 ,, 涂卫, 王海波, 应静文, 杜鹃, 赵喜娟, 赵庆浩, 黄维, 蔡兴奎
,, 涂卫, 王海波, 应静文, 杜鹃, 赵喜娟, 赵庆浩, 黄维, 蔡兴奎 ,*, 宋波涛
,*, 宋波涛 ,*华中农业大学园艺林学学院 / 园艺植物生物学教育部重点试验室 / 农业农村部马铃薯生物学与生物技术重点试验室, 湖北武汉 430070
,*华中农业大学园艺林学学院 / 园艺植物生物学教育部重点试验室 / 农业农村部马铃薯生物学与生物技术重点试验室, 湖北武汉 430070Establishment of a high efficient method for chromosome doubling and exploration of cold-resistant resources in potato
DONG Jian-Ke ,, TU Wei, WANG Hai-Bo, YING Jing-Wen, DU Juan, ZHAO Xi-Juan, ZHAO Qing-Hao, HUANG Wei, CAI Xing-Kui
,, TU Wei, WANG Hai-Bo, YING Jing-Wen, DU Juan, ZHAO Xi-Juan, ZHAO Qing-Hao, HUANG Wei, CAI Xing-Kui ,*, SONG Bo-Tao
,*, SONG Bo-Tao ,*College of Horticulture and Forestry Sciences, Huazhong Agricultural University / Key Laboratory of Horticultural Plant Biology (HZAU), Ministry of Education / Key Laboratory of Potato Biology and Biotechnology, Ministry of Agriculture and Rural Affairs, Wuhan 430070, Hubei, China
,*College of Horticulture and Forestry Sciences, Huazhong Agricultural University / Key Laboratory of Horticultural Plant Biology (HZAU), Ministry of Education / Key Laboratory of Potato Biology and Biotechnology, Ministry of Agriculture and Rural Affairs, Wuhan 430070, Hubei, China通讯作者:
收稿日期:2020-03-19接受日期:2020-07-2网络出版日期:2020-11-12
| 基金资助: |
Received:2020-03-19Accepted:2020-07-2Online:2020-11-12
| Fund supported: |
作者简介 About authors
E-mail:

摘要
关键词:
Abstract
Keywords:
PDF (1940KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
董建科, 涂卫, 王海波, 应静文, 杜鹃, 赵喜娟, 赵庆浩, 黄维, 蔡兴奎, 宋波涛. 马铃薯高效染色体加倍方法建立与抗寒资源创制[J]. 作物学报, 2020, 46(11): 1659-1666. doi:10.3724/SP.J.1006.2020.04073
DONG Jian-Ke, TU Wei, WANG Hai-Bo, YING Jing-Wen, DU Juan, ZHAO Xi-Juan, ZHAO Qing-Hao, HUANG Wei, CAI Xing-Kui, SONG Bo-Tao.
