The Relationship Between Anthocyanins and Flower Colors of Bud Mutation in Camellia japonica
LI XinLei, YIN HengFu, FAN ZhengQi, LI JiYuanResearch Institute of Subtropical Forestry, Chinese Academy of Forestry, Hangzhou 311400责任编辑: 赵伶俐
收稿日期:2018-12-29接受日期:2019-03-5网络出版日期:2019-06-01
| 基金资助: |
Received:2018-12-29Accepted:2019-03-5Online:2019-06-01
作者简介 About authors
李辛雷,Tel:15968855095;E-mail:lixinlei2020@163.com

摘要
关键词:
Abstract
Keywords:
PDF (379KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
李辛雷, 殷恒福, 范正琪, 李纪元. 山茶芽变花色与花青苷的关系[J]. 中国农业科学, 2019, 52(11): 1961-1969 doi:10.3864/j.issn.0578-1752.2019.11.010
LI XinLei, YIN HengFu, FAN ZhengQi, LI JiYuan.
0 引言
【研究意义】山茶(Camellia japonica)冬春季节开花,花色、花型和花径等变异丰富,观赏价值高、适应性强,是园林绿化及盆栽重要的木本花卉,在国内外广泛应用[1]。山茶花色变异丰富,其原始花色为红色,通过芽变可产生粉色、白色及复色品种,如通过芽变已从‘红宝珠’‘红芙蓉’和‘红嫦娥彩’等开红色花的品种中选育出相应的粉色和白色品种[2]。植物花色色素主要包括类黄酮、类胡萝卜素和生物碱[3],山茶属植物花色色素成分主要为类黄酮,包括金花茶(C. nitidissima)黄色花瓣中的黄酮醇类等[4],及山茶红色花瓣中的花青苷类[5,6,7,8,9,10,11]。因此,研究山茶及其芽变品种不同花色花瓣中花青苷成分与含量,明确山茶芽变花色与花青苷关系对山茶花色的芽变育种具有重要意义。【前人研究进展】山茶属花瓣中花青苷的早期研究方面,HAYASHI等[5]利用纸层析技术从山茶(C. japonica)中发现矢车菊素-3-O-β-葡萄糖苷,SAKATA等[6]从短柄山茶(C. japonica ssp. rusticana)花瓣中发现矢车菊素-3-O-β-半乳糖苷,SAITO等[7]从山茶物种和品种中分离并鉴定出矢车菊素-3-O-[6-O-(E)-p-香豆酰]-β-葡萄糖苷等花青苷。近年来,LI等[8,9]从滇山茶(C. reticulata)和滇山茶品种‘大理茶’中检测到10种花青苷,其部分花青苷Cy结构上连接特有的3-(2-xylosyl)-单糖基;LI等[10]从怒江红山茶(C. saluenensis)中鉴定出7种花青苷,部分花青苷Cy结构上连接特有的3,5-二糖基;LI等[11]从香港红山茶(C. hongkongensis)中鉴定出12种花青苷,除8种矢车菊素糖苷外,还检测到4种飞燕草素糖苷。【本研究切入点】山茶花青苷相关研究的开展为其花色研究奠定了基础,但到目前为止,山茶属花青苷研究主要集中于部分山茶物种及开红色花的品种,山茶品种花瓣中花青苷成分与花色的关系及不同花色形成机制尚不清楚。山茶花色主要为红色,通过芽变极易形成粉色和白色变异品种[1,2]。芽变选育为山茶育种重要方法,但目前关于山茶及其粉色、白色芽变品种花色形成的物质基础尚不清楚,极大地限制了山茶花色的芽变育种。【拟解决的关键问题】本试验利用高效液相色谱-光电二极管阵列检测和超高效液相色谱-四极杆-飞行时间质谱联用技术对山茶品种红色花瓣及其粉色与白色芽变品种花瓣中花青苷成分与含量进行研究,结合花色表型分析,探讨山茶芽变花色与花青苷之间的关系,揭示山茶芽变花色形成的物质基础,从而为山茶花色的芽变育种提供科学依据。1 材料与方法
试验于2018年在中国林业科学研究院亚热带林业研究所开展。1.1 材料
2018年3月开始收集试验材料,采集新鲜花朵测定其花色。试验材料为山茶及其芽变品种(表1),山茶品种花色均为红色,其芽变品种分别为相应的粉色和白色品种,如‘红宝珠’花色为红色,其芽变品种为粉色‘粉宝珠’和白色‘白宝珠’;‘红芙蓉’花色为红色,其芽变品种为粉色‘粉芙蓉’和白色‘白芙蓉’等。山茶及其芽变品种均选取生长状况一致的植株5株,每株取树冠外围盛开期花朵3朵。Table 1
表1
表1山茶品种花色数据
Table 1
| 品种 Cultivars | CIE L*a*b*表色系统 CIE L*a*b*coordinate | |||||
|---|---|---|---|---|---|---|
| 花色 Flower color | L* | a* | b* | C* | h | |
| 白宝珠Baibaozhu | 白色 White | 93.09±1.23 | -0.70±0.02 | 1.65±0.38 | 1.80 ±0.25 | 92.72±2.01 |
| 白芙蓉Baifurong | 白色 White | 89.10±2.01 | -0.51±0.01 | 3.21±0.83 | 3.27±0.35 | 101.21±1.09 |
| 白嫦娥彩Baichangecai | 白色 White | 88.21±1.57 | -0.93±0.12 | 4.77±0.47 | 4.86±1.21 | 101.19±1.37 |
| 白五宝Baiwubao | 白色 White | 90.13±1.46 | -0.71±0.05 | 3.21±0.53 | 3.31±0.23 | 105.04±3.11 |
| 白碧玉Baibiyu | 白色 White | 87.57±1.82 | -0.59±0.05 | 6.53±1.18 | 6.57±0.51 | 95.22± 2.25 |
| 白七仙女Baiqixiannv | 白色 White | 88.65± 3.12 | -0.68±0.06 | 4.46±0.45 | 4.64±0.29 | 98.19±1.85 |
| 平均 Average | 89.46 | -0.69 | 3.97 | 4.07 | 98.93 | |
