 ,1, 王志慧1, 淮东欣1, 高华援2, 晏立英1, 李建国1, 李威涛1, 陈玉宁1, 康彦平1, 刘海龙2, 王欣1, 薛晓梦1, 姜慧芳1, 廖伯寿
,1, 王志慧1, 淮东欣1, 高华援2, 晏立英1, 李建国1, 李威涛1, 陈玉宁1, 康彦平1, 刘海龙2, 王欣1, 薛晓梦1, 姜慧芳1, 廖伯寿 ,1,*
,1,*Development and application of a near infrared spectroscopy model for predicting high sucrose content of peanut seed
LEI Yong ,1, WANG Zhi-Hui1, HUAI Dong-Xin1, GAO Hua-Yuan2, YAN Li-Ying1, LI Jian-Guo1, LI Wei-Tao1, CHEN Yu-Ning1, KANG Yan-Ping1, LIU Hai-Long2, WANG Xin1, XUE Xiao-Meng1, JIANG Hui-Fang1, LIAO Bo-Shou
,1, WANG Zhi-Hui1, HUAI Dong-Xin1, GAO Hua-Yuan2, YAN Li-Ying1, LI Jian-Guo1, LI Wei-Tao1, CHEN Yu-Ning1, KANG Yan-Ping1, LIU Hai-Long2, WANG Xin1, XUE Xiao-Meng1, JIANG Hui-Fang1, LIAO Bo-Shou ,1,*
,1,*通讯作者:
收稿日期:2020-05-14接受日期:2020-08-19网络出版日期:2020-09-21
| 基金资助: |
Received:2020-05-14Accepted:2020-08-19Online:2020-09-21
| Fund supported: |
作者简介 About authors
E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (4965KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
雷永, 王志慧, 淮东欣, 高华援, 晏立英, 李建国, 李威涛, 陈玉宁, 康彦平, 刘海龙, 王欣, 薛晓梦, 姜慧芳, 廖伯寿. 花生籽仁蔗糖含量近红外模型构建及在高糖品种培育中的应用[J]. 作物学报, 2021, 47(2): 332-341. doi:10.3724/SP.J.1006.2021.04106
LEI Yong, WANG Zhi-Hui, HUAI Dong-Xin, GAO Hua-Yuan, YAN Li-Ying, LI Jian-Guo, LI Wei-Tao, CHEN Yu-Ning, KANG Yan-Ping, LIU Hai-Long, WANG Xin, XUE Xiao-Meng, JIANG Hui-Fang, LIAO Bo-Shou.
花生是我国重要的油料和经济作物。近10年来, 我国花生年均总产约1680万吨, 居世界首位, 占全球花生总产的38%[1]。我国花生总产约50%用于榨油, 40%作为食用, 欧美等发达国家生产和进口的花生则70%以上作为食用和食品加工原料。随着人民生活水平的提高和加工技术的进步, 我国花生食用及食品加工量将越来越大, 对原料的需求越来越多样化[2]。含糖量高低对于花生的食用品质、加工风味有重要影响, 含糖量和甜度高是鲜食花生的关键品质指标。研究表明, 花生籽仁含糖量可作为预测烤花生风味和甜度的重要指标, 与花生籽仁口味品质相关系数达0.88, 糖含量的微小变化也会影响最终的烘烤质量[3,4,5]。花生干籽仁中蔗糖含量达到6%以上时, 口感较好。成熟花生籽仁中的糖类主要是蔗糖, 占90%左右。因此, 发掘和培育高蔗糖含量的种质和品种, 对于食用型花生品质的提升具有重要价值。
已有研究表明, 花生含糖量是可以遗传的性状[3,4,5,6], 不同市场型花生的含糖量有显著差异, 兰娜型(runner type)、弗吉尼亚型(virginia type)、瓦伦西亚型(valencia type)的总糖量均值分别为2.90%、3.51%和2.88%, 西班牙型(spanish type)花生资源的蔗糖含量在2.44%~7.61%之间[7,8]。如何对资源材料和育种后代的蔗糖含量进行高通量、规模化的检测分析, 是选育高糖优异食用型花生品种的关键技术。目前, 传统的花生籽仁蔗糖含量测定方法多采用比色法和液相色谱法, 耗时、费力且不能进行高通量检测。
近红外光谱法具有非破坏性、快速、高效的特点, 在农作物品质分析中已得到广泛应用[9,10,11]。花生中已经建立了含油量、脂肪酸、蛋白质、氨基酸等重要品质指标的近红外模型, 但在籽仁蔗糖检测方面应用的较少[12,13,14,15,16,17,18,19]。秦利等[20]和唐月异等[21]分别利用瑞典波通DA7200和德国布鲁克Matris-I近红外光谱仪建立了多粒花生籽仁的蔗糖含量近红外模型, 但建立模型与测试时需要的籽仁量较大(30~50粒), 不能对籽仁量较少的单株进行蔗糖含量检测, 并且以上2种型号光谱仪采集的光谱无法在其他公司生产的光谱仪上应用[20,21]。
为了深入探索近红外光谱分析法的仪器广适性, 本研究通过对185份花生样品蔗糖含量的测定, 利用Unity近红外仪扫描样品的光谱, 建立和优化花生成熟籽仁蔗糖含量的近红外分析模型, 以期为花生蔗糖含量测定提供更加快捷有效的方法, 加快食用型花生品种选育进程。
1 材料与方法
1.1 研究材料
建模所用花生材料系本团队收集的国内育成品种和培育的高含糖量中间材料, 总计185份, 其中S001~S121是团队选育的蔗糖含量较高的育种中间材料, 种植地点为湖北武汉, S122~S185是筛选的含糖量有显著差异的品种在多个地方种植的样品(表1)。另取20份样品, 编号为ST01~ST20, 作为外部样品用于模型验证。Table 1
表1
表1建模和模型验证所用花生品种(系)编号及名称
Table 1
| 样品编号 Sample ID | 品种(系) Variety (line) | 种植地点 Location | 样品编号 Sample ID | 品种(系) Variety (line) | 种植地点 Location |
|---|---|---|---|---|---|
| S001 | A1435-3 | 湖北武汉 Wuhan, Hubei | S104 | A1516-1 | 湖北武汉 Wuhan, Hubei |
| S002 | A1435-4 | 湖北武汉 Wuhan, Hubei | S105 | A1516-2 | 湖北武汉 Wuhan, Hubei |
| S003 | A1442-1 | 湖北武汉 Wuhan, Hubei | S106 | A1518-1 | 湖北武汉 Wuhan, Hubei |
| S004 | A1444-1 | 湖北武汉 Wuhan, Hubei | S107 | A1518-2 | 湖北武汉 Wuhan, Hubei |
| S005 | A1444-2 | 湖北武汉 Wuhan, Hubei | S108 | A1520-1 | 湖北武汉 Wuhan, Hubei |
