 ,, 石延霞
,, 石延霞 ,, 朱发娣, 谢学文, 柴阿丽, 李宝聚
,, 朱发娣, 谢学文, 柴阿丽, 李宝聚 ,中国农业科学院蔬菜花卉研究所,北京 100081
,中国农业科学院蔬菜花卉研究所,北京 100081Establishment of AS-real-time PCR for Quantitatively Detecting the H278R Allele in the SdhB Associated with Corynespora cassiicola in Cucumber
SUN BingXue ,, SHI YanXia
,, SHI YanXia ,, ZHU FaDI, XIE XueWen, CHAI ALi, LI BaoJu
,, ZHU FaDI, XIE XueWen, CHAI ALi, LI BaoJu ,Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Beijing 100081
,Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Beijing 100081通讯作者:
第一联系人:
收稿日期:2018-06-22接受日期:2018-09-15网络出版日期:2018-12-26
| 基金资助: |
Received:2018-06-22Accepted:2018-09-15Online:2018-12-26

摘要
关键词:
Abstract
Keywords:
PDF (1995KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
孙炳学, 石延霞, 朱发娣, 谢学文, 柴阿丽, 李宝聚. 多主棒孢SdhB-H278R突变位点AS-real-time PCR 定量检测体系的建立[J]. 中国农业科学, 2018, 51(24): 4647-4658 doi:10.3864/j.issn.0578-1752.2018.24.006
SUN BingXue, SHI YanXia, ZHU FaDI, XIE XueWen, CHAI ALi, LI BaoJu.
0 引言
【研究意义】多主棒孢(Corynespora cassiicola)隶属于格孢菌目(Pleosporales)棒孢科(Corynesporascaceae)棒孢属(Corynespora),由其引起的黄瓜棒孢叶斑病发生普遍,该病害在温室、大棚和露地中均不断加重,已成为黄瓜栽培中主要的病害之一[1]。目前大多采用化学药剂防治黄瓜棒孢叶斑病,由于多主棒孢繁殖速度快、遗传变异大,加之多年连续使用单一药剂防治,导致其对苯并咪唑类、二羧酰亚胺类、N-苯氨基甲酸酯类和QoI类的大部分杀菌剂产生了抗药性[2,3]。啶酰菌胺是德国巴斯夫公司1996年开发的新型烟酰胺类内吸性杀菌剂,该药剂能够阻断电子在复合体II琥珀酸脱氢酶[3Fe-4S]结构域与泛醌之间的传递,从而影响病原真菌的电子传递,阻碍病原菌的能量代谢[3,4]。因该药剂作用位点单一,FRAC已将其归为中度抗性风险杀菌剂。自2008年啶酰菌胺在中国登记防治灰霉病并在黄瓜栽培中大量使用,间接导致多主棒孢对啶酰菌胺产生抗药性,出现大量的抗性突变株,因此,建立一种快速、准确、定量的多主棒孢SdhB-H278R突变检测方法,对监测啶酰菌胺田间的抗性频率和病害防治具有重要意义。【前人研究进展】随着啶酰菌胺的广泛使用,在不同蔬菜和作物上出现了对啶酰菌胺有抗性的病原菌。2007年,首次在开心果上发现了对啶酰菌胺产生抗性的链格孢(Alternaria alternata)[5],之后在灰葡萄孢(Botrytis cinerea)、小双胞腔菌(Didymella bryoniae)和核盘菌(Sclerotinia sclerotiorum)上也发现了对啶酰菌胺产生抗性的菌株[6,7,8]。2009年,MIYAMOTO等[9]在黄瓜上发现了对啶酰菌胺产生抗性的多主棒孢并在2010[10]年发现了SdhB-H278R/Y、SdhC-C73P、SdhD-S89P和SdhD-G109V等多种抗性突变类型,且SdhB-H278R基因型在田间的频率为0.64%。传统的抗药性检测方法包括离体测定和活体测定法,其中离体测定法主要有菌落生长速率法和孢子萌发法,均是通过测定药剂对植物病原菌的EC50值以区分敏感和抗性菌株,比较费时费力。近年来,随着分子生物学技术的发展,PCR-RFLP(restriction fragment length polymorphism PCR)、AS-PCR(allele specific PCR)、AS-real-time PCR、HRM(high resolution melting)和多重等位基因PCR等分子鉴定手段广泛应用于植物病原菌对药剂的抗药性检测。FURUYA等[11]和AOKI等[12]在霜霉病菌(Plasmopara viticola)上分别建立了对QoI杀菌剂抗性Cytb-G143A和对CAA杀菌剂PvCesA3-G1105S突变位点PCR-RFLP的检测体系。基于等位基因特异性核苷酸引物,AS-PCR和AS-real-time PCR抗药性检测技术已在核盘菌、茄链格孢(A. solani)、大麦云纹病菌(Rhynchosporium secalis)和灰葡萄孢等病原菌上应用[13,14,15,16]。最近,新兴的HRM抗药性检测技术因具有高通量、快速的特点并可用于SNP位点的筛选,已开始在核盘菌对甲基硫菌灵和灰葡萄孢对环酰菌胺以及啶酰菌胺的抗药性检测上应用[17,18],准确性高达100%,但该方法只能定性检测。【本研究切入点】目前为止尚未建立一种检测多主棒孢抗性突变位点的检测体系,基于笔者课题组前期发现的一种抗啶酰菌胺的黄瓜多主棒孢SdhB-H278R抗性突变位点,使用荧光定量PCR仪,建立一种定量、高效、快速检测该突变位点的AS-real-time PCR体系,并对此体系的特异性、可重复性和灵敏度进行评价。【拟解决的关键问题】建立一种定量、高效、快速检测黄瓜多主棒孢SdhB-H278R突变位点的AS-real-time PCR体系,以期为该病原菌对啶酰菌胺的田间抗性检测和病害防控提供更好的技术支持。1 材料与方法
试验于2017年3月至2018年4月在中国农业科学院蔬菜花卉研究所完成。1.1 试验材料
1.1.1 菌株 2017年3月从北京市大兴区采集黄瓜多主棒孢叶斑病病样,在室内进行分离培养,单孢纯化后得到24株多主棒孢菌株,其他5株多主棒孢菌株采自河北和辽宁。所有菌株由中国农业科学院蔬菜花卉研究所蔬菜病害综合防治课题组提供。1.1.2 试剂和培养基 96%啶酰菌胺原药(陕西美邦农药有限公司)、植物组织DNA提取试剂盒、SuperReal PreMix Plus(天根生化科技(北京)有限公司),2×Taq MasterMix (北京博迈德生物技术有限公司)、PDA培养基、YBA培养基。
1.1.3 主要仪器设备 NanoDrop2000超微量分光光度计(Thermo Fisher Scientific Inc.,USA)、3-18K型离心机(Sigma,德国)、7500 Real time PCR system(ABI,美国)、S1000 Thermal Cycle PCR仪(BIO- RAD,美国)。
1.1.4 引物合成测序 荧光定量PCR和普通PCR所用引物及基因序列由北京博迈德基因技术有限公司合成并测定。
1.2 方法