| 粉宝珠Fenbaozhu | 粉色Pink | 65.90±1.23 | 39.90±0.86 | 2.93±0.25 | 40.03±1.38 | 4.11±1.22 |
| 粉芙蓉Fenfurong | 粉色Pink | 69.75±0.95 | 36.95±1.56 | 9.87±1.08 | 37.00± 1.26 | 6.82±2.05 |
| 粉嫦娥彩Fenchangecai | 粉色Pink | 67.62±1.26 | 41.71±1.87 | 9.94±0.74 | 33.81±2.18 | 3.72± 0.96 |
| 粉五宝Fenwubao | 粉色Pink | 83.87±1.85 | 31.73±1.35 | 8.62±0.69 | 37.23± 1.56 | 7.68±1.63 |
| 粉碧玉Fenbiyu | 粉色Pink | 71.06±1.63 | 35.57±1.65 | 2.43±0.15 | 35.56± 1.29 | 3.90±0.28 |
| 粉七仙女Fenqixiannv | 粉色Pink | 76.77±2.14 | 32.19±1.34 | 7.17±0.42 | 26.89± 1.18 | 6.37±1.45 |
| 平均 Average | 72.49 | 36.34 | 6.83 | 35.09 | 5.43 | |
| 红宝珠Hongbaozhu | 红色 Red | 54.08±2.15 | 55.91±2.24 | 20.22±1.65 | 57.07±2.31 | 21.24±1.92 |
| 红芙蓉Hongfurong | 红色 Red | 54.99±1.43 | 52.70±1.78 | 18.48±2.46 | 53.39±1.54 | 19.14±2.26 |
| 红嫦娥彩Hongchangecai | 红色 Red | 50.62±1.28 | 48.71±1.53 | 21.94±1.09 | 53.81±1.66 | 23.72±1.88 |
| 红五宝Hongwubao | 红色 Red | 56.00±3.26 | 50.54±1.64 | 15.50± 1.16 | 50.85±1.25 | 16.16±1.25 |
| 红碧玉Hongbiyu | 红色 Red | 62.18±1.27 | 55.44±2.13 | 13.85±0.87 | 45.61±1.29 | 14.80±1.36 |
| 红七仙女Hongqixiannv | 红色 Red | 62.33±1.88 | 49.35±1.39 | 18.66±1.91 | 49.42±1.16 | 16.76±1.11 |
| 平均 Average | 56.70 | 52.11 | 18.11 | 51.69 | 18.64 | |
新窗口打开|下载CSV
1.2 方法
1.2.1 花色测定 花色测定按照国际照明委员会制定的CIE L*a*b*表色系法,用分光色差仪(NF555,Spectrophotometer,TRISUM)测定花色的L*、色相a*值、色相b*值、彩度C*值和色调角h。CIE L*a*b*体系中,L*表示花色明暗变化程度;色相a*值表示花瓣红、绿色变化,a*为正值时且数值越大,花色越红;色相b*值表示花瓣黄蓝色变化程度;彩度C*表示色彩鲜艳程度,C*值越大,颜色越深;色调角h是对红、橙、黄、绿、青、蓝、紫7种颜色色调的描述。花色测定位置为花瓣上表皮中央部位,光源为C/2°。每样品测量5朵花的花色,取平均值[12,13]。1.2.2 定性分析 取新鲜花瓣0.6 g,液氮研磨至粉末,按照HASHIMOTO等[14]方法加甲醇﹕水﹕甲酸﹕三氟乙酸(TFA)(70﹕27﹕2﹕1,体积比)提取液2 mL,浸提24 h后用0.22 μm滤膜过滤,滤液保存在-20℃冰箱备用[15]。
利用高效液相色谱-光电二极管阵列检测(HPLC-DAD)和超高效液相色谱-四极杆-飞行时间质谱(UPLC-Q-TOF-MS)联用技术对花瓣中花青苷成分进行定性与定量分析。ACQUITYTM UPLC I-Class 超高效液相色谱系统(Waters Corporation,Milford,MA,USA),Xevo G2-XS QTof MS质谱系统(Waters Corporation,Manchester,UK),UNIFI 1.8软件系统。色谱柱为ACQUITY BEH C18(2.1 mm×100 mm,1.7 μm)。流速:0.3 mL·min-1,进样量:2 μL,柱温40℃。以0.1%甲酸水溶液(A)和乙腈(B)为流动相,洗脱程序为0—1.5 min,5% B;1.5—11 min,5%—40% B;11—14 min,40%—95% B;14—16.5 min,95% B;16.5—16.8 min,95%—5% B;16.8—20 min,5% B。电喷雾电离离子源(ESI),正离子模式,全离子扫描,扫描范围(m/z):50—1 200 u。脱溶剂气体为高纯度氮气,温度为450℃,流速为600 L·h-1,毛细管电压为1 kV,锥孔电压为40 V。低能量扫描电压为6 eV,高能量扫描电压为20—45 eV。
1.2.3 定量分析 运用HPLC-DAD方法,在525 nm处检测花瓣中花青苷。采用标准品半定量法分别计算花瓣中含有的相对于标准品矢车菊素-3-O-β-葡萄糖苷(cyanidin-3-O-β-glucoside,Cy3G)的花青苷含量[16,17],重复3次。标准品矢车菊素-3-O-β-葡萄糖苷和矢车菊素-3-O-β-半乳糖苷(cyanidin-3-O-β- galactoside,Cy3Ga)购于Sigma公司,纯度≥98%。应用SPSS 17.0进行多元逐步回归分析。
2 结果
2.1 山茶品种花色
山茶品种与花色测定数据见表1,从表1可以发现,CIE L*a*b*体系中,白色、粉色和红色花瓣L*值的平均值分别为89.46、72.49和56.70,随花色加深L*值降低,花色明亮度降低。白色花瓣a*值介于-0.51— -0.93,平均值为-0.69;粉色花瓣a*值介于31.73— 41.71,平均值为36.34;红色花瓣a*值介于48.71— 55.91,平均值为52.11;随花色加深,a*值升高,花瓣红色程度增加。白色花瓣b*值介于1.65—6.53,平均值为3.97;粉色花瓣b*值介于2.43—9.94,平均值为6.83;红色花瓣b*值介于13.85—21.94,平均值为18.11;随花色加深,b*值升高,花瓣红色程度增加。白色、粉色和红色花瓣C*值平均值分别为4.07、35.09和51.69,随花色加深,C*值升高,花瓣鲜艳程度增加。白色花瓣h值均处于90°—180°,平均值为98.93;粉色和红色花瓣h值均处于0°—90°,平均值分别为5.43和18.64。2.2 山茶花青苷定性分析