| S006 | A1444-4 | 湖北武汉 Wuhan, Hubei | S109 | A1520-2 | 湖北武汉 Wuhan, Hubei |
| S007 | A1444-5 | 湖北武汉 Wuhan, Hubei | S110 | A1529-1 | 湖北武汉 Wuhan, Hubei |
| S008 | A1444-6 | 湖北武汉 Wuhan, Hubei | S111 | A1529-2 | 湖北武汉 Wuhan, Hubei |
| S009 | A1447-1 | 湖北武汉 Wuhan, Hubei | S112 | A1532 | 湖北武汉 Wuhan, Hubei |
| S010 | A1447-2 | 湖北武汉 Wuhan, Hubei | S113 | A1538 | 湖北武汉 Wuhan, Hubei |
| S011 | A1447-3 | 湖北武汉 Wuhan, Hubei | S114 | A1541 | 湖北武汉 Wuhan, Hubei |
| S012 | A1447-4 | 湖北武汉 Wuhan, Hubei | S115 | A1543-1 | 湖北武汉 Wuhan, Hubei |
| S013 | A1447-5 | 湖北武汉 Wuhan, Hubei | S116 | A1543-2 | 湖北武汉 Wuhan, Hubei |
| 样品编号 Sample ID | 品种(系) Variety (line) | 种植地点 Location | 样品编号 Sample ID | 品种(系) Variety (line) | 种植地点 Location |
| S014 | A1447-7 | 湖北武汉 Wuhan, Hubei | S117 | A1547-1 | 湖北武汉 Wuhan, Hubei |
| S015 | A1449-1 | 湖北武汉 Wuhan, Hubei | S118 | A1547-2 | 湖北武汉 Wuhan, Hubei |
| S016 | A1449-2 | 湖北武汉 Wuhan, Hubei | S119 | A1547-3 | 湖北武汉 Wuhan, Hubei |
| S017 | A1449-3 | 湖北武汉 Wuhan, Hubei | S120 | A1547-4 | 湖北武汉 Wuhan, Hubei |
| S018 | A1449-4 | 湖北武汉 Wuhan, Hubei | S121 | A1547-5 | 湖北武汉 Wuhan, Hubei |
| S019 | A1449-5 | 湖北武汉 Wuhan, Hubei | S122 | 花育23 Huayu 23 | 湖北武汉 Wuhan, Hubei |
| S020 | A1449-6 | 湖北武汉 Wuhan, Hubei | S123 | 远杂9102 Yuanza 9102 | 湖北武汉 Wuhan, Hubei |
| S021 | A1449-7 | 湖北武汉 Wuhan, Hubei | S124 | 锦花15 Jinhua 15 | 湖北武汉 Wuhan, Hubei |
| S022 | A1449-8 | 湖北武汉 Wuhan, Hubei | S125 | 贵州红 Guizhouhong | 湖北武汉 Wuhan, Hubei |
| S023 | A1449-10 | 湖北武汉 Wuhan, Hubei | S126 | 扶余四粒红 Fuyusilihong | 湖北武汉 Wuhan, Hubei |
| S024 | A1449-11 | 湖北武汉 Wuhan, Hubei | S127 | 花育16 Huayu 16 | 湖北武汉 Wuhan, Hubei |
| S025 | A1449-12 | 湖北武汉 Wuhan, Hubei | S128 | 云花3号 Yunhua 3 | 湖北武汉 Wuhan, Hubei |
| S026 | A1449-13 | 湖北武汉 Wuhan, Hubei | S129 | 山东仔 Shandongzai | 湖北武汉 Wuhan, Hubei |
| S027 | A1449-14 | 湖北武汉 Wuhan, Hubei | S130 | 麻阳小子 Mayangxiaozi | 湖北武汉 Wuhan, Hubei |
| S028 | A1454-1 | 湖北武汉 Wuhan, Hubei | S131 | 扶余四粒红 Fuyusiliong | 湖北武汉 Wuhan, Hubei |
| S029 | A1454-2 | 湖北武汉 Wuhan, Hubei | S132 | 青花505 Qinhua 505 | 湖北武汉 Wuhan, Hubei |
| S030 | A1454-3 | 湖北武汉 Wuhan, Hubei | S133 | 赣花7号 Ganhua 7 | 湖北武汉 Wuhan, Hubei |
| S031 | A1454-4 | 湖北武汉 Wuhan, Hubei | S134 | 红豆花生 Hongdouhuasheng | 湖北武汉 Wuhan, Hubei |
| S032 | A1454-5 | 湖北武汉 Wuhan, Hubei | S135 | 团风小红粒 Tuanfengxiaoli | 湖北武汉 Wuhan, Hubei |
| S033 | A1454-6 | 湖北武汉 Wuhan, Hubei | S136 | 沿河本地种 Yanhebendizhong | 湖北武汉 Wuhan, Hubei |
| S034 | A1458-1 | 湖北武汉 Wuhan, Hubei | S137 | 贵州红花生 Guizhouhonghuasheng | 湖北武汉 Wuhan, Hubei |
| S035 | A1458-3 | 湖北武汉 Wuhan, Hubei | S138 | 贵州红皮 Guizhouhongpi | 湖北武汉 Wuhan, Hubei |
| S036 | A1458-4 | 湖北武汉 Wuhan, Hubei | S139 | 红花生 Honghuasheng | 湖北武汉 Wuhan, Hubei |
| S037 | A1463 | 湖北武汉 Wuhan, Hubei | S140 | 14-511855 | 湖北武汉 Wuhan, Hubei |
| S038 | A1464-1 | 湖北武汉 Wuhan, Hubei | S141 | 皖花2号 Wanhua 2 | 湖北武汉 Wuhan, Hubei |
| S039 | A1464-2 | 湖北武汉 Wuhan, Hubei | S142 | 日照瓜子 Rizhaoguazi | 湖北武汉 Wuhan, Hubei |
| S040 | A1464-3 | 湖北武汉 Wuhan, Hubei | S143 | 天府3号 Tianfu 3 | 湖北武汉 Wuhan, Hubei |
| S041 | A1464-4 | 湖北武汉 Wuhan, Hubei | S144 | 天府22 Tianfu 22 | 湖北武汉 Wuhan, Hubei |
| S042 | A1464-5 | 湖北武汉 Wuhan, Hubei | S145 | 开封水果 Kaifengshuiguo | 湖北武汉 Wuhan, Hubei |
| S043 | A1464-6 | 湖北武汉 Wuhan, Hubei | S146 | 冀花10号 Jihua 10 | 湖北武汉 Wuhan, Hubei |