1.2.1 多主棒孢对啶酰菌胺敏感性的测定 参照MIYAMOTO等[9]方法,采用菌丝生长速率法测定北京大兴24株及河北、辽宁的5株多主棒孢对啶酰菌胺的敏感性。抗药性测定设置7个浓度梯度(0、0.01、0.1、1、5、10、30 μg·mL-1),以不加药剂为空白对照;在新鲜的菌落边缘打取直径5 mm的菌饼,接种于含药培养基的平板上,在26℃黑暗培养5 d,测量菌落直径,每个处理重复3次。比较试验组和对照组的菌落直径,并计算EC50值、毒力回归方程和相关系数。根据MIYAMOTO等[9]的敏感基线(0.04—0.59 μg·mL-1)判定多主棒孢对啶酰菌胺的敏感性。1.2.2 抗性和敏感菌株的生物学特性测定 菌丝生长速率的测定:从24株多主棒孢菌中随机选取4株敏感菌株和8株抗性菌株作为测定生物学特性的菌株。在多主棒孢菌生长5 d的PDA平板上制备5 mm的菌饼,采用灭菌牙签将菌碟菌丝面朝下贴于PDA平板中央,密封后置于26℃培养。分别于第2—11天,采用十字交叉法测量菌落生长直径。每个菌株3次重复,试验重复2次。
产孢能力的测定:待多主棒孢菌生长至第11天,用直径5 mm的打孔器在菌落边缘打取5个菌饼置于1 mL的无菌水中,加入5个直径2 mm的研磨珠用涡旋振荡机振荡2 min洗脱分生孢子,用血球计数板计数计算分生孢子的浓度。每个菌株3次重复,试验重复2次。
致病性的测定:采集离体组织测定法,采集叶龄一致,形状、大小相似的健康黄瓜叶片,用灭菌的接种针刺破形成微伤口。将制备好直径5 mm的菌饼分别接种于黄瓜叶片,接种后放置于保鲜盒中,置于相对湿度85%,自然光照的26℃温室中,培养5 d后采用十字交叉法测量病斑直径。每个菌株测6片叶片,试验重复2次。
1.2.3 DNA提取 将多主棒孢接种到PDA平板培养5 d,刮取菌丝作为DNA提取样本。采取植物组织DNA提取试剂盒,根据试剂盒中的说明书提取DNA,提取后用NanoDrop 2000c测定DNA浓度,用ddH2O稀释至40 ng·μL-1,4℃保存备用。
1.2.4 AS-real-time PCR定量检测体系的建立 SdhB序列测定:根据GenBank多主棒孢SdhB序列(AB548739.1), 应用Primer Premier 5.0设计引物Cc-SdhB-F和Cc-SdhB-R(表1),以表2中所有多主棒孢的DNA为模板,用Cc-SdhB-F和Cc-SdhB-R引物扩增SdhB基因片段,PCR产物进行测序。50 μL PCR体系:2×Taq PCR MasterMix 25 μL,Cc-SdhB-F和Cc-SdhB-R引物2 μL,模板DNA 2 μL,ddH2O补足50 μL。PCR反应程序:94℃ 5 min,94℃ 45 s,58℃ 45 s,72℃ 90 s,步骤2—4进行30个循环,72℃ 10 min。
引物设计:根据SdhB的测序信息,针对SNP位点和相同的序列信息,用Primer Premier 5.0设计特异性引物B-H278R-2F/2R14和内参引物B-H278R- TY-F/R(表1),引物设计策略和引物信息如图1和表1所示。为增加引物的特异性,引物3′端与突变碱基G配对,在靠近3′端的第2、3位引入错配碱基[13-16,19]。
图1
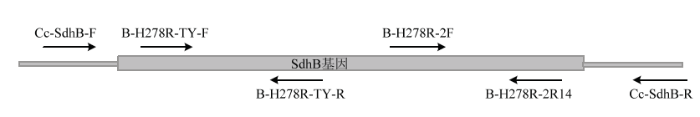 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1引物设计策略
Fig. 1Primer design strategy
Table 1
表1
表1引物信息
Table 1
| 引物名称 Primer name | 序列 Sequence | 引物长度 Length of primer (bp) | 产物长度 Length of product (bp) |
|---|---|---|---|
| Cc-SdhB-F | CACTCTTCTTCGCCATCC | 18 | 1422 |
| Cc-SdhB-R | CATCACACTCACGGTCAC | 18 | |
| B-H278R-2F | GTGAATACCGCCAGTCCAA | 19 | 244 |
| B-H278R-2R14 | CAGTTGAGAATCGCGC | 16 | |
| B-H278R-TY-F | GACCTTTAGGCGAAGTTGC | 19 | 246 |
| B-H278R-TY-R | CTGCTTGTAGAAGAGCGTCAT | 21 |
新窗口打开|下载CSV
引物特异性检测:以含有H278R突变、其他点突变菌株以及常见的8种植物病原菌DNA为模板,以ddH2O为阴性对照,用引物B-H278R-2F和B-H278R-2R14进行PCR扩增,检测引物特异性。20 μL PCR反应体系:2×Taq PCR MasterMix 10 μL,引物B-H278R-2F和B-H278R-2R14 0.4 μL,模板DNA 1.6 μL,ddH2O补足10 μL。PCR反应程序:94℃ 5 min,94℃ 45 s,65℃ 45 s,72℃ 30 s,步骤2—4进行35个循环,72℃ 10 min。PCR产物经1%琼脂糖凝胶电泳检测后,凝胶成像系统分析结果。
标准曲线的建立:为了定量检测多主棒孢SdhB- H278R突变株在田间的比例,以含有H278R突变菌株(R278)和不含有H278R突变菌株(H278)的混合DNA作为模板,以R278 DNA摩尔质量分别占总量DNA的6.25%、12.5%、25%、50%、75%和100%作为不同的处理,3次重复,分别用特异性引物B-H278R-2F/2R14和内参引物B-H278R-TY-F/R在7500 real-time PCR system仪中进行AS-real-time PCR,计算ΔCT(ΔCT=CT特异性引物-CT内参引物),以ΔCT值为纵坐标,以R278 DNA相对摩尔质量的对数为横坐标,制作标准曲线。
2 结果
2.1 多主棒孢对啶酰菌胺的敏感性测定
采用菌丝生长速率法测定采集的多主棒孢对啶酰菌胺的敏感性,结果表明,采自北京的24株多主棒孢分为敏感和抗性2个群体,其中敏感菌株16株,占66.7%,其EC50值为0.057—0.563 μg?mL-1,平均值为0.368 μg?mL-1。抗性菌株8株,占33.3%,其EC50值为5.395—11.710 μg?mL-1,平均值为7.586 μg?mL-1(图2、表2)。图2
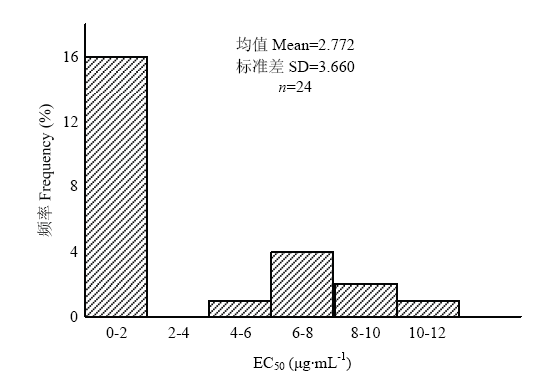 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图224株多主棒孢菌的敏感性分布
Fig. 2Sensitivity distribution of 24 C. cassiicola strains
Table 2
表2
表2供试菌株敏感性测定
Table 2
| 序号 Number | 菌株编号 Strain code | 采集时间 Collection time | 采集地 Collection area | EC50 (μg·mL-1) | 毒力回归方程Toxicity regression equation (y=) | 相关系数 Coefficient (R2) | 突变类型Mutation type | 敏感性 Sensitivity |
|---|---|---|---|---|---|---|---|---|