利用HPLC-DAD和UPLC-Q-TOF-MS对山茶品种花瓣中花青苷成分进行定性分析,根据UPLC-Q-TOF-MS图谱,参考相关文献,对其主要花青苷成分进行结构鉴定。共检测到7种花青苷成分(图1),其紫外-可见光谱及质谱数据见表2。根据Cy糖苷在513—520 nm 有特征吸收峰及碎片离子m/z 287,推测7种花青苷均为Cy型花青苷[18,19,20]。峰P1和峰P2质谱数据为分子离子m/z 449,碎片离子m/z 287(Cy苷元特征质荷比),根据峰P1和峰P2与标准品Cy3Ga和Cy3G共洗脱特性,及花青素半乳糖洗脱时间小于花青素葡萄糖苷特性[18,21],确定峰P1为矢车菊素-3-O-β-半乳糖苷(tR,8.33 min),简称Cy3Ga,峰P2为矢车菊素-3-O-β-葡萄糖苷(tR,9.43 min),简称Cy3G。图1
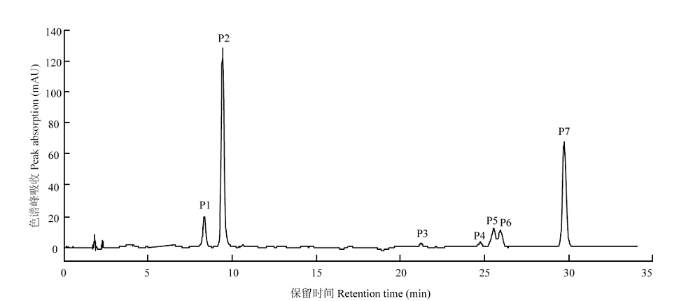 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1山茶品种花青苷成分的HPLC图谱
Fig. 1HPLC chromatogram of anthocyanin components in C. japonica cultivars
Table 2
表2
表2山茶花青苷成分的紫外-可见吸收光谱与质谱数据
Table 2
| 色谱峰 No.Peaks | 保留时间 Retention time (min) | 吸收波长 λmax (nm) | A440/Avis-max (%) | 分子离子 Molecular ions (m/z) | 碎片离子 Fragment ions (m/z) | 推定结果 Tentative identification |
|---|---|---|---|---|---|---|
| P1 | 8.33 | 281, 516 | 31 | 449 | 287 | Cy3Ga |
| P2 | 9.43 | 282, 517 | 32 | 449 | 287 | Cy3G |
| P3 | 21.22 | 283, 315, 516 | 33 | 611 | 449, 287 | Cy3GaECaf |
| P4 | 24.75 | 283, 316, 515 | 33 | 611 | 449, 287 | Cy3GECaf |
| P5 | 25.54 | 282, 311, 514 | 32 | 595 | 449, 287 | Gy3GZpC |
| P6 | 25.95 | 284, 312, 516 | 32 | 595 | 449, 287 | Cy3GaEpC |
| P7 | 29.74 | 285, 312, 513 | 32 | 595 | 449, 287 | Cy3GEpC |
新窗口打开|下载CSV
峰P3—峰P7的A440/Avis-max变化范围为32%— 33%,确定其为3-O-糖苷类型[22];根据峰P3—峰P7在311—316 nm波长下肩峰的出现,推定化合物被芳香酸酰化[23,24]。峰P3和峰P4质谱数据为分子离子m/z 611,碎片离子m/z 449、287。m/z 287为Cy苷元特征质荷比,m/z 611至m/z 449丢失162 u,m/z 449至m/z 287丢失162 u,推定其为Cy-3-O-咖啡酰型葡萄糖苷或半乳糖苷[24];根据LI等[11]关于山茶、香港红山茶和南山茶(C. semiserrata)中矢车菊素-3-O-[6-O-(E)-咖啡酰]-β-半乳糖苷(Cy3GaECaf)和矢车菊素-3-O-[6-O-(E)-咖啡酰]-β-葡萄糖苷(Cy3GECaf)的报道及峰P3洗脱时间小于峰P4,判断峰P3和峰P4分别为矢车菊素-3-O-[6-O-(E)-咖啡酰]-β-半乳糖苷和矢车菊素-3-O-[6-O-(E)-咖啡酰]-β-葡萄糖苷。
峰P5、峰P6和峰P7质谱数据为分子离子m/z 595,碎片离子m/z 449、287。m/z 287为Cy苷元特征质荷比,m/z 595至m/z 449丢失146 u,m/z 449至m/z 287丢失162 u,推定其为Cy-3-O-p-香豆酰型葡萄糖苷或半乳糖苷[24]。根据LI等[11]关于山茶、香港红山茶和南山茶中矢车菊素-3-O-[6-O-(Z)-p-香豆酰]-β-葡萄糖苷(Cy3GZpC)、矢车菊素-3-O-[6-O-(E)-p-香豆酰]-β-半乳糖苷(Cy3GaEpC)和矢车菊素-3-O-[6-O-(E)-p-香豆酰]-β-葡萄糖苷(Cy3GEpC)的报道,以及顺式花青苷洗脱时间小于反式花青苷[25,26]及花青素半乳糖苷洗脱时间小于花青素葡萄糖苷特性,判断峰P5、峰P6和峰P7分别为矢车菊素-3-O-[6-O-(Z)-p-香豆酰]-β-葡萄糖苷、矢车菊素-3-O-[6-O-(E)-p-香豆酰]-β-半乳糖苷和矢车菊素-3-O-[6-O-(E)-p-香豆酰]-β-葡萄糖苷。
2.3 山茶品种花青苷定量分析
山茶品种花瓣中花青苷成分含量见表3。山茶各系列芽变品种中,白色花瓣中均未检测到花青苷,红色花瓣中花青苷成分与粉色花瓣一致,但其各成分含量及花青苷总量均远高于粉色花瓣。如‘红宝珠’系列芽变品种中,‘白宝珠’白色花瓣中未检测到花青苷,‘红宝珠’红色花瓣和‘粉宝珠’粉色花瓣中均检测到7种花青苷,但‘红宝珠’中7种花青苷含量及花青苷总量均远高于‘粉宝珠’,其中‘红宝珠’Cy3G、Cy3GEpC含量和花青苷总量分别为‘粉宝珠’的6.99倍、2.65倍和5.32倍。‘白五宝’花瓣中未检测到花青苷,‘红五宝’和‘粉五宝’花瓣中均检测到6种花青苷,‘红五宝’中6种花青苷含量及花青苷总量均远高于‘粉五宝’,如‘红五宝’中Cy3G、Cy3GEpC含量和花青苷总量分别为‘粉五宝’的2.77倍、1.74倍和1.99倍。可见,各种花青苷含量及花青苷总量越大,山茶花瓣红色越深。Table 3
表3
表3不同山茶品种花青苷成分含量
Table 3