随着农业产业结构不断调整和马铃薯主粮化战略的实施, 马铃薯的需求量日益增大, 种植的范围与面积也将进一步扩大, 而低温霜冻是当前马铃薯产业发展面临的主要胁迫之一。自然界中的普通马铃薯栽培种在抗寒方面几乎不存在遗传变异, 均对霜冻敏感且无驯化能力, 野生种中则存在丰富的抗寒资源, 已报道Solanum acaule、S. commersonii、S. demissum、S. chomatophilum、S. boliviense等35个野生种中均有抗寒耐低温株系的存在[1,2]。但这些野生种大多数自交不亲和, 且高度杂合, 它们等位基因多样性对最大限度地提高栽培种马铃薯的杂合性、扩大栽培种遗传资源、将野生种的遗传多样性和目标性状导入栽培种具有非常重要的作用[3,4]。但野生资源中多为二倍体, 约占75% [5,6], 由于倍性差异难以杂交成功, 倍性水平的调节为解决这一难题提供了技术与材料基础。
Johnstone[7]利用0.5%的秋水仙素在25℃培养箱中处理包括S. acaule、S. commersoni、S. demissum、S. fendleri、S. jamesii、S. maglia和S. neoantipovichii在内的共13个野生种, 处理时间3~5 d, 直到每批处理种子种皮破裂长出幼芽, 发芽种子种植后观察叶片气孔大小和植株形态, 保留具有明显变异的材料, 进一步结合花粉粒大小和染色体计数鉴定植株倍性, 最终成功得到S. neoantipovichii、S. andigenum、S. chacoense和S. jamesii野生种的体细胞染色体加倍材料, 但加倍率极低。Karp等[8]利用秋水仙素分别对马铃薯单倍体和二倍体材料带腋芽茎段进行染色体加倍处理, 获得马铃薯双单倍体和双二倍体材料, 证明利用秋水仙素进行马铃薯体细胞染色体加倍对于纯化马铃薯基因型是简单有效的。张艳萍等[9]利用秋水仙素诱导马铃薯野生种S. acaule加倍, 利用0.3%秋水仙素浓度处理14 d, 能获得最大诱导率31.6%, 加倍材料克服了由于胚乳平衡数影响造成的杂交败育现象。但这些研究都存在其操作难度大、加倍率低、加倍处理时间长等问题, 因此, 建立高效地马铃薯染色体加倍技术体系显得尤为重要。
本试验在马铃薯二倍体野生种S. boliviense抗寒性鉴定基础上, 通过与二倍体栽培种杂交获得了一批具有S. boliviense抗寒资源的新种质材料, 但这些材料倍性鉴定结果均为二倍体, 由于自然界栽培种材料大多为四倍体, 很难直接应用到育种中来。本研究通过利用秋水仙素结合摇床转动的处理方式对上述二倍体材料茎段进行了染色体加倍处理, 探索适合马铃薯染色体加倍的高效技术方法, 以期为马铃薯倍性操作提供理论依据和技术支持。
1 材料与方法
1.1 试验材料
本研究所用材料包括AC142、BLV29-2、E3和FT073-4共4份马铃薯材料, 其中加倍处理材料FT073-4是二倍体抗寒野生种S. boliviense与二倍体栽培种S. tuberosum的种间杂种, 其加倍后材料命名为T-FT073-4-X (X为株系编号); AC142和E3分别为二倍体和四倍体栽培种对照, 且对低温霜冻敏感; BLV29-2 (S. boliviense)为耐低温霜冻的野生种。所有材料均来自于华中农业大学农业农村部马铃薯生物学与生物技术重点实验室种质资源库。采用组织培养的方法进行材料茎段扩繁, 在无菌工作台上将带芽茎段通过扦插的方式, 转入新的装有培养基的培养盒内保存备用。所用培养基为普通MS培养基, 培养基含糖量为4%, 将扩繁好的组培苗在植物组织培养室内(16 h光照/8 h黑暗, 20℃/18℃, 湿度50% ± 10%)培养4周, 用于后期加倍处理。1.2 染色体加倍方法
利用剪孔的PCR板和组培盒自行设计加倍处理装置, PCR板起支撑作用, 避免处理过程中茎段浸没造成无氧呼吸。染色体加倍所用培养基分为固体培养基和液体培养基, 固体培养基在液体培养基基础上加琼脂8 g L-1, 用于秋水仙素处理后的恢复生长。液体培养基高温灭菌后在超净工作台上加入过滤灭菌的秋水仙素母液, 根据设置浓度梯度使最终秋水仙素浓度分别为0.05% (A)、0.1% (B)、0.25% (C)和0.5% (D)后分装到培养盒中, 液体培养基厚度不超过PCR板高度, 用于材料加倍处理。选取生长4周左右长势健壮的组培苗进行加倍处理, 在超净工作台上剪去植株顶端, 剪取第2到第4节带叶片单节间转入含不同秋水仙素浓度的液体培养基的PCR板孔中, 剪取茎段长度约1.5 cm, 使茎段基部完全浸没, 腋芽和叶片在PCR板面位置略高于液体培养基表面, 每个组培盒内放置12个处理茎段, 每个处理组合4个重复, 操作完成后盖上组培盒盖, 标记处理组合名称, 放入组培室(16 h光照/8 h黑暗, 20℃/18℃, 湿度50% ± 10%), 处理时间梯度依次为3、5、7和10 d, 同时设置不同处理方式, 一部分材料置于转速120转min-1摇床上, 一部分放置于转速为0, 即不转动摇床上。到达处理时间后, 在超净工作台用灭菌水清洗处理茎段2~3次, 洗干净茎段表面的秋水仙素残留, 转入正常固体MS培养基, 继续放入植物培养室, 后期进行倍性检测, 试验处理以不加秋水仙素其他操作完全一致的处理方式作为对照。统计处理材料的成活率和加倍率, 成活率=处理成活株系数/总处理株系数, 加倍率=检测加倍株系数/处理后成活株系数。1.3 倍性鉴定