| 1 | HG17031006-1 | 2017 | 北京Beijing | 9.261 | 1.2509x+3.7941 | 0.9995 | + | R |
| 2 | HG17031006-2 | 2017 | 北京Beijing | 5.925 | 1.1082x+4.1659 | 0.9788 | + | R |
| 3 | HG17031006-3 | 2017 | 北京Beijing | 0.482 | 0.4848x+5.1535 | 0.9899 | - | S |
| 4 | HG17031006-4 | 2017 | 北京Beijing | 0.227 | 0.4565x+5.2941 | 0.9910 | - | S |
| 5 | HG17031006-5 | 2017 | 北京Beijing | 5.395 | 0.6544x+4.4397 | 0.9276 | + | R |
| 6 | HG17031007-1 | 2017 | 北京Beijing | 0.337 | 0.4846x+5.2290 | 0.9905 | - | S |
| 7 | HG17031007-2 | 2017 | 北京Beijing | 0.057 | 1.6824x+7.0839 | 0.9777 | - | S |
| 8 | HG17031007-3 | 2017 | 北京Beijing | 0.261 | 2.1962x+4.9202 | 0.9905 | - | S |
| 9 | HG17031007-4 | 2017 | 北京Beijing | 0.383 | 0.4912x+5.2047 | 0.9331 | - | S |
| 10 | HG17031007-5 | 2017 | 北京Beijing | 0.221 | 0.5069x+5.9325 | 0.9746 | - | S |
| 11 | HG17031008-1 | 2017 | 北京Beijing | 7.585 | 0.9496x+4.1644 | 0.9998 | + | R |
| 12 | HG17031008-2 | 2017 | 北京Beijing | 0.535 | 1.5481x+5.8387 | 0.9219 | - | S |
| 13 | HG17031008-3 | 2017 | 北京Beijing | 6.089 | 1.0695x+4.1608 | 0.9998 | + | R |
| 14 | HG17031008-4 | 2017 | 北京Beijing | 7.408 | 1.0990x+4.0752 | 0.9961 | + | R |
| 15 | HG17031008-5 | 2017 | 北京Beijing | 7.309 | 1.3476x+3.8337 | 0.9967 | + | R |
| 16 | HG17031009-1 | 2017 | 北京Beijing | 0.495 | 1.1440x+5.6638 | 0.9038 | - | S |
| 17 | HG17031009-2 | 2017 | 北京Beijing | 0.469 | 1.4358x+6.3270 | 0.9929 | - | S |
| 18 | HG17031009-3 | 2017 | 北京Beijing | 0.550 | 1.1084x+5.9661 | 0.9944 | - | S |
| 19 | HG17031009-4 | 2017 | 北京Beijing | 0.484 | 0.5298x+5.1666 | 0.9791 | - | S |
| 20 | HG17031009-5 | 2017 | 北京Beijing | 11.710 | 0.6667x+4.2859 | 0.9154 | + | R |
| 21 | HG17031010-1 | 2017 | 北京Beijing | 0.216 | 0.5158x+5.3436 | 0.9991 | - | S |
| 22 | HG17031010-2 | 2017 | 北京Beijing | 0.563 | 1.3191x+5.6942 | 0.9225 | - | S |
| 23 | HG17031010-4 | 2017 | 北京Beijing | 0.296 | 0.6165x+5.3264 | 0.9897 | - | S |
| 24 | HG14102524-4 | 2014 | 河北Hebei | 0.316 | 2.9056x+3.5592 | 0.9945 | - | S |
| 25 | HG17031010-5 | 2017 | 北京Beijing | 0.274 | 1.0630x+5.7384 | 0.9129 | - | S |
| 26 | HG14102430-1 | 2014 | 河北Hebei | 22.371 | 0.9039x+3.7312 | 0.9652 | SdhB-H278Y | R |
| 27 | HG15050729-5 | 2015 | 辽宁Liaoning | 3.954 | 0.9434x+4.4106 | 0.9960 | SdhB-I280V | R |
| 28 | HG14102415-1 | 2014 | 河北Hebei | 1.111 | 0.8091x+4.4362 | 0.9967 | SdhB-P199S | R |
| 29 | HG15050701-1 | 2015 | 辽宁Liaoning | 19.526 | 0.7921x+4.0567 | 0.9967 | SdhC-P73S | R |
新窗口打开|下载CSV
2.2 抗性和敏感菌株的生物学特性
随机选取的12株多主棒孢之间的平均菌丝生长速率存在差异,敏感菌株菌丝生长速率为0.49—0.61 cm·d-1,抗性菌株菌丝生长速率为0.30—0.56 cm·d-1(表3)。与敏感菌株相比,抗性菌株除第9天的菌丝生长速率相等外,其他时间段均比敏感菌株生长慢(图3),且抗性菌株的平均生长速率与敏感菌株差异极显著(P<0.01)。菌丝生长速率与EC50之间呈显著负相关(表4)。图3
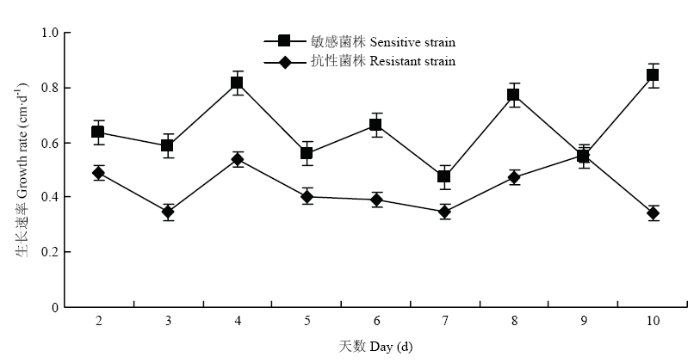 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3抗性和敏感菌株菌丝生长速率比较
Fig. 3Comparison of growth rate of resistant and sensitive strains
由表3可见,敏感菌株的产孢量在0.33×105—7.00×105个/mL,抗性菌株的产孢量在1.33×105—7.46×105个/mL,抗性菌株和敏感菌株在产孢量上不存在显著差异(P=0.949)。产孢量与生长速率和致病性存在低度相关,但不显著(表4)。
Table 3
Table 3The biological characteristics of resistance and sensitivity strains
| 菌株编号 Strain code | 敏感性 Sensitivity | 菌丝生长速率(11d) Mycelium growth rate (cm·d-1) | 产孢量 Spore outputs (×105/mL) | 病斑直径 Lesion diameter (cm) |