| 品种 Cultivars | 花青苷 Anthocyanin (μg·100 mg-1) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cy3Ga | Cy3G | Cy3GaECaf | Cy3GECaf | Gy3GZpC | Cy3GaEpC | Cy3GEpC | 合计 Total | |
| 白宝珠Baibaozhu | - | - | - | - | - | - | - | - |
| 粉宝珠Fenbaozhu | 1.23±0.13 | 8.91±0.47 | 0.25±0.12 | 0.16±0.00 | 0.59±0.04 | 0.98±0.42 | 5.12±0.23 | 17.24 |
| 红宝珠Hongbaozhu | 7.42±0.35 | 62.32±0.83 | 1.12±0.03 | 0.65±0.01 | 1.44±0.00 | 5.14±0.16 | 13.58±0.26 | 91.67 |
| 白芙蓉Baifurong | - | - | - | - | - | - | - | - |
| 粉芙蓉Fenfurong | 1.30±0.04 | 9.16±0.35 | 0.34±0.00 | 0.28±0.00 | 0.91±0.17 | 0.68±0.01 | 3.37±0.11 | 16.04 |
| 红芙蓉Hongfurong | 5.89±0.33 | 45.91±0.78 | 0.77±0.02 | 0.45±0.06 | 2.04±0.05 | 2.54±0.15 | 9.86±0.67 | 67.46 |
| 白嫦娥彩Baichangecai | - | - | - | - | - | - | - | - |
| 粉嫦娥彩Fenchangecai | 1.12±0.02 | 3.12±0.05 | - | - | 0.76±0.00 | 0.56±0.01 | 2.82±0.51 | 8.38 |
| 红嫦娥彩Hongchangecai | 5.71±0.05 | 18.72±0.45 | - | - | 1.91±0.12 | 2.42±0.08 | 8.19±0.71 | 36.95 |
| 白五宝Baiwubao | - | - | - | - | - | - | - | - |
| 粉五宝Fenwubao | 0.11±0.00 | 5.25±0.19 | - | 0.18±0.00 | 2.27±0.15 | 0.64±0.07 | 15.75±0.43 | 24.2 |
| 红五宝Hongwubao | 0.42±0.05 | 14.52±0.35 | - | 0.56±0.07 | 4.16±0.32 | 1.08±0.01 | 27.42±0.37 | 48.16 |
| 白碧玉Baibiyu | - | - | - | - | - | - | - | - |
| 粉碧玉Fenbiyu | 0.12±0.00 | 2.07±0.05 | - | - | 0.83±0.01 | 0.23±0.00 | 2.21±0.28 | 5.46 |
| 红碧玉Hongbiyu | 0.71±0.06 | 7.86±0.33 | - | - | 2.08±0.05 | 0.82±0.17 | 8.56±0.45 | 20.03 |
| 白七仙女Baiqixiannv | - | - | - | - | - | - | - | - |
| 粉七仙女Fenqixiannv | 0.16±0.00 | 2.23±0.18 | - | - | 0.51±0.13 | 0.25±0.01 | 2.71±0.23 | 5.86 |
| 红七仙女Hongqixiannv | 0.44±0.01 | 4.74±0.18 | - | - | 0.63±0.00 | 0.39±0.01 | 5.16±0.16 | 11.36 |
新窗口打开|下载CSV
山茶品种花瓣中花青苷成分比例见表4。山茶各系列芽变品种中,主要花青苷成分均为Cy3G和Cy3GEpC,如‘粉宝珠’花瓣中Cy3G和Cy3GEpC分别占51.68%和29.70%,合计为81.38%;‘红宝珠’花瓣中Cy3G和Cy3GEpC分别占67.98%和14.81%,合计为82.79%。山茶各系列芽变品种花瓣中Cy3Ga和Cy3G比例均随花瓣红色加深而增大,Cy3GEpC等花青苷成分比例随花瓣红色加深而减小,如‘粉宝珠’和‘红宝珠’花瓣中Cy3Ga分别占7.13%和8.09%,Cy3G分别占51.68%和67.98%,Cy3GEpC分别占29.70%和14.81%。可见,山茶品种主要花青苷为Cy3G和Cy3GEpC;随花瓣红色加深,Cy3Ga和Cy3G比例增大,其余花青苷比例减小。
Table 4
表4
表4不同山茶品种花青苷成分比例
Table 4
| 品种 Cultivars | 花青苷 Anthocyanin (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cy3Ga | Cy3G | Cy3GaECaf | Cy3GECaf | Gy3GZpC | Cy3GaEpC | Cy3GEpC | 合计 Total | |
| 白宝珠Baibaozhu | - | - | - | - | - | - | - | - |
| 粉宝珠Fenbaozhu | 7.13 | 51.68 | 1.45 | 0.93 | 3.42 | 5.68 | 29.70 | 100.00 |
| 红宝珠Hongbaozhu | 8.09 | 67.98 | 1.22 | 0.71 | 1.57 | 5.61 | 14.81 | 100.00 |
| 白芙蓉Baifurong | - | - | - | - | - | - | - | - |
| 粉芙蓉Fenfurong | 8.10 | 57.11 | 2.12 | 1.75 | 5.67 | 4.24 | 21.01 | 100.00 |
| 红芙蓉Hongfurong | 8.73 | 68.06 | 1.14 | 0.67 | 3.02 | 3.77 | 14.62 | 100.00 |
| 白嫦娥彩Baichangecai | - | - | - | - | - | - | - | - |
| 粉嫦娥彩Fenchangecai | 13.37 | 37.23 | 0.00 | 0.00 | 9.07 | 6.68 | 33.65 | 100.00 |
