"><I ><SPAN lang=EN-US>Ha_BtR</SPAN></I><SPAN lang=EN-US> dsRNA</SPAN></FONT><SPAN >后</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >干扰后的幼虫对</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Cry<st1:chmetcnv UnitName="ac" SourceValue="1" HasSpace="False" Negative="False" NumberType="1" TCSC="0" w:st="on">1Ac</st1:chmetcnv></FONT></SPAN><SPAN >活化毒素和原毒素的敏感性均显著下降。本研究进一步证明了棉铃虫</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Haapn1</FONT></SPAN><SPAN >和</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Ha_BtR</FONT></SPAN><SPAN >均是</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Bt</FONT></SPAN><SPAN >毒素</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Cry<st1:chmetcnv UnitName="ac" SourceValue="1" HasSpace="False" Negative="False" NumberType="1" TCSC="0" w:st="on">1Ac</st1:chmetcnv></FONT></SPAN><SPAN >的功能受体</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, </FONT></SPAN><SPAN >这两种受体蛋白共同参与</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Cry<st1:chmetcnv UnitName="ac" SourceValue="1" HasSpace="False" Negative="False" NumberType="1" TCSC="0" w:st="on">1Ac</st1:chmetcnv></FONT></SPAN><SPAN >的毒杀作用过程。该结果也提示</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">, <I >Haapn1</I></FONT></SPAN><SPAN >或</SPAN><I ><SPAN lang=EN-US><FONT face="Times New Roman">Ha_BtR</FONT></SPAN></I><SPAN >基因产生突变都可能导致棉铃虫对</SPAN><SPAN lang=EN-US><FONT face="Times New Roman">Cry<st1:chmetcnv UnitName="ac" SourceValue="1" HasSpace="False" Negative="False" NumberType="1" TCSC="0" w:st="on">1Ac</st1:chmetcnv></FONT></SPAN><SPAN >产生抗性。</SPAN></FONT></P>
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