|---|---|---|---|---|
| HG17031007-2 | S | 0.60±0.09f | 0.92±1.38a | 0.78±0.09efg |
| HG17031010-1 | S | 0.61±0.09f | 7.00±4.61e | 0.65±0.26de |
| HG14102524-4 | S | 0.61±0.01f | 3.85±1.99bcd | 0.84±0.16fg |
| HG17031008-2 | S | 0.49±0.06de | 0.33±0.52a | 0.38±0.10ab |
| HG17031006-2 | R | 0.49±0.04de | 5.15±2.41de | 0.70±0.26def |
| HG17031008-4 | R | 0.45±0.05cd | 4.25±2.67bcd | 0.75±0.17efg |
| HG17031006-5 | R | 0.30±0.03a | 2.31±2.06abc | 0.56±0.19cd |
| HG17031008-1 | R | 0.37±0.02ab | 1.33±1.87a | 0.45±0.22abc |
| HG17031008-3 | R | 0.56±0.08ef | 7.46±2.70e | 0.91±0.12g |
| HG17031008-5 | R | 0.52±0.01de | 4.82±2.93cd | 0.54±0.05bcd |
| HG17031006-1 | R | 0.37±0.02ab | 1.85±1.28ab | 0.80±0.09efg |
| HG17031009-5 | R | 0.40±0.01bc | 4.00±2.80bcd | 0.33±0.07a |
根据Duncan’s最小显著性差异测定,同列数值后不同字母表示差异显著(P<0.05)According to a Duncan’s least-signi?cant difference, different lowercases after data in the same column indicate significant difference (P<0.05)
新窗口打开|下载CSV
Table 4
表4
表4EC50与生物学特性的相关性分析
Table 4
| EC50 | 生长速率 Growth rate | 产孢量 Spore outputs | 致病性 Pathogenicity | |
|---|---|---|---|---|
| EC50 | 1 | -0.700* | 0.141 | -0.21 |
| 生长速率Growth rate | 0.42 | 0.457 | ||
| 产孢量Spore outputs | 0.51 | |||
| 致病性Pathogenicity | 1 |
新窗口打开|下载CSV
敏感菌株的病斑直径在0.38—0.84 cm,抗性菌株的病斑直径在0.33—0.91 cm。抗性和敏感菌株在致病性上不存在显著差异(表3)。致病性与产孢量和生长速率之间存在低度相关性,但不显著(表4)。
2.3 AS-real-time PCR定量检测体系的建立
应用引物Cc-SdhB-F和Cc-SdhB-R测定SdhB的序列,结果如图4 所示,抗性菌株(R)在SdhB第956位的A突变到G,导致在密码子的第278组氨酸(H)突变成精氨酸(R),进一步发现含有H278R突变的菌株对啶酰菌胺具有抗性。图4
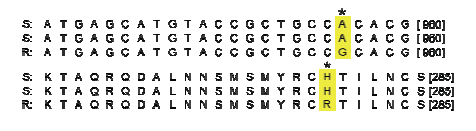 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4在SdhB上抗性和敏感菌株的核酸序列和氨基酸序列信息
Fig. 4Comparison of nucleotide and amino acid of gene SdhB in resistant and sensitive strains
以携带不同点突变的多主棒孢和8株常见的病原菌基因组DNA为模板,采用引物B-H278R-2F和B-H278R-2R14进行特异性验证(图5),结果表明仅有携带SdhB-H278R突变的多主棒孢基因组DNA有扩增条带,大小为244 bp,携带其他点突变的多主棒孢以及8种病原菌DNA均未检测到扩增条带,可用于不同菌种间多主棒孢H278R基因型的检测。
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5引物特异性检测
Fig. 5The specificity of the selected primers to H278R of SdhB
利用10倍梯度稀释的基因组DNA对引物B-H278R-2F和B-H278R-2R14进行普通PCR和AS-real-time PCR灵敏度检测,结果表明普通PCR检测的灵敏度为91 pg·μL-1(图6-A),AS-real-time PCR检测的灵敏度为9.1 pg·μL-1(图6-B)。AS-real-time PCR的灵敏度为普通PCR的10倍。
图6
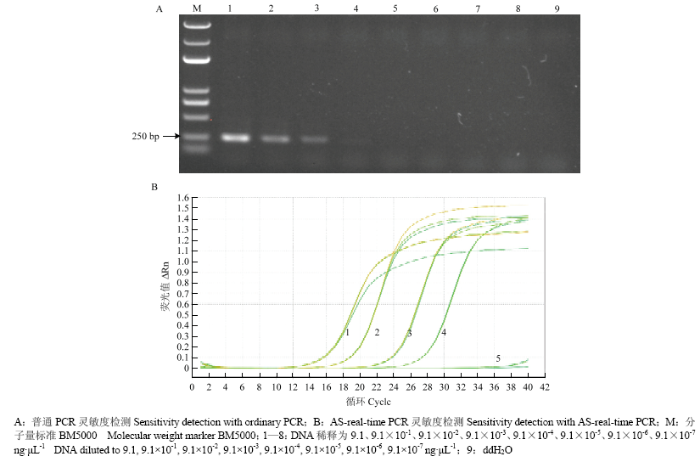 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6灵敏度检测
Fig. 6Sensitivity detection
利用优化的条件进行AS-real-time PCR,特异性引物B-H278R-2F/2R14扩增产物在熔解温度为91.62℃处出现单一的特异性峰(图7-A),内参引物B-H278R- TY-F/R扩增产物在熔解温度为87.81℃处出现单一的特异性峰(图7-B)。
图7
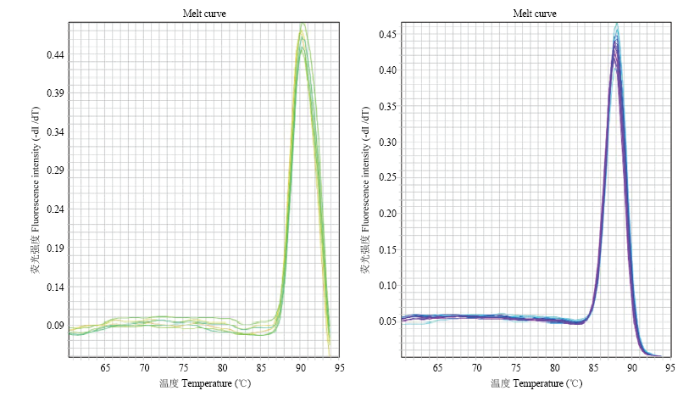 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7AS-real-time PCR熔解曲线分析
Fig. 7The melt curve analysis of AS-real-time PCR
以R278 DNA摩尔质量分别占总量DNA的6.25%、12.5%、25%、50%、75%和100%作为不同的处理,用引物B-H278R-2F/2R14、B-H278R-TY/R进行AS- real-time PCR检测,建立了标准曲线y=-3.513x+9.807,斜率为-3.513,截距为9.807,相关系数R2=0.9857,扩增效率为92.59%。R278 DNA占总DNA的6.25%—100%的比例中,呈现出良好的线性关系(图8)。
图8
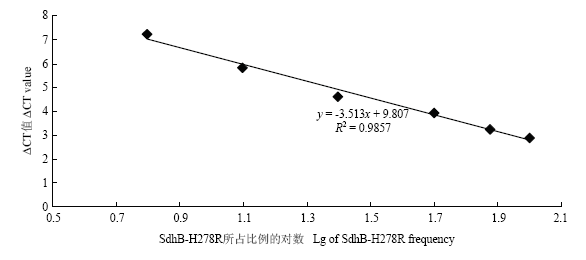 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图8AS-real-time PCR建立检测SdhB-H278R突变的标准曲线
Fig. 8Standard calibration curves for quantitative detection of SdhB-H278R variants by AS-real-time PCR assay
2.4 AS-real-time PCR定量检测体系的验证