| S044 | A1464-7 | 湖北武汉 Wuhan, Hubei | S147 | 河北水果 Hebeishuiguo | 湖北武汉 Wuhan, Hubei |
| S045 | A1467-1 | 湖北武汉 Wuhan, Hubei | S148 | 红仁花生 Hongrenhuasheng | 湖北武汉 Wuhan, Hubei |
| S046 | A1467-2 | 湖北武汉 Wuhan, Hubei | S149 | 四粒红 Silihong | 湖北武汉 Wuhan, Hubei |
| S047 | A1467-3 | 湖北武汉 Wuhan, Hubei | S150 | 杜皮红 Dupihong | 湖北武汉 Wuhan, Hubei |
| S048 | A1467-4 | 湖北武汉 Wuhan, Hubei | S151 | 钟山红豆 Zhongshanhongdou | 湖北武汉 Wuhan, Hubei |
| S049 | A1467-5 | 湖北武汉 Wuhan, Hubei | S152 | 吉花18 Jihua 18 | 湖北武汉 Wuhan, Hubei |
| S050 | A1467-6 | 湖北武汉 Wuhan, Hubei | S153 | 吉花19 Jihua 19 | 湖北武汉 Wuhan, Hubei |
| S051 | A1471-1 | 湖北武汉 Wuhan, Hubei | S154 | 吉花20 Jihua 20 | 湖北武汉 Wuhan, Hubei |
| S052 | A1471-2 | 湖北武汉 Wuhan, Hubei | S155 | 吉花23 Jihua 23 | 湖北武汉 Wuhan, Hubei |
| S053 | A1471-3 | 湖北武汉 Wuhan, Hubei | S156 | 吉花24 Jihua 24 | 湖北武汉 Wuhan, Hubei |
| S054 | A1471-4 | 湖北武汉 Wuhan, Hubei | S157 | 阜花25 Fuhua 25 | 湖北武汉 Wuhan, Hubei |
| S055 | A1473-1 | 湖北武汉 Wuhan, Hubei | S158 | 阜花26 Fuhua 26 | 湖北武汉 Wuhan, Hubei |
| S056 | A1473-2 | 湖北武汉 Wuhan, Hubei | S159 | 阜花30 Fuhua 30 | 湖北武汉 Wuhan, Hubei |
| S057 | A1473-3 | 湖北武汉 Wuhan, Hubei | S160 | 花育23 Huayu 23 | 安徽合肥 Hefei, Anhui |
| S058 | A1473-4 | 湖北武汉 Wuhan, Hubei | S161 | 四粒红 Silihong | 河北石家庄 Shijiazhuang, Hebei |
| 样品编号 Sample ID | 品种(系) Variety (line) | 种植地点 Location | 样品编号 Sample ID | 品种(系) Variety (line) | 种植地点 Location |
| S059 | A1473-5 | 湖北武汉 Wuhan, Hubei | S162 | 冀113 Ji 113 | 河北石家庄 Shijiazhuang, Hebei |
| S060 | A1473-6 | 湖北武汉 Wuhan, Hubei | S163 | 远杂9102 Yuanza 9102 | 河北石家庄 Shijiazhuang, Hebei |
| S061 | A1473-7 | 湖北武汉 Wuhan, Hubei | S164 | 冀11 Ji 11 | 河北石家庄 Shijiazhuang, Hebei |
| S062 | A1473-8 | 湖北武汉 Wuhan, Hubei | S165 | 冀花18 Jihua 18 | 河北石家庄 Shijiazhuang, Hebei |
| S063 | A1476 | 湖北武汉 Wuhan, Hubei | S166 | 冀甜1号 Jitian 1 | 河北石家庄 Shijiazhuang, Hebei |
| S064 | A1478-1 | 湖北武汉 Wuhan, Hubei | S167 | 中花16 Zhonghua 16 | 四川成都 Chengdu, Sichuan |
| S065 | A1480-1 | 湖北武汉 Wuhan, Hubei | S168 | 蜀花3号 Shuhua 3 | 四川成都 Chengdu, Sichuan |
| S066 | A1480-2 | 湖北武汉 Wuhan, Hubei | S169 | 天府11 Tianfu 11 | 四川成都 Chengdu, Sichuan |
| S067 | A1480-3 | 湖北武汉 Wuhan, Hubei | S170 | 花育23 Huayu 23 | 四川成都 Chengdu, Sichuan |
| S068 | A1480-4 | 湖北武汉 Wuhan, Hubei | S171 | 远杂9102 Yuanza 9102 | 四川成都 Chengdu, Sichuan |
| S069 | A1483-1 | 湖北武汉 Wuhan, Hubei | S172 | 中花6号 Zhonghua 16 | 四川成都 Chengdu, Sichuan |
| S070 | A1483-2 | 湖北武汉 Wuhan, Hubei | S173 | 四粒红 Silihong | 四川成都 Chengdu, Sichuan |
| S071 | A1483-3 | 湖北武汉 Wuhan, Hubei | S174 | 中花24 Zhonghua 24 | 四川成都 Chengdu, Sichuan |
| S072 | A1485-1 | 湖北武汉 Wuhan, Hubei | S175 | 中花21 Zhonghua 21 | 四川成都 Chengdu, Sichuan |
| S073 | A1485-2 | 湖北武汉 Wuhan, Hubei | S176 | 罗汉果 Luohanguo | 四川成都 Chengdu, Sichuan |
| S074 | A1485-3 | 湖北武汉 Wuhan, Hubei | S177 | 中花21 Zhonghua 21 | 安徽合肥 Hefei, Anhui |
| S075 | A1485-4 | 湖北武汉 Wuhan, Hubei | S178 | 罗汉果 Luohanguo | 安徽合肥 Hefei, Anhui |
| S076 | A1488 | 湖北武汉 Wuhan, Hubei | S179 | 中花215 Zhonghua 215 | 安徽合肥 Hefei, Anhui |
| S077 | A1490-1 | 湖北武汉 Wuhan, Hubei | S180 | 中花16 Zhonghua 16 | 安徽合肥 Hefei, Anhui |
| S078 | A1490-2 | 湖北武汉 Wuhan, Hubei | S181 | 四粒红 Silihong | 安徽合肥 Hefei, Anhui |
| S079 | A1493-1 | 湖北武汉 Wuhan, Hubei | S182 | 中花21 Zhonghua 21 | 河北石家庄 Shijiazhuang, Hebei |