| 红嫦娥彩Hongchangecai | 15.45 | 50.66 | 0.00 | 0.00 | 5.17 | 6.55 | 22.17 | 100.00 |
| 白五宝Baiwubao | - | - | - | - | - | - | - | - |
| 粉五宝Fenwubao | 0.45 | 21.69 | 0.00 | 0.74 | 9.38 | 2.64 | 65.08 | 100.00 |
| 红五宝Hongwubao | 0.87 | 30.15 | 0.00 | 1.16 | 8.64 | 2.24 | 56.94 | 100.00 |
| 白碧玉Baibiyu | - | - | - | - | - | - | - | - |
| 粉碧玉Fenbiyu | 2.20 | 37.91 | 0.00 | 0.00 | 15.20 | 4.21 | 40.48 | 100.00 |
| 红碧玉Hongbiyu | 3.54 | 39.24 | 0.00 | 0.00 | 10.38 | 4.09 | 42.74 | 100.00 |
| 白七仙女Baiqixiannv | - | - | - | - | - | - | - | - |
| 粉七仙女Fenqixiannv | 2.73 | 38.05 | 0.00 | 0.00 | 8.70 | 4.27 | 46.25 | 100.00 |
| 红七仙女Hongqixiannv | 3.87 | 41.73 | 0.00 | 0.00 | 5.55 | 3.43 | 45.42 | 100.00 |
新窗口打开|下载CSV
2.4 山茶品种花青苷成分与花色关系
以山茶花瓣中花青苷成分Cy3Ga、Cy3G、Cy3GaECaf、Cy3GECaf、Cy3GZpC、Cy3GaEpC和Cy3GEpC为自变量,分别对应于x1、x2、x3、x4、x5、x6、x7,用描述花色的L*、a*、b*、C*、h为因变量,进行多元逐步回归分析,研究花青苷成分与花色的关系。回归方程为:L*=82.850-0.442x1-0.531x2,R=0.810,P=0.001;a*=13.547+0.415x2+0.565x7+8.584,R=0.770,P=0.001;C*=15.906+0.403x1+0.590x7,R=0.808,P=0.001。从回归方程的系数R及显著度P可以发现,山茶花青苷成分与花色之间极显著相关(P<0.01),根据回归系数绝对值大小可判断花青苷成分对花色形成贡献的大小。明度L*值表示花色的明亮程度,其回归方程表明,Cy3Ga和Cy3G与L*值呈负相关;山茶各系列芽变品种花瓣从白色、粉色到红色,Cy3Ga和Cy3G含量增加,L*值降低,花色明度降低。色相a*值正方向反映花瓣红色程度,a*值越大,花色越红,其回归方程表明,Cy3G和Cy3GEpC与色相a*值正相关;Cy3G和Cy3GEpC含量增加,a*值升高,红色加深。彩度C*值与Cy3Ga和Cy3GEpC正相关;山茶各系列芽变品种花瓣从白色、粉色到红色,Cy3Ga和Cy3GEpC含量增加,C*值升高,红色加深。
3 讨论
研究表明,山茶属植物花色色素成分主要为类黄酮,其中金花茶类植物花瓣为黄色,相关成分主要为黄酮醇类的槲皮素-3-O-葡萄糖苷、槲皮素-3-O-芸香糖苷和山柰酚-3-O-葡萄苷等[27,28,29],而山茶红色系花瓣中主要为花青苷类[5,6,7,8,9,10,11]。山茶属植物花瓣花青苷主要为Cy型和Dp型,Cy型花青苷在所有山茶属植物红色花瓣中均存在,而Dp型花青苷主要存在于香港红山茶和部分茶梅(C. sasanqua)品种的红色花瓣中[7,11]。山茶属植物Cy型花青苷研究表明,不同物种的花青苷成分有明显差异。滇山茶和窄叶西南红山茶(C. pitardii var. yunnanica)Cy结构上多具有3-(2-xylosyl)-单糖基[8,9],怒江红山茶和西南红山茶(C. pitardii)Cy结构上有3,5-二糖基[10],山茶、浙江红山茶(C. chekiangoleosa)、南山茶及日本的山茶红色花瓣中Cy上通常连有3-单糖基[30,31]。LI等[11]在山茶物种中鉴定出8种花青苷,山茶品种及其芽变品种均来源于山茶,白色花瓣中均未检测到花青苷,粉色和红色花瓣中共检测到7种Cy-3-O-糖苷型花青苷,除未检测到矢车菊素-3-O-[6-O-(Z)-p-香豆酰]-β-半乳糖苷(Cy3GaZpC)外,本试验鉴定出的7种花青苷成分均与其研究结果一致。菊花花青苷的研究表明,从白色、粉色到红色,花瓣中花青苷含量迅速增加,花色越深花青苷含量越高[32]。山茶花青苷研究表明,矢车菊素-3-O-葡萄糖苷等是其红色花瓣中主要的花青苷成分[11]。本试验红色品种花瓣中花青苷成分与粉色芽变品种一致,不同花色差异主要在于其各成分含量及花青苷总量的不同。多元线性回归结果表明,Cy3Ga和Cy3G与L*值显著负相关,其含量的积累显著降低花色明度;Cy3G和Cy3GEpC与色相a*值显著正相关,且Cy3GEpC回归系数大于Cy3G,对花瓣红色贡献较大;Cy3Ga和Cy3GEpC与彩度C*值正相关,Cy3Ga和Cy3GEpC含量增加,C*值升高,表现出增色效应。因此,Cy3Ga、Cy3G和Cy3GEpC是决定山茶花色变异的主要花青苷,其含量的积累增加花瓣的红色程度。研究表明,贴梗海棠(Chaenomeles speciosa)中Pg及Cy色素的比例直接影响花瓣红色的程度[20],月季属(Rosa)花色亦表现出相同的呈色规律[33]。本试验中随花瓣红色加深,山茶及其芽变品种总花青苷及主要花青苷含量增大,Cy3Ga和Cy3G比例增大,芳香族有机酸酰化花青苷Cy3GEpC等比例减小,说明除色素成分、含量外,色素成分比例亦可能影响其花色呈现,但山茶色素成分比例及芳香族有机酸酰化花青苷对花色的影响尚不清楚,有待于进一步研究。
芽变育种为山茶品种选育的重要方法,尤其山茶花色变异丰富,生产上大量应用的品种极易发生花色变异,通过芽变选育进而有望培育出花色各异的品种,如山茶‘赤丹’花色多变,通过芽变育种已选育出粉色‘粉丹’、白色‘玉丹’、黑色‘金碧辉煌’及复色‘五色赤丹’和‘鸳鸯凤冠’等品种10余个[2]。著名的山茶品种‘马卡德’‘明天’和‘赛牡丹’等通过花色变异均选育出10多个芽变品种[1]。可见,利用山茶丰富的花色变异,对变异枝条进行嫁接、扦插等无性繁殖,有望分离并保持其变异性状,进而选育出特异花色新品种。同时,以花青苷总量及Cy3G和Cy3GEpC等含量较高的品种为亲本,通过杂交、回交可进一步选育出深红色或鲜红色的山茶品种。此外,山茶花色色素还具有抑制肿瘤[34]、抗氧化[35]和增强人体免疫力[36]等生理功效,B环具邻二羟基苯结构的花青苷如Cy衍生物能抑制肿瘤细胞生长[37]。本试验中山茶品种粉色和红色花瓣中花青苷均为Cy衍生物,可用于花青苷功能色素的开发利用。