以R278菌株DNA摩尔质量和孢子数量分别占总量的80%、40%、20%、10%和5%作为不同的处理,进行AS-real-time PCR检测并验证标准曲线的可行性。结果显示预期值为80%、40%、20%、10%和5%时,相应的DNA检测值依次为85.95%、44.64%、22.24%、11.86%和6.40%,孢子检测值依次为116.45%、48.39%、26.38%、11.86%和6.83%(表5)。随着预期值逐渐降低检测值的标准差也依次下降,且预期值在5%时具有最小的标准差。同时预期值和检测值具有很高的相关性(R2=0.9998和R2=0.9922),说明此检测体系具有可行性,在检测SdhB-H278R突变比例为5%时具有更高的准确性。Table 5
表5
表5AS-real-time PCR验证
Table 5
| 预期值Expected percentage (%) | 80 | 40 | 20 | 10 | 5 | |
|---|---|---|---|---|---|---|
| 检测值Detected percentage (%) | DNA | 85.95±1.45 | 44.64±0.69 | 22.24±0.85 | 11.86±0.60 | 6.40±0.24 |
| 孢子Spore | 116.45±4.83 | 48.39±6.15 | 26.38±4.59 | 11.86±0.98 | 6.83±1.43 |
新窗口打开|下载CSV
3 讨论
多主棒孢是一种重要的植物病原菌,是棒孢属内发现最早、寄主范围最广的种,可侵染葫芦科、茄科和豆科等大部分蔬菜,园艺花卉,橡胶等经济作物以及大豆等粮食作物,约380属530多种植物[20]。黄瓜棒孢叶斑病在我国北京、山东、甘肃、河北、广东和黑龙江等多个省份[21,22,23]以及在日本和韩国[9,10,24]均大范围发生,一般田间发病率为10%—25%,严重时可达60%—70%,甚至100%。因此由多主棒孢引起的黄瓜棒孢叶斑病在黄瓜上已成为一种重要的病害。啶酰菌胺是德国巴斯夫公司开发的一种SDHIs类杀菌剂,自2006年开始在日本登记防治黄瓜棒孢叶斑病,随后很快出现了对啶酰菌胺的抗药性[9,10]。但在国内该药剂并未登记在防治黄瓜棒孢叶斑病上,只登记用于防治黄瓜灰霉病,随着啶酰菌胺在防治黄瓜灰霉病中的广泛使用,灰葡萄孢对啶酰菌胺产生了抗药性[25],间接地多主棒孢对啶酰菌胺也相应产生了抗药性,导致黄瓜棒孢叶斑病大量发生。目前多主棒孢上的抗性突变包括SdhB-P199S、H278Y/R、I280V、SdhC-S73P、SdhD-S89P、D95E、H108R、G109V[10],而H278R为首次在国内报道,因此建立一种AS-real-time PCR检测方法非常必要,可用于指导后续的抗性治理。若H278R抗性频率升高,应使该药剂减少甚至停止使用,并采用不同作用机制的杀菌剂轮换使用或具有负交互抗药性药剂复配使用,可延长啶酰菌胺的使用寿命,减少黄瓜棒孢叶斑病的发生。通过测定在北京市大兴区采集的黄瓜多主棒孢对啶酰菌胺的敏感性并参考MIYAMOTO等[9]的研究结果,将多主棒孢菌株分为敏感和抗性菌株两个群体,通过对黄瓜多主棒孢SdhB序列的测定,抗性菌株均为SdhB-H278R突变。MIYAMOTO等[9]检测到SdhB-H278R共4株且划分为高等抗性菌株,EC50范围为8.9—10.7 μg·mL-1,而本文检测到SdhB-H278R突变共8株且EC50范围更广(5.395—11.710 μg·mL-1),推测可能与检测到H278R基因型的数量有关。通过对抗性和敏感菌株的生物学特性分析,发现抗性和敏感菌株除在菌丝生长速率方面存在显著差异外,在产孢量和致病性方面差异不显著,说明H278R突变株与敏感菌株之间的田间适合度差异不大,在药剂选择压力下易发展成优势群体导致黄瓜叶斑病的大量发生。SdhB-H272Y/R/L、H277Y/R/Y和H278R/Y几种点突变类型已经在小双胞腔菌、链格孢和灰葡萄孢等多种病原菌中被报道[7,14,16,26-28]。这几种点突变均发生在泛醌结合口袋及其附近[29],且氨基酸序列具有高度的保守性,可以将这几种突变归为一类。LALèVE等[30]研究发现,灰葡萄孢H272R/L/Y突变在以葡萄糖作为碳源时,前期菌丝生长速率较慢,后期一致;另外H272Y突变不影响SDH和呼吸活性。推测这类点突变在田间的适应性较强,极易导致田间病害的发生。结合AS-real-time PCR检测体系,预测含有H278R突变位点菌株的发展趋势,可指导药剂的合理使用及制定合理的田间管理策略。
本研究建立的AS-real-time PCR检测方法可以快速定量检测田间多主棒孢SdhB-H278R突变位点的频率,经验证AS-real-time PCR检测方法预期值和试验值的线性相关性为R2=0.9998和R2=0.9922。此方法可以检测到5%的SdhB-H278R抗性突变,灵敏度是普通PCR的10倍。相比高苇等[31,32]建立的检测黄瓜多主棒孢实时荧光定量PCR检测技术和普通PCR检测技术,此检测体系可以定量检测SdhB-H278R抗性突变位点以及抗性突变在田间频率的变化,克服了PCR-RFLP检测体系中变异系数大的难题[33,34,35]。与常规PCR检测方法相比较,该检测体系具有快速、无污染和灵敏度高的特点,并可根据检测结果指导科学用药以及降低用药成本和减少环境污染。
4 结论
建立了快速检测黄瓜多主棒孢菌SdhB-H278R突变位点的AS-real-time PCR技术体系,该体系自动化程度高,准确性强、灵敏度高,整个过程可在一个封闭的体系中进行,无污染、操作简单,可用于检测该突变位点在田间的频率。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1094/PHYTO-01-11-0016URLPMID:21469935 [本文引用: 1]

Abstract Botrytis cinerea isolates obtained from apple orchards were screened for resistance to boscalid. Boscalid-resistant (BosR) isolates were classified into four phenotypes based on the levels of the concentration that inhibited fungal growth by 50% relative to control. Of the 220 isolates tested, 42 were resistant to boscalid, with resistant phenotypes ranging from low to very high resistance. There was cross resistance between boscalid and carboxin. Analysis of partial sequences of the iron-sulfur subunit of succinate dehydrogenase gene in B. cinerea (BcSdhB) from 13 BosR and 9 boscalid-sensitive (BosS) isolates showed that point mutations in BcSdhB leading to amino acid substitutions at the codon position 272 from histidine