| S080 | A1493-2 | 湖北武汉 Wuhan, Hubei | S183 | 罗汉果 Luohanguo | 河北石家庄 Shijiazhuang, Hebei |
| S081 | A1495-1 | 湖北武汉 Wuhan, Hubei | S184 | 中花16 Zhonghua 16 | 河北石家庄 Shijiazhuang, Hebei |
| S082 | A1495-2 | 湖北武汉 Wuhan, Hubei | S185 | 冀花16 Jihua 16 | 河北石家庄 Shijiazhuang, Hebei |
| S083 | A1497 | 湖北武汉 Wuhan, Hubei | ST01 | 中花16 Zhonghua 16 | 湖北武汉 Wuhan, Hubei |
| S084 | A1504-1 | 湖北武汉 Wuhan, Hubei | ST02 | 中花21 Zhonghua 21 | 湖北武汉 Wuhan, Hubei |
| S085 | A1504-2 | 湖北武汉 Wuhan, Hubei | ST03 | A1458-2 | 湖北武汉 Wuhan, Hubei |
| S086 | A1504-3 | 湖北武汉 Wuhan, Hubei | ST04 | 宛花2号 Wanhua 2 | 湖北武汉 Wuhan, Hubei |
| S087 | A1504-4 | 湖北武汉 Wuhan, Hubei | ST05 | 中花15 Zhonghua 15 | 湖北武汉 Wuhan, Hubei |
| S088 | A1504-5 | 湖北武汉 Wuhan, Hubei | ST06 | 中花12 Zhonghua 12 | 湖北武汉 Wuhan, Hubei |
| S089 | A1504-6 | 湖北武汉 Wuhan, Hubei | ST07 | 19A1385 | 湖北武汉 Wuhan, Hubei |
| S090 | A1504-7 | 湖北武汉 Wuhan, Hubei | ST08 | 皖花9号 Wanhua 9 | 安徽合肥 Hefei, Anhui |
| S091 | A1507-1 | 湖北武汉 Wuhan, Hubei | ST09 | 徐花甜29 Xuhuatian 29 | 江苏徐州 Xuzhou, Jiangsu |
| S092 | A1507-2 | 湖北武汉 Wuhan, Hubei | ST10 | 中花9号 Zhonghua 9 | 湖北武汉 Wuhan, Hubei |
| S093 | A1507-4 | 湖北武汉 Wuhan, Hubei | ST11 | 远杂9102 Yuanza 9102 | 江苏南京 Nanjing, Jiangsu |
| S094 | A1507-5 | 湖北武汉 Wuhan, Hubei | ST12 | 宁泰9922 Ningtai 9922 | 江苏南京 Nanjing, Jiangsu |
| S095 | A1510-1 | 湖北武汉 Wuhan, Hubei | ST13 | A1447-6 | 湖北武汉 Wuhan, Hubei |
| S096 | A1510-2 | 湖北武汉 Wuhan, Hubei | ST14 | 苏花0537 Suhua 0537 | 江苏南京 Nanjing, Jiangsu |
| S097 | A1513-1 | 湖北武汉 Wuhan, Hubei | ST15 | 大四粒红 Dasilihong | 湖北武汉 Wuhan, Hubei |
| S098 | A1513-2 | 湖北武汉 Wuhan, Hubei | ST16 | 冀花16 Jihua 16 | 湖北武汉 Wuhan, Hubei |
| S099 | A1513-3 | 湖北武汉 Wuhan, Hubei | ST17 | 苏花1713 Suhua 1713 | 江苏南京 Nanjing, Jiangsu |
| S100 | A1513-4 | 湖北武汉 Wuhan, Hubei | ST18 | 冀花11 Jihua 11 | 湖北武汉 Wuhan, Hubei |
| S101 | A1513-5 | 湖北武汉 Wuhan, Hubei | ST19 | A1478-2 | 湖北武汉 Wuhan, Hubei |
| S102 | A1513-6 | 湖北武汉 Wuhan, Hubei | ST20 | 徐花甜30 Xuhuatian 30 | 江苏徐州 Xuzhou, Jiangsu |
| S103 | A1513-7 | 湖北武汉 Wuhan, Hubei |
新窗口打开|下载CSV
1.2 研究方法
1.2.1 光谱采集 采用美国Unity科技公司生产的 SpectraStar XL近红外光谱仪采集光谱, 光谱仪扫描波长范围为1100~2500 nm。调整环境温度在20℃左右, 样品在20℃左右恒温放置48 h以上, 仪器开机预热30 min后, 每个样品取15~20粒籽仁, 装入直径为3 cm的小样品杯, 重复装样3次, 获得平均光谱用于建模(图1)。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1本研究所用的近红外仪及小样品杯
Fig. 1Near infrared reflectance spectroscopy instrument Unity-SpectrastarXL and small cup used in this experiment
1.2.2 蔗糖含量测定 将采集光谱所用的花生籽仁用磨样器磨碎, 称取1 g粉末样品(重复3次), 至50 mL的离心管中, 加入10 mL的80%乙醇溶液, 80℃水浴30 min; 冷却至室温, 取上清2 mL, 14,000×g离心5 min; 离心后取上清700 μL, 过滤至上样瓶中待上机检测。高效液相色谱型号为Agilent 1290, 示差折光检测器(RID)检测, 柱子型号20RBAX NH2 (4.6 mm × 250 mm, 5 μm), 流动相为75∶25 (v/v)乙腈/水溶液, 流速1 mL min-1, 柱温40℃, 进样量10 μL。按照GB 5009.8-2016 [22]中高效液相法的标准溶液配制方法, 配制2.0、4.0、6.0、8.0和10.0 mg mL-1浓度的标准溶液, 过滤至上样瓶进行上机测定, 记录不同浓度标准溶液的峰面积, 以峰面积对浓度进行线性回归, 构建标准曲线。试样溶液上机检测, 记录目标峰面积, 从标准曲线中获得试样溶液中蔗糖的浓度。1.2.3 模型构建与优化 采用Unity科技公司自带的UCAL近红外定标软件构建模型, 将测得的蔗糖含量化学值与采集的近红外光谱导入UCAL软件进行拟合光谱处理, 采用最小二乘法优化建模, 建模过程中自动剔除较大剩余值的异常样品, 然后再进行内部交叉验证剔除异常值, 通过比较模型的决定系数(R2)和标准差(RMSECV)衡量模型质量, 筛选最佳模型, 并比较样品预测值与化学值的决定系数(R2)和均方差(Mean Square Deviation, MSD)来衡量模型的质量。1.2.4 模型的外部验证 选取20个蔗糖含量显著差异的花生品种(系), 利用建立的近红外方法检测其蔗糖含量, 记录近红外模型的预测值, 再利用液相色谱法(HPLC)分析样品的蔗糖含量, 比较NIR预测值与HPLC测定化学值的相关性和准确性。1.2.5 高糖高油酸品系选育过程 2015年春季, 以普通油酸花生品种“吉花02-1-4” (蔗糖含量为4.0%)为母本, 高油酸品种“中花26” (蔗糖含量2.3%)为父本配制杂交组合, 并收获F1种子。2015年秋季, 将F1种植于广东湛江, 收获F2种子。2016年春季, 使用近红外检测单粒F2种子中油酸含量, 选择油酸含量在75%以上的种子, 种植于湖北武汉, 并收获F3单株。2017年春季, 使用近红外检测F3单株种子油酸含量, 选择油酸含量在75%以上的单株, 种植于湖北武汉, 收获F4。2018年春季, 将收获的F4按株系种植于武汉, 调查农艺性状, 收获F5, 2018年冬季, 将F5种植于海南三亚, 收获F6株系。2019年, 利用本研究建立的单株蔗糖含量近红外模型测定F6株系的蔗糖含量, 并经液相色谱分析验证, 获得蔗糖含量7%以上、油酸78%以上的优良株系。