4 结论
山茶各系列芽变品种中,白色花瓣中均未检测到花青苷,红色花瓣中花青苷成分与粉色花瓣一致,但其各成分含量及花青苷总量均远高于粉色花瓣。粉色和红色花瓣中主要花青苷成分均为Cy3G和Cy3GEpC;随花色加深,花瓣中Cy3Ga和Cy3G比例增大,其余花青苷比例减小。山茶各系列芽变品种中花青苷总量越大,花瓣红色越深;Cy3Ga、Cy3G和Cy3GEpC是决定山茶花色芽变的主要花青苷成分,相关成分含量的积累增加花瓣的红色程度。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 3]
[本文引用: 3]
[本文引用: 3]
[本文引用: 3]
DOI:10.1093/oxfordjournals.pcp.a029312URL [本文引用: 1]
DOI:10.1007/s11101-017-9537-xURL [本文引用: 1]
[本文引用: 3]
DOI:10.2503/jjshs.55.82URL [本文引用: 3]
[本文引用: 4]
DOI:10.1271/bbb.70124URL [本文引用: 4]
DOI:10.1016/j.phytochem.2008.03.014URL [本文引用: 4]
DOI:10.2503/jjshs1.77.75URL [本文引用: 4]
[本文引用: 8]
DOI:10.1016/j.scienta.2003.07.002URL [本文引用: 1]
DOI:10.1016/j.scienta.2006.05.007URL [本文引用: 1]
DOI:10.1271/bbb.66.1652URL [本文引用: 1]
DOI:10.1007/s10265-003-0130-6URL [本文引用: 1]
DOI:10.1007/PL00013966URL [本文引用: 1]
Magsci [本文引用: 1]

以从内蒙古自治区赤峰市克什克腾旗引种的野生芍药(<em>Paeonia lactiflora</em>)为材料,采用英国皇家园艺学会比色卡和分光色差计测量不同开花阶段的花色,利用高效液相色谱—光电二极管阵列检测(HPLC-DAD)和高效液相色谱—电喷雾离子化—二级质谱联用技术(HPLC-ESI-MS<sup>2</sup>)定性定量分析其花色素组分,运用多元线性回归方法分析了花色与花瓣中色素组成之间的关系。结果表明:开花过程中花色的明度增加,红色减退,彩度变小,颜色由红紫(N57A)变为淡紫红色(75C)。从其花瓣中共检出6种花青苷,4种黄酮苷和15种黄酮醇苷。其中两种花青苷(芍药花素–3–没食子酰葡萄糖苷–5–葡萄糖苷、芍药花素–3–丙二酰葡萄糖苷–5–葡萄糖苷),两种黄酮苷(木犀草素–7–没食子酰葡萄糖苷、木犀草素–7–阿拉伯糖苷)和13种黄酮醇苷在芍药中属首次报道。分析结果表明其花青苷的主要成分是芍<a name="OLE_LINK9">药花素–</a>3, 5–二葡萄糖苷,约占总花青苷含量(total anthocyanins content,TA)的93.93%;其花黄素(包括黄酮和黄酮醇)的主要成分是山奈酚–3–葡萄糖苷,占总黄酮含量(total flavones content,TF)的48.78%。不同的开花阶段,从露色期到松瓣期TA含量略增加,松瓣期后开始降低,TF则先略减少后增加。多元回归结果显示,芍药花素–3, 5–二葡萄糖苷、槲皮素–3–葡萄糖苷、槲皮素–7–葡萄糖苷、异鼠李素–3–葡萄糖苷的含量与花色变化具有线性相关性。
Magsci [本文引用: 1]

以从内蒙古自治区赤峰市克什克腾旗引种的野生芍药(<em>Paeonia lactiflora</em>)为材料,采用英国皇家园艺学会比色卡和分光色差计测量不同开花阶段的花色,利用高效液相色谱—光电二极管阵列检测(HPLC-DAD)和高效液相色谱—电喷雾离子化—二级质谱联用技术(HPLC-ESI-MS<sup>2</sup>)定性定量分析其花色素组分,运用多元线性回归方法分析了花色与花瓣中色素组成之间的关系。结果表明:开花过程中花色的明度增加,红色减退,彩度变小,颜色由红紫(N57A)变为淡紫红色(75C)。从其花瓣中共检出6种花青苷,4种黄酮苷和15种黄酮醇苷。其中两种花青苷(芍药花素–3–没食子酰葡萄糖苷–5–葡萄糖苷、芍药花素–3–丙二酰葡萄糖苷–5–葡萄糖苷),两种黄酮苷(木犀草素–7–没食子酰葡萄糖苷、木犀草素–7–阿拉伯糖苷)和13种黄酮醇苷在芍药中属首次报道。分析结果表明其花青苷的主要成分是芍<a name="OLE_LINK9">药花素–</a>3, 5–二葡萄糖苷,约占总花青苷含量(total anthocyanins content,TA)的93.93%;其花黄素(包括黄酮和黄酮醇)的主要成分是山奈酚–3–葡萄糖苷,占总黄酮含量(total flavones content,TF)的48.78%。不同的开花阶段,从露色期到松瓣期TA含量略增加,松瓣期后开始降低,TF则先略减少后增加。多元回归结果显示,芍药花素–3, 5–二葡萄糖苷、槲皮素–3–葡萄糖苷、槲皮素–7–葡萄糖苷、异鼠李素–3–葡萄糖苷的含量与花色变化具有线性相关性。
DOI:10.1016/j.lwt.2005.09.018URL [本文引用: 2]
[本文引用: 1]
Magsci [本文引用: 2]

以贴梗海棠[<EM>Chaenomeles speciosa</EM>(Sweet)Nakai]不同花色的24份种质为材料,调查了其花色在CIE L*a*b*表色系的分布状况,利用高效液相色谱—光电二极管阵列检测(HPLC–DAD)和高效液相色谱—电喷雾离子化—多级质谱联用技术(HPLC–ESI–MSn)定性定量分析其花青苷组分,运用多元线性回归方法分析了花色与花青苷组成之间的关系。结果表明,贴梗海棠花瓣中共含有6种花青苷,分别是矢车菊素–3–O–半乳糖苷(Cy3Ga)、矢车菊素–3–O–葡萄糖苷、天竺葵素–3–O–半乳糖苷、天竺葵素–3–O–(半乳糖葡萄糖苷)[Pg3(Ga-G)]、矢车菊素–3–O–(半乳糖葡萄糖苷)以及矢车菊素–3–O–琥珀酸–阿拉伯糖苷(Cy3SucAra)。Cy3SucAra为首次发现。其中,Cy3Ga、Pg3(Ga-G)和Cy3SucAra是决定贴梗海棠花色的主要色素,这3种色素含量的增加导致花色显著变红。基于花青苷的组成信息,探讨了贴梗海棠的花色改良和蓝色花创制的策略。 <BR>