to either tyrosine (H272Y) or arginine (H272R) were correlated with boscalid resistance. Allele-specific polymerase chain reaction (PCR) analysis of 66 BosR isolates (including 24 additional isolates obtained from decayed apple fruit) showed that 19 carried the point mutation H272Y and 46 had the point mutation H272R, but 1 BosR isolate gave no amplification product. Analysis of the BcSdhB sequence of this isolate revealed a different point mutation at codon 225, resulting in a substitution of proline (P) by phenylalanine (F) (P225F). The results indicated that H272R/Y in BcSdhB were the dominant genotypes of mutants in field BosR isolates from apple. A multiplex allele-specific PCR assay was developed to detect point mutations H272R/Y in a single PCR amplification. Levels of boscalid resistance ranged from low to very high within isolates carrying either the H272R or H272Y mutation, indicating that, among BosR isolates, different BosR phenotypes (levels of resistance) were not associated with particular types of point mutations (H272R versus H272Y) in BcSdhB. Analysis of genetic relationships between 39 BosR and 56 BosS isolates based on three microsatellite markers showed that 39 BosR isolates and 30 BosS isolates were clustered into two groups, and the third group consisted of only BosS isolates, suggesting that the development of resistance to boscalid in B. cinerea likely is not totally random, and resistant populations may come from specific genetic groups.
URL [本文引用: 2]
[本文引用: 1]
DOI:10.1111/j.1365-3059.2009.02151.xURL [本文引用: 7]

A total of 651 isolates of cucumber corynespora leaf spot fungus (Corynespora cassiicola) collected from cucumber in Japan, either with (438 isolates) or without (213 isolates) a prior history of boscalid use, were tested for their sensitivity to boscalid by using a mycelial growth inhibition method on YBA agar medium. Additionally, seven isolates of C. cassiicola obtained from tomato, soybean, eggplant (aubergine) and cowpea in different locations in Japan were tested before boscalid registration. Minimum inhibitory concentration (MIC) and 50% effective concentration (EC50) values for 220 isolates from crops without a prior history of boscalid use ranged from 0·5 to 7·5 μg mL611 and from 0·04 to 0·59 μg mL611, respectively. Two hundred and fourteen out of 438 isolates collected from ten cucumber greenhouses in Ibaraki Prefecture, Japan, which received boscalid spray applications showed boscalid resistance, with MIC values higher than 30 μg mL611. Moreover, resistant isolates were divided into two groups: a moderately resistant (MR) group consisting of 189 isolates with EC50 values ranging from 1·1 to 6·3 μg mL611, and a very highly resistant (VHR) group consisting of 25 isolates with EC50 values higher than 24·8 μg mL611. MR isolates were detected from all ten greenhouses, but VHR isolates were detected from only three. As a result of fungus inoculation tests which used potted cucumber plants, control failures of boscalid were observed against resistant isolates. Efficacy of boscalid was remarkably low against VHR isolates in particular. This is the first known report on boscalid resistance in Japan.