2 结果与分析
2.1 样品中蔗糖含量的化学分析结果
采用高效液相色谱法测定了185份花生籽仁的蔗糖含量, 样品的蔗糖测定值见表2。蔗糖含量平均4.82%, 变异范围1.02%~8.48%, 标准差为1.96, 标准误0.011, 变异系数40.66%, 表明本试验选择花生材料的蔗糖含量分布范围广, 变异系数大, 代表性好。Table 2
表2
表2HPLC法测定的定标样品蔗糖含量化学值
Table 2
| 样品编号 Sample ID | 蔗糖含量 Sucrose content (%) | 样品编号 Sample ID | 蔗糖含量 Sucrose content (%) | 样品编号 Sample ID | 蔗糖含量 Sucrose content (%) | 样品编号 Sample ID | 蔗糖含量 Sucrose content (%) | 样品编号 Sample ID | 蔗糖含量 Sucrose content (%) |
|---|---|---|---|---|---|---|---|---|---|
| S001 | 6.43 | S038 | 7.22 | S075 | 5.82 | S112 | 5.50 | S149 | 2.47 |
| S002 | 7.66 | S039 | 7.02 | S076 | 6.25 | S113 | 6.50 | S150 | 1.68 |
| S003 | 5.46 | S040 | 6.91 | S077 | 5.61 | S114 | 3.95 | S151 | 2.07 |
| S004 | 6.48 | S041 | 6.86 | S078 | 6.35 | S115 | 5.77 | S152 | 2.45 |
| S005 | 5.93 | S042 | 6.66 | S079 | 5.68 | S116 | 5.77 | S153 | 1.75 |
| S006 | 5.36 | S043 | 6.58 | S080 | 5.46 | S117 | 4.77 | S154 | 1.33 |
| S007 | 6.17 | S044 | 6.43 | S081 | 6.12 | S118 | 5.68 | S155 | 2.10 |
| S008 | 5.38 | S045 | 6.26 | S082 | 6.33 | S119 | 6.53 | S156 | 1.71 |
| S009 | 5.05 | S046 | 6.04 | S083 | 6.47 | S120 | 5.15 | S157 | 1.68 |
| S010 | 6.51 | S047 | 5.97 | S084 | 5.59 | S121 | 5.70 | S158 | 1.70 |
| S011 | 6.67 | S048 | 6.42 | S085 | 5.45 | S122 | 2.38 | S159 | 2.01 |
| S012 | 6.77 | S049 | 8.48 | S086 | 5.74 | S123 | 1.87 | S160 | 2.12 |
| S013 | 8.01 | S050 | 6.22 | S087 | 6.58 | S124 | 1.68 | S161 | 2.80 |
| S014 | 6.86 | S051 | 6.06 | S088 | 6.16 | S125 | 2.13 | S162 | 2.05 |
| S015 | 5.85 | S052 | 5.86 | S089 | 6.45 | S126 | 2.16 | S163 | 2.72 |
| S016 | 5.03 | S053 | 5.90 | S090 | 5.68 | S127 | 1.65 | S164 | 4.00 |
| S017 | 5.44 | S054 | 6.20 | S091 | 5.83 | S128 | 2.09 | S165 | 3.52 |
| S018 | 5.94 | S055 | 6.00 | S092 | 6.09 | S129 | 1.27 | S166 | 8.39 |
| S019 | 6.20 | S056 | 6.24 | S093 | 7.48 | S130 | 1.29 | S167 | 2.67 |
| S020 | 6.31 | S057 | 6.58 | S094 | 7.55 | S131 | 1.65 | S168 | 2.99 |
| S021 | 4.94 | S058 | 5.44 | S095 | 6.56 | S132 | 1.56 | S169 | 3.04 |
| S022 | 5.23 | S059 | 5.30 | S096 | 7.32 | S133 | 2.27 | S170 | 3.00 |
| S023 | 6.49 | S060 | 5.41 | S097 | 6.34 | S134 | 1.61 | S171 | 3.31 |
| S024 | 5.24 | S061 | 5.31 | S098 | 6.16 | S135 | 1.34 | S172 | 2.80 |
| S025 | 5.83 | S062 | 5.33 | S099 | 6.39 | S136 | 1.87 | S173 | 3.12 |
| S026 | 4.93 | S063 | 6.34 | S100 | 6.42 | S137 | 1.26 | S174 | 2.95 |
| S027 | 5.53 | S064 | 5.42 | S101 | 6.19 | S138 | 1.02 | S175 | 3.57 |
| S028 | 6.20 | S065 | 6.49 | S102 | 5.74 | S139 | 1.20 | S176 | 2.64 |
| S029 | 5.07 | S066 | 5.93 | S103 | 5.28 | S140 | 2.32 | S177 | 2.21 |
| S030 | 7.22 | S067 | 6.19 | S104 | 5.36 | S141 | 1.56 | S178 | 3.13 |
| S031 | 7.36 | S068 | 6.67 | S105 | 5.71 | S142 | 2.36 | S179 | 3.96 |
| S032 | 5.73 | S069 | 7.24 | S106 | 5.42 | S143 | 2.54 | S180 | 2.38 |