Magsci [本文引用: 2]

以贴梗海棠[<EM>Chaenomeles speciosa</EM>(Sweet)Nakai]不同花色的24份种质为材料,调查了其花色在CIE L*a*b*表色系的分布状况,利用高效液相色谱—光电二极管阵列检测(HPLC–DAD)和高效液相色谱—电喷雾离子化—多级质谱联用技术(HPLC–ESI–MSn)定性定量分析其花青苷组分,运用多元线性回归方法分析了花色与花青苷组成之间的关系。结果表明,贴梗海棠花瓣中共含有6种花青苷,分别是矢车菊素–3–O–半乳糖苷(Cy3Ga)、矢车菊素–3–O–葡萄糖苷、天竺葵素–3–O–半乳糖苷、天竺葵素–3–O–(半乳糖葡萄糖苷)[Pg3(Ga-G)]、矢车菊素–3–O–(半乳糖葡萄糖苷)以及矢车菊素–3–O–琥珀酸–阿拉伯糖苷(Cy3SucAra)。Cy3SucAra为首次发现。其中,Cy3Ga、Pg3(Ga-G)和Cy3SucAra是决定贴梗海棠花色的主要色素,这3种色素含量的增加导致花色显著变红。基于花青苷的组成信息,探讨了贴梗海棠的花色改良和蓝色花创制的策略。 <BR>
DOI:10.1021/jf048068bURL [本文引用: 1]
DOI:10.1042/bj0700022URL [本文引用: 1]
DOI:10.1016/S0031-9422(98)00064-8URL [本文引用: 1]
URL [本文引用: 3]

Anthocyanins are important water-soluble pigments, which have significant influence in foodprocessing industry. Anthocyanins have anti-oxidant, anti-inflammatory, anti-antimicrobial, inhibition of plateletaggregation and anticancer activity, and have been the focus as important functional ingredients. Separation andidentification of anthocyanins is the base for studying their bioactive effects. However, the complexity and varietyof anthocyanins make it difficult to characterize the composition of anthocyanins. High pressure liquid chromato-graphy (HPLC) coupled with mass spectrometry has become the standard and most powerful method for routineanthocyanin analysis. This paper reviewed the golden rules for separation and identification of anthocyanins byhigh pressure liquid chromatography-tandem mass spectrometry (HPLC-DAD-MS), which might provide thechemical evidence for clarification of structure-activity relationship and screening for high value-added resources.
URL [本文引用: 3]

Anthocyanins are important water-soluble pigments, which have significant influence in foodprocessing industry. Anthocyanins have anti-oxidant, anti-inflammatory, anti-antimicrobial, inhibition of plateletaggregation and anticancer activity, and have been the focus as important functional ingredients. Separation andidentification of anthocyanins is the base for studying their bioactive effects. However, the complexity and varietyof anthocyanins make it difficult to characterize the composition of anthocyanins. High pressure liquid chromato-graphy (HPLC) coupled with mass spectrometry has become the standard and most powerful method for routineanthocyanin analysis. This paper reviewed the golden rules for separation and identification of anthocyanins byhigh pressure liquid chromatography-tandem mass spectrometry (HPLC-DAD-MS), which might provide thechemical evidence for clarification of structure-activity relationship and screening for high value-added resources.