[本文引用: 4]
[本文引用: 1]
DOI:10.1002/ps.2214URLPMID:21674751 [本文引用: 1]

BACKGROUND: The occurrence of carboxylic acid amide (CAA)-fungicide-resistant Plasmopara viticola populations is becoming a serious problem in the control of grapevine downy mildew worldwide.RESULTS: The authors have developed a method, which utilises PCR-RFLP, for the rapid detection of resistance to the CAA fungicide mandipropamid in P. viticola populations. With this method, a glycine-to-serine substitution at codon 1105 of the cellulose synthase gene PvCesA3 of CAA-fungicide-resistant P. viticola was easily detected, although no resistant P. viticola was detected from 398 isolates in Japan.CONCLUSION: It is proposed that the PCR-RFLP method is a reliable tool for the rapid detection of CAA-fungicide-resistant P. viticola isolates. Only 4 h was required from the sampling of symptoms to the phenotyping of fungicide resistance. Copyright 2011 Society of Chemical Industry
[本文引用: 2]
[本文引用: 2]
DOI:10.1094/PHYTO-02-13-0041-RURLPMID:23901829 [本文引用: 2]

Early blight, caused by Alternaria solani, is an economically important foliar disease of potato in several production areas of the United States. Few potato cultivars possess resistance to early blight; therefore, the application of fungicides is the primary means of achieving disease control. Previous work in our laboratory reported resistance to the succinate dehydrogenase-inhibiting (SDHI) fungicide boscalid in this plant pathogen with a concomitant loss of disease control. Two phenotypes were detected, one in which A. solani isolates were moderately resistant to boscalid, the other in which isolates were highly resistant to the fungicide. Resistance in other fungal plant pathogens to SDHI fungicides is known to occur due to amino acid exchanges in the soluble subunit succinate dehydrogenase B (SdhB), C (SdhC), and D (SdhD) proteins. In this study, the AsSdhB, AsSdhC, and AsSdhD genes were analyzed and compared in sensitive (50% effective concentration [EC50] 100 g ml(-1)) A. solani isolates. In total, five mutations were detected, two in each of the AsSdhB and AsSdhD genes and one in the AsSdhC gene. The sequencing of AsSdhB elucidated point mutations cytosine (C) to thymine (T) at nucleotide 990 and adenine (A) to guanine (G) at nucleotide 991, leading to an exchange from histidine to tyrosine (H278Y) or arginine (H278R), respectively, at codon 278. The H278R exchange was detected in 4 of 10 A. solani isolates moderately resistant to boscalid, exhibiting EC50 values of 6 to 8 g ml(-1). Further genetic analysis also confirmed this mutation in isolates with high and very high EC50 values for boscalid of 28 to 500 g ml(-1). Subsequent sequencing of AsSdhC and AsSdhD genes confirmed the presence of additional mutations from A to G at nucleotide position 490 in AsSdhC and at nucleotide position 398 in the AsSdhD, conferring H134R and H133R exchanges in AsSdhC and AsSdhD, respectively. The H134R exchange in AsSdhC was observed in A. solani isolates with sensitive, moderate, highly resistant, and very highly resistant boscalid phenotypes, and the AsSdhD H133R exchange was observed in isolates with both moderate and very high EC50 value boscalid phenotypes. Detection and differentiation of point mutations in AsSdhB resulting in H278R and H278Y exchanges in the AsSdhB subunit were facilitated by the development of a mismatch amplification mutation assay. Detection of these two mutations in boscalid-resistant isolates, in addition to mutations in AsSdhC and AsSdhD resulting in an H134R and H133R exchange, respectively, was achieved by the development of a multiplex polymerase chain reaction to detect and differentiate the sensitive and resistant isolates based on the single-nucleotide polymorphisms present in all three genes. A single A. solani isolate with resistance to boscalid did not contain any of the above-mentioned exchanges but did contain a substitution of aspartate to glutamic acid at amino acid position 123 (D123E) in the AsSdhD subunit. Among A. solani isolates possessing resistance to boscalid, point mutations in AsSdhB were more frequently detected than mutations in genes coding for any other subunit.
[本文引用: 1]
[本文引用: 3]
URL [本文引用: 1]
DOI:10.3389/fmicb.2016.01815URLPMID:27895633 [本文引用: 1]

Botrytis cinerea, is a high risk pathogen for fungicide resistance development. Pathogen resistance to SDHIs is associated with several mutations insdhgene. The diversity of mutations and their differential effect on cross-resistance patterns among SDHIs and the fitness of resistant strains necessitate the availability of a tool for their rapid identification. This study was initiated to develop and validate a high-resolution melting (HRM) analysis for the identification of P225H/F/L//T, N230I, and H272L/R/Y mutations. Based on the sequence ofsdhB subunit of resistant and sensitive isolates, a universal primer pair was designed. The specificity of the HRM analysis primers was verified to ensure against the cross-reaction with other fungal species and its sensitivity was evaluated using concentrations of known amounts of mutant DNA. The melting curve analysis generated nine distinct curve profiles, enabling the discrimination of all the four mutations located at codon 225, the N230I mutation, the three mutations located in codon 272, and the non-mutated isolates (isolates of wild-type sensitivity). Similar results were obtained when DNA was extracted directly from artificially inoculated strawberry fruit. The method was validated by monitoring the presence ofsdhB mutations in samples of naturally infected strawberry fruits and stone fruit rootstock seedling plants showing damping-off symptoms. HRM analysis data were compared with a standard PIRA CR technique and an absolute agreement was observed suggesting that in both populations the H272R mutation was the predominant one, while H272Y, N230I, and P225H were detected in lower frequencies. The results of the study suggest that HRM analysis can be a useful tool for sensate, accurate, and rapid identification of severalsdhB mutations inB. cinereaand it is expected to contribute in routine fungicide resistance monitoring or assessments of the effectiveness of anti-resistance strategies implemented in crops heavily treated with botryticides.
[本文引用: 1]
[本文引用: 1]
DOI:10.1094/PHYTO-99-9-1015URLPMID:19671003 [本文引用: 1]

Abstract The fungus Corynespora cassiicola is primarily found in the tropics and subtropics, and is widely diverse in substrate utilization and host association. Isolate characterization within C. cassiicola was undertaken to investigate how genetic diversity correlates with host specificity, growth rate, and geographic distribution. C. cassiicola isolates were collected from 68 different plant species in American Samoa, Brazil, Malaysia, and Micronesia, and Florida, Mississippi, and Tennessee within the United States. Phylogenetic analyses using four loci were performed with 143 Corynespora spp. isolates, including outgroup taxa obtained from culture collections: C. citricola, C. melongenae, C. olivacea, C. proliferata, C. sesamum, and C. smithii. Phylogenetic trees were congruent from the ribosomal DNA internal transcribed spacer region, two random hypervariable loci (caa5 and ga4), and the actin-encoding locus act1, indicating a lack of recombination within the species and asexual propagation. Fifty isolates were tested for pathogenicity on eight known C. cassiicola crop hosts: basil, bean, cowpea, cucumber, papaya, soybean, sweet potato, and tomato. Pathogenicity profiles ranged from one to four hosts, with cucumber appearing in 14 of the 16 profiles. Bootstrap analyses and Bayesian posterior probability values identified six statistically significant phylogenetic lineages. The six phylogenetic lineages correlated with host of origin, pathogenicity, and growth rate but not with geographic location. Common fungal genotypes were widely distributed geographically, indicating long-distance and global dispersal of clonal lineages. This research reveals an abundance of previously unrecognized genetic diversity within the species and provides evidence for host specialization on papaya.