| S033 | 5.85 | S070 | 6.59 | S107 | 5.91 | S144 | 1.36 | S181 | 2.97 |
| S034 | 8.06 | S071 | 6.56 | S108 | 5.89 | S145 | 1.52 | S182 | 3.50 |
| S035 | 6.45 | S072 | 5.28 | S109 | 6.36 | S146 | 2.22 | S183 | 2.89 |
| S036 | 5.61 | S073 | 7.44 | S110 | 4.91 | S147 | 3.24 | S184 | 2.75 |
| S037 | 5.60 | S074 | 6.41 | S111 | 5.22 | S148 | 2.50 | S185 | 3.17 |
新窗口打开|下载CSV
2.2 近红外模型构建
采集的185份花生籽仁近红外光谱如图2所示, 建模样品近红光谱曲线趋势大致相同, 但不同样品的吸收峰强度不同。表明花生小样品杯扫描获得的近红外光谱图可以用于花生籽粒蔗糖含量的定量分析。对花生中蔗糖的化学值和采集的近红外光谱数据进行拟合光谱处理, 采用偏最小二乘法(PLS)的化学计量学方法建立数学模型, 反复采用内部交叉验证剔除异常值, 通过比较模型决定系数(R2)和均方差(MSD)衡量模型质量, 筛选最佳模型。去除异常值后, 176份样品建立模型的决定系数(R2)为0.962, 均方差(MSD)为0.383 (图3)。图2
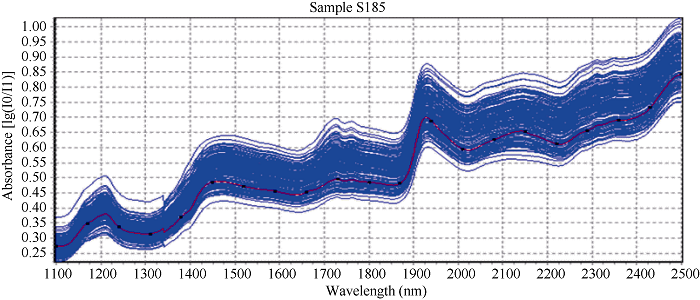 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2花生样品的近红外扫描光谱
Fig. 2NIR spectrums of peanut kernel samples
图3
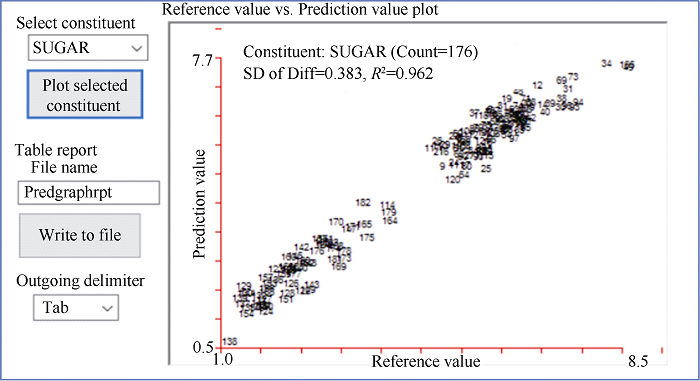 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3花生成熟籽仁蔗糖含量定标曲线决定系数
Fig. 3Determination coefficient of predicted sucrose content in peanut seed by near-infrared reflectance spectroscopy
2.3 模型的外部验证
利用所建立的定标模型, 用近红外分析仪和化学方法对20份没有参加定标的花生材料作为外部验证样品集, 检验模型预测效果。蔗糖含量的预测值与化学值的绝对偏差在-1.44%~0.72%之间, 决定系数(R2) 0.947, t = 0.233< t0.05 = 2.093 (图4), 相关性达到极显著水平, 表明利用这个模型得到的预测结果准确, 具有很高的可信度, 可用于花生籽仁蔗糖含量的快速鉴定。图4
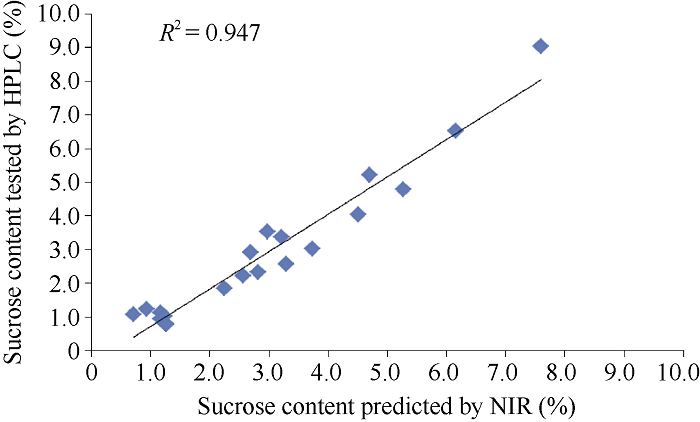 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4外部验证花生样品的蔗糖含量与测定化学值的相关性
Fig. 4Correlation coefficients of sucrose content between HPLC and NIR testing in the external peanut samples
2.4 模型在高含糖量、高油酸优良品系中的应用效果
2019年利用本研究建立的单株蔗糖含量近红外模型, 测定了“吉花02-1-4×中花26”的168个F6株系的蔗糖含量, 并经液相色谱分析, 获得蔗糖含量6.8%以上、油酸78%以上的优良株系(表3)。从表3可以看出, 获得的高蔗糖材料的含油量均低于48%, 最低的仅42%, 属于低油品系, 油酸含量均在78%以上, 属于高油酸材料。从图5可以看出, 本研究筛选出的6份高蔗糖、低含油量、高油酸品系在荚果大小、每荚果粒数、荚果和籽仁形状等食用品质相关的指标上也有很大差别, 具有培育成不同食用型花生品种的潜力。Table 3
表3
表3花生种子蔗糖含量近红外模型的验证
Table 3
| 样品编号 Sample ID | 化学值 Chemical value | 预测值 Prediction value | 偏差 Deviation | 样品编号 Sample ID | 化学值 Chemical value | 预测值 Prediction value | 偏差 Deviation |
|---|---|---|---|---|---|---|---|
| ST01 | 0.780 | 1.263 | 0.483 | ST11 | 2.220 | 2.563 | 0.343 |
| ST02 | 0.818 | 1.256 | 0.438 | ST12 | 2.320 | 2.810 | 0.490 |
| ST03 | 9.030 | 7.590 | -1.440 | ST13 | 6.520 | 6.152 | -0.368 |
| ST04 | 0.940 | 1.174 | 0.234 | ST14 | 2.570 | 3.290 | 0.720 |
| ST05 | 1.021 | 1.234 | 0.213 | ST15 | 2.910 | 2.696 | -0.214 |
| ST06 | 1.078 | 0.716 | -0.362 | ST16 | 3.020 | 3.730 | 0.710 |
| ST07 | 1.119 | 1.164 | 0.045 | ST17 | 3.370 | 3.213 | -0.157 |
| ST08 | 1.246 | 0.927 | -0.320 | ST18 | 3.540 | 2.982 | -0.558 |
| ST09 | 4.773 | 5.268 | 0.495 | ST19 | 4.045 | 4.518 | 0.473 |
| ST10 | 1.851 | 2.246 | 0.395 | ST20 | 5.206 | 4.706 | -0.500 |
新窗口打开|下载CSV
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5获得的高糖高油酸优良花生品系
Fig. 5Selected peanut lines with high sugar and high oleic acid content
Table 4
表4
表4获得的高蔗糖高油酸花生品系的主要性状
Table 4
| 品系名称 Lines name | 蔗糖含量 Sucrose content (%) | 含油量 Oil content (%) | 油酸 Oleate content (%) | 百果重 100-pod weight (g) | 百仁重 100-seed weight (g) | 出仁率 Shelling percentage (%) | 荚果长 Pod length (cm) | 籽仁长 Seed length (cm) |
|---|---|---|---|---|---|---|---|---|
| SYT5-1 | 8.45 | 46.8 | 79.6 | 212 | 67 | 76.4 | 5.1 | 1.6 |