DOI:10.1016/j.chroma.2008.06.002URLPMID:18573501 [本文引用: 1]

A limitation of large-scale viticultural trials is the time and cost of comprehensive compositional analysis of the fruit by high-performance liquid chromatography (HPLC). In addition, separate methods have generally been required to identify and quantify different classes of metabolites. To address these shortcomings a reversed-phase HPLC method was developed to simultaneously separate the anthocyanins and flavonols present in grape skins. The method employs a methanol and water gradient acidified with 10% formic acid with a run-time of 48 min including re-equilibration. Identity of anthocyanins and flavonols in Shiraz ( Vitis vinifera L.) skin was confirmed by mass spectral analysis.
DOI:10.1002/pca.v23.1URL [本文引用: 1]
DOI:10.1021/jf4029877URLPMID:24001127 [本文引用: 1]

Yellow camellia, with its golden yellow flowers, is rare in the world. Most studies of yellow camellia have focused on its ornamental properties; however; there are fewer published studies on its medical values. The purpose of this study was to define the chemical constituents and the biological potential of the water extract of leaves in six species of yellow camellia. The data showed that Camellia murauchii had significantly higher total catechins and total polyphenol content than others; Camellia euphlebia had the highest total amino acids and gamma-aminobutyric acid. The results indicated that Camellia tunghinensis exhibited the highest free radical scavenging capacity and showed potent anticancer activities. Camellia nitidissima had stronger inhibitory effect than other species on fatty acid synthesis. In addition to catechins, 3-p-coumaroylquinic acid, kaempferol-3-O-glucoside, and quercetin-3-O-glucoside were detected in C. tunghinensis using liquid chromatography tandem mass spectrometry. Taken together, yellow camellias possess biological activity and are worthy of continued study.
DOI:10.1016/j.foodchem.2011.04.083URL [本文引用: 1]
[本文引用: 1]
DOI:10.2503/jjshs.56.208URL [本文引用: 1]
DOI:10.2503/jjshs.61.375URL [本文引用: 1]
DOI:10.3969/j.issn.1674-3466.2010.03.004Magsci [本文引用: 1]

<FONT face=Verdana>应用高效液相色谱和多级质谱联用技术(HPLC-ESI-MS<SUP>n</SUP>), 分析菊花(<EM>Chrysanthemum</EM> ×<EM> morifolium</EM>)白色、粉色、红色、紫色、红紫色和墨色6个色系共计82个品种中花青素苷合成过程的中间产物和最终产物, 发现从白色、粉色、红色、紫色、红紫色到墨色花青素苷含量快速增加, 分别为4.68、111.60、366.89、543.56、1 220.36和2 674.95 μg·g<SUP>–1</SUP>, 不同色系间花青素苷的含量差异显著(<EM>P</EM><0.01), 花青素苷含量越高花色越深; 墨色菊花品种中总类黄酮含量显著高于其它花色品种(<EM>P</EM><0.01), 其它不同色系间总类黄酮含量差异不显著(<EM>P</EM>>0.05); 随着菊花花色变深, 从柚皮素分支到圣草酚的代谢流, 以及从圣草酚分支到矢车菊素苷的代谢流比例增加。花青素苷成分分析发现: 菊花中只含有矢车菊素苷类化合物。根据花青素苷代谢成分分析结果绘制了菊花中花青素苷代谢路径图, 即在菊花类黄酮代谢途径中只存在矢车菊素苷代谢分支途径;菊花不同色系在柚皮素和圣草酚2个关键代谢分支点上向不同方向代谢流的分配比例不同, 造成花青素苷产物含量不同,导致不同花色。以上研究结果为菊花花色改良的分子育种提供了理论依据。</FONT>
DOI:10.3969/j.issn.1674-3466.2010.03.004Magsci [本文引用: 1]

<FONT face=Verdana>应用高效液相色谱和多级质谱联用技术(HPLC-ESI-MS<SUP>n</SUP>), 分析菊花(<EM>Chrysanthemum</EM> ×<EM> morifolium</EM>)白色、粉色、红色、紫色、红紫色和墨色6个色系共计82个品种中花青素苷合成过程的中间产物和最终产物, 发现从白色、粉色、红色、紫色、红紫色到墨色花青素苷含量快速增加, 分别为4.68、111.60、366.89、543.56、1 220.36和2 674.95 μg·g<SUP>–1</SUP>, 不同色系间花青素苷的含量差异显著(<EM>P</EM><0.01), 花青素苷含量越高花色越深; 墨色菊花品种中总类黄酮含量显著高于其它花色品种(<EM>P</EM><0.01), 其它不同色系间总类黄酮含量差异不显著(<EM>P</EM>>0.05); 随着菊花花色变深, 从柚皮素分支到圣草酚的代谢流, 以及从圣草酚分支到矢车菊素苷的代谢流比例增加。花青素苷成分分析发现: 菊花中只含有矢车菊素苷类化合物。根据花青素苷代谢成分分析结果绘制了菊花中花青素苷代谢路径图, 即在菊花类黄酮代谢途径中只存在矢车菊素苷代谢分支途径;菊花不同色系在柚皮素和圣草酚2个关键代谢分支点上向不同方向代谢流的分配比例不同, 造成花青素苷产物含量不同,导致不同花色。以上研究结果为菊花花色改良的分子育种提供了理论依据。</FONT>
DOI:10.1016/S0305-1978(99)00127-1URL [本文引用: 1]
DOI:10.1080/10286020.2012.691475URL [本文引用: 1]
DOI:10.1016/j.foodchem.2011.05.001URL [本文引用: 1]
DOI:10.1016/S1674-6384(16)60012-6URL [本文引用: 1]
[本文引用: 1]