[D].
[本文引用: 1]
[D].
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1094/PDIS-04-11-0317URL [本文引用: 1]

Botrytis cinerea isolates (n = 122) were collected from strawberry fields located in northern Greece during a 3-year period (2008–10) and tested for their sensitivity to the succinate dehydrogenase inhibitor boscalid. Sensitivity measurements showed three distinct phenotypes consisting of isolates highly sensitive (fungicide concentration causing inhibition of germ tube growth by 50% [EC6368 values] of 0.05 to 0.21 μg ml–01), moderately resistant (EC6368 values of 1.37 to 7.79 μg ml–01), or highly resistant (EC6368 values of >50 μg ml–01) to boscalid. Sequence analysis of the sdhB gene revealed five mutations leading to amino acid substitutions in the SdhB subunit in isolates moderately resistant and highly resistant to boscalid. Three moderately resistant isolates showed a nucleotide change from A to T at codon 230, resulting in an asparagine to isoleucine (N230I) substitution. Several moderately resistant isolates showed a nucleotide change from C to T at codon 272, resulting in a substitution from histidine to arginine (H272R) whereas, in another set of isolates, a nucleotide change from A to G was found at the same codon, leading to a substitution from histidine to tyrosine (H272Y). One highly resistant isolate had a nucleotide change from A to T at codon 272, leading to a substitution from histidine to leucine (H272L), whereas, in three other highly resistant isolates, a double nucleotide change from CC to TT was observed at codon 225, resulting in a substitution from proline to phenylalanine (P225F). To facilitate rapid detection of these mutations associated with resistance to boscalid, a primer-introduced restriction analysis polymerase chain reaction was developed. The method was successfully applied to the moderately and highly resistant subpopulations and showed that the H272R mutation was predominant with relative frequencies of 28.5, 37.5, and 30% during 2008, 2009, and 2010, respectively. In contrast, the H272L mutation was detected at a frequency of 2.5% only in the 2009 population, whereas the P225F mutation was detected at a frequency of 7.5% only in the 2010 population.
[D].
[D].
URL [本文引用: 1]
URL [本文引用: 1]
DOI:10.1111/1462-2920.12282URLPMID:24119086225230272 [本文引用: 1]

Abstract Carboxamide fungicides target succinate dehydrogenase (SDH). Recent field monitoring studies have identified Botrytis cinerea isolates resistant to one or several SDH inhibitors (SDHIs) with amino acid substitutions in the SDH B subunit. We confirmed, by site-directed mutagenesis of the sdhB gene, that each of the mutations identified in field strains conferred resistance to boscalid in B.cinerea, and in some cases cross-resistance to other SDHIs (fluopyram, carboxin). Enzyme inhibition studies showed that the studied modifications (SdhB_P225T/L/F, N230I, H272Y/R/L) affected the inhibition of SDH activity by SDHIs, directly contributing to resistance. Our results confirm the importance of H272, P225 and N230 for carboxamide binding. Modifications of P225 and N230 conferred resistance to the four carboxamides tested (boscalid, fluopyram, carboxin, bixafen). Modifications of H272 had differential effects on the susceptibility of SDH to SDHIs. SdhB(H272L) , affected susceptibility to all SDHIs, SdhB(H272R) conferred resistance to all SDHIs tested except fluopyram, and SdhB(H272Y) conferred fluopyram hypersensitivity. Affinity-binding studies with radiolabelled fluopyram revealed strong correlations among the affinity of SDHIs for SDH, SDH inhibition and in vivo growth inhibition in the wild type. The sdhB(H272Y) mutation did not affect SDH and respiration activities, whereas all the other mutations affected respiration by decreasing SDH activity. 2013 Society for Applied Microbiology and John Wiley & Sons Ltd.
[本文引用: 1]
[本文引用: 1]
[D].
[本文引用: 1]
[D].
[本文引用: 1]
DOI:10.1111/j.1439-0434.2011.01805.xURL [本文引用: 1]

Fourteen primers were designed specific to SNP (G→C mutation) in the cytochrome b gene of Venturia inaequalis, corresponding to G143A substitution related to strobilurin resistance. Specificity of the primers and amplification efficiency were preliminarily tested in conventional PCR at different annealing temperatures. The performance of several preselected primer sets was verified in real-time PCR with SYBR Green I dye. Finally, two primer sets (‘2Wt’ and ‘5Mt’) were successfully applied for discrimination between wild-type and mutated allele. Different sensitivity of the detection of homologous and non-homologous DNA corresponded to the difference in Ct values equalled 10.5 for ‘2Wt’ and 12.0 for ‘5Mt’ primer set. Primers specific to Mycosphaerella fijensis cytochrome b sequence (Wille et al. 2002) were applied for additional control of PCR inhibitors. The reliability of the new method was evaluated and verified using two series of reference DNA dilutions and artificial mixtures of both types of DNA. Developed real-time PCR assay was applied to measure the ratio of mutated to non-mutated allele in field samples, collected in Poland in 2009, using two series of reference DNA in every run. The measured mutation level for the samples derived from orchards with conventional chemical control was very high (50–100%). For two populations originating from one organic orchard, the measured level of mutation was 1% and 46%. The combination of molecular and traditional tests for the evaluation of mutation level and monitoring of resistance levels in orchards is recommended.
DOI:10.1002/ps.1425URLPMID:17912681 [本文引用: 1]

BACKGROUND: Thiophanate-methyl, a member of the benzimidazole class of fungicides, is used in California to control brown rot of stone fruit caused by Monilinia fructicola (G. Wint.) Honey. The goal of this study was to develop a real-time polymerase chain reaction (PCR) assay as an efficient method to quantify the E198A allele of -tubulin that confers benzimidazole resistance. RESULTS: Using the real-time PCR assay, the frequency of allele E198A (FEA) in a population was determined from the quantities of DNA amplified with the E198A allele-specific primer pair HRF/HRR and the M. fructicola - specific primer pair MfF6/MfR6. The average proportions of highly resistant isolates determined with the conventional fungicide sensitivity method were within the range of average FEA values determined with the real-time PCR assay. We also determined the FEAs of M. fructicola populations sampled from 21 stone fruit orchards in California. Only one orchard showed a high FEA over 0.20, seven orchards had values between 0.01 and 0.1, and 13 orchards had values less than 0.01. CONCLUSION: The real-time PCR assay developed in this study provides a potentially useful tool to efficiently quantify benzimidazole resistance for large M. fructicola populations. Copyright 2007 Society of Chemical Industry
[本文引用: 1]