| SYT7-1 | 7.63 | 45.3 | 80.2 | 204 | 56 | 76.6 | 4.6 | 1.5 |
| SYT3-1 | 7.12 | 44.6 | 78.6 | 162 | 76 | 70.3 | 3.9 | 1.8 |
| SYT3-2 | 8.02 | 47.8 | 81.5 | 176 | 80 | 74.9 | 3.7 | 1.8 |
| SYT4-1 | 7.88 | 45.3 | 79.8 | 159 | 67 | 76.7 | 3.0 | 1.5 |
| SYT4-2 | 9.07 | 42.2 | 81.1 | 168 | 80 | 78.6 | 3.2 | 1.5 |
新窗口打开|下载CSV
3 讨论
近年来, 花生油用和食用品质及其遗传改良受到越来越多的关注, 品质指标从最初的含油量、蛋白质含量逐步扩展到脂肪酸、氨基酸、蔗糖含量、白藜芦醇含量等。虽然这些品质指标均建有精准的化学测定方法, 但普遍费时费力, 因而推动了近红外技术在花生品质改良上的应用, 针对不同的近红外仪型号, 国内建立了多个品质性状的近红外模型[13-14,16,18]。同时, 模型检测需要的花生籽仁量越来越少, 甚至建立了单粒籽仁主要脂肪酸含量的近红外模型[15,19], 模型预测结果的准确性也越来越好, 显著提升了品质育种效率。本研究利用美国Unity公司的近红外仪, 建立了小样品杯的蔗糖含量预测模型, 能对杂交后代早期的单株籽仁进行检测, 并具有较好的预测结果, 是对已有蔗糖含量模型的改进和丰富[20,21]。为更好地进行蔗糖含量的预测和种子纯度分析, 在以后的工作中, 还需要建立单粒花生蔗糖含量的近红外模型。利用一个近红外仪对多个品质指标进行跟踪检测已经成为可能。如本研究就同时利用了含油量、油酸含量、含糖量3个近红外检测模型对同一样品进行检测, 获得了兼具高含糖量、低含油量、高油酸的优良食用型花生新品系, 这得益于当前花生重要品质参数近红外模型越来越丰富。随着花生品质研究的深入, 一些含量不高、目前关注较少、具有重要价值的品质性状也需要逐步建立近红外预测模型。
本研究在2个蔗糖含量并不很高的花生品种吉花02-1-4 (4.0%)和中花26 (2.3%)的杂交后代中筛选出一批含糖量超过7%的优良品系, 蔗糖含量表现出明显的超亲现象, 推测可能的原因是不同材料中控制蔗糖含量的位点不同, 杂交聚合产生了超亲效应所致。同时, 我们也看到, 蔗糖含量高的品系普遍含油量显著降低, 说明这一杂交组合中产生蔗糖含量超亲的后代与含油量合成受阻直接或间接相关, 因此, 深入研究高糖、低油品系形成的分子基础也十分必要。另外, 虽然成熟花生种仁的蔗糖含量与总含糖量及甜度相关, 但成熟花生种仁中还存在葡萄糖、果糖等其他糖类以及影响甜度的单宁等苦味成分, 这些成分对甜度亦有一定的正向和负向影响, 食用型花生的甜味及适口性评价仍需在蔗糖含量测定的基础上, 由经过训练的人员进行描述性感官风味分析[3,5]。
4 结论
本研究利用籽仁蔗糖含量差异显著的花生材料, 构建了花生籽仁蔗糖含量的小样品杯近红外定标模型, 模型的决定系数R2 = 0.962, 验证样品集的决定系数为R2 = 0.947, 可以较好地预测花生籽仁的蔗糖含量。利用该模型配合含油量、油酸含量近红外模型, 高效的在“吉花02-1-4×中花26”杂交后代群体中发掘出6份高蔗糖、低油、高油酸、农艺性状优良的食用型花生新品系。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.3146/i0095-3679-25-2-2URL [本文引用: 3]
DOI:10.1021/jf9910739URLPMID:10725144 [本文引用: 2]

Carbohydrates are known to be important precursors in the development of roasted peanut quality. However, little is known about their genotypic variation. A better understanding of the role of carbohydrates in roasted peanut quality requires first an understanding of the genotypic variation in the soluble carbohydrate components. Ion exchange chromatography was used to isolate 20 different carbohydrate components in 52 genotypes grown in replicated trials at two locations. Inositol, glucose, fructose, sucrose, raffinose, and stachyose were quantitated, and 12 unknown peaks were evaluated on the basis of the peak height of the unknown relative to the cellobiose internal standard peak height. Peaks tentatively identified as verbascose and ajugose could not be properly integrated because of tailing. Of the 18 carbohydrates that were estimated, 9 exhibited significant variation between test environments, 5 among market types, 14 among genotypes within market types, and 11 exhibited some significant form of genotype x environment interaction. Genotypes accounted for 38-78% of the total variation for the known components, suggesting that broad-sense heritability for these components is high. The observed high genotypic variation in carbohydrate components is similar to the high genotypic variation observed for the sweetness attribute in roasted peanuts, which raises the question regarding possible interrelationships. The establishment of such interrelationships could be most beneficial to peanut breeding programs to ensure the maintenance of flavor quality in future peanut varieties.
DOI:10.1021/jf9910741URLPMID:10725145 [本文引用: 3]

Certain roasted peanut quality sensory attributes have been shown to be heritable. Currently the only means of measuring these traits is the use of a trained sensory panel. This is a costly and time-consuming process. It is desirable, from a cost, time, and sample size perspective, to find other methodologies for estimating these traits. Because sweetness is the most heritable trait and it has a significant positive relationship to the roasted peanut trait, the possible relationships between heritable sensory traits and 18 carbohydrate components (inositol, glucose, fructose, sucrose, raffinose, stachyose, and 12 unknown peaks) in raw peanuts from 52 genotypes have been investigated. Previously reported correlations among sweet, bitter, and roasted peanut attributes were evident in this study as well. Where there was positive correlation of total sugars with sweetness, there also was positive correlation of total sugars with roasted peanut attribute and negative correlation of total sugars with bitterness and astringency. The expected generalized relationship of total sugars or sucrose to sweetness could not be established because the relationship was not the same across all market-types. Further work is needed to determine the nature of the chemical components related to the bitter principle, which appear to modify the sweet response and interfere with the sensory perception of sweetness, particularly in the Virginia market-type. Also, certain carbohydrate components showed significant relationships with sensory attributes in one market-type and not another. These differential associations demonstrate the complexity of the interrelationships among sweet, bitter, and roasted peanut sensory attributes. Within two market-types it is possible to improve the efficiency of selection for sweetness and roasted peanut quality by assaying for total carbohydrates. On the basis of the regression values the greatest efficiency would occur in the fastigiate market-type and then the runner.
DOI:10.1021/jf035465yURLPMID:15137870 [本文引用: 1]

Peanut seeds contain approximately 50% oil on a dry weight basis, making them a high fat food. Reduction of the oil content would make peanuts a more desirable food to fat conscious consumers. Removal of existing oil by processing is not feasible for in-shell peanuts, the dominant product of the North Carolina-Virginia area. To reduce oil content in in-shell peanuts, a genetic solution must be found. However, while reduced oil content is a desirable objective, changes in oil must not be accompanied by significant decreases in any of the desirable aspects of peanut flavor. Because the impact of selection for low or high oil on flavor is not known, it would be useful to know in what form dry matter is being stored in the seed, particularly if it is not being stored as oil. Screening of 584 accessions identified two lines (PI 269723 and PI 315608) with high and two (Robusto 2 and Robusto 3) with low oil contents, each pair differing in sugar content. The four parents were crossed in diallel fashion to investigate patterns of inheritance. General combining abilities (GCA) for oil content closely followed values of the parental lines. One low oil parent (Robusto 2) had a correspondingly elevated GCA for sugar content, but neither low oil parent had the effect of elevating starch in progeny. Reciprocal cross differences were found for starch and sugar contents, suggesting influences of cytoplasmic genes on those traits. These lines serve as resource material for researchers interested in the genetic and physiological aspects of the oil-sugar-starch relationship in peanuts.
DOI:10.1016/j.indcrop.2013.08.050URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1002/(ISSN)1097-0010URL [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
DOI:10.1255/jnirs.559URL [本文引用: 2]
DOI:10.2135/cropsci2006.01.0031URL [本文引用: 2]
[本文引用: 2]
[本文引用: 2]
DOI:10.1071/AR9610001URL [本文引用: 1]
[本文引用: 2]
[本文引用: 2]
DOI:10.3724/SP.J.1006.2019.94016URL [本文引用: 2]

R 2)为0.907, 均方差为3.463; 亚油酸模型的决定系数为0.918, 均方差为2.824; 棕榈酸模型的决定系数为0.824, 均方差为0.782。使用100粒花生验证该模型的准确性, 结果油酸、亚油酸和棕榈酸的近红外预测值与化学值的相关系数分别为0.88、0.90和0.71, 表明此模型可以准确地预测单粒花生中这3种脂肪酸的含量。本研究借助该模型建立了一种不依赖分子标记的快速、高效选育高油酸花生的方法, 并成功应用于高油酸花生育种, 选育出高油酸花生品种中花215。]]>
[本文引用: 2]
DOI:10.7505/j.issn.1007-9084.2016.05.018URL [本文引用: 3]

对72份国内外优质食用花生种质资源进行花生籽仁蔗糖含量的化学测定,利用瑞典波通DA7200 型近红外分析仪采集近红外光谱,采用偏最小二乘法(Modified PLS)建立近红外光谱定标模型,以寻找花生籽仁蔗糖含量的快速测定方法。本研究结果表明:所建模型中蔗糖含量的定标决定系数为0.822,定标标准误差为0.386,决定系数较高,误差较小,表明该模型代替化学分析进行花生籽仁蔗糖含量的测定是可行的。
[本文引用: 3]
[本文引用: 3]
[本文引用: 3]
[本文引用: 1]
[本文引用: 1]