采用流式细胞仪和染色体计数2种方法进行倍性鉴定。取植株顶端新鲜的功能叶片, 参照Dpoole?el等[10]的原理和方法, 以马铃薯二倍体AC142和四倍体E3材料为对照, 使用BD FACSVerse 细胞分析仪(上海微速生物科技有限公司), 试剂为Cystain UV Precise T试剂盒进行倍性鉴定。参考Zhao等[11]的原理和方法, 用荧光显微镜观察染色体制片, 选取具有较多中期染色体分裂相、分散良好且很少出现细胞质背景的视野照相, 每个株系选取至少30个视野进行卡方检测。染色体计数卡方检测公式:$x^{2}=\sum_{i=1}^{n}\frac{(A-T)^{2}}{T}$
式中, A代表实际观察染色体数, T代表理论染色体数, i代表检测次数。
1.4 农艺学性状统计
于华中农业大学内国家蔬菜改良中心华中分中心的塑料大棚统计加倍材料田间植物学性状, 每份材料种植5钵。具体种植方法为: 组培苗首先在植物组织培养室生长4周左右后, 玻璃温室内炼苗2 d, 移栽到20 cm×30 cm的塑料钵中, 栽培基质为山东商道生物科技有限公司生产的育苗基质, 并在相应的时期完成相关性状的鉴定,参考Ugborogho等[12]的方法统计农艺学性状和鉴定花粉活力。1.5 抗寒性检测方法
将生长4周左右的组培苗经炼苗后, 移栽到10 cm×10 cm的塑料钵中, 置于植物生长室(16 h光照/8 h黑暗, 20℃/18℃, 湿度50% ± 10%)内生长3~4周后用于抗寒能力LT50鉴定。室内电导率LT50鉴定方法釆用Steffen等[13]并加以适当的修改。LT50计算方法为: 将每个温度点的3次电导率平均值与相应的温度点用Logistic方程分析, 该材料的半致死温度点即为方程的拐点。y = l/(l + eb(c - x)), 其中, y代表相对电导率; x代表处理的温度; b代表方程在拐点c的斜率; c代表LT50值。1.6 数据处理
使用Microsoft Excel 2007整理与分析试验数据, 使用GraphPad Prism 8作图和进行差异显著性分析, 使用PowerPoint 2007组合图片。2 结果与分析
2.1 加倍处理对植株长势的影响
对FT073-4株系进行加倍处理。根据不同处理组合, 先处理对照组(CK), 再按浓度从低到高处理试验组。由图1可知, 对照组植株长势良好, 且气生根增多, 表明装置及处理方式不影响植株生长。秋水仙素处理浓度、处理时间、处理方式均严重影响植株生长状态。相同处理浓度相同处理时间, 不同处理方式转速为0转 min-1比转速120转 min-1植株生长更快; 相同处理浓度相同处理方式, 不同处理时间对植株生长影响差异很大, 处理时间越长, 秋水仙素对植株胁迫越严重, 植株生长越慢; 在处理方式及处理时间相同的情况下, 处理浓度越高, 植株生长受抑制情况越严重, 植株生长越缓慢。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1加倍处理装置及对照处理植株长势
A: 加倍处理装置, 红色箭头所指位置为PCR板剪孔位置; B: 对照处理植株长势。
Fig. 1Chromosome doubling processing devices and plants growth of control
A: devices of chromosome doubling treatment; the red arrows refer to the position of PCR plate shear hole. B: plants growth of the control.
2.2 加倍处理材料成活率统计
随着处理时间的延长, 成活率降低, 当茎段处理时间达到7 d时, 植株成活率明显下降, 当处理时间达到10 d时, 几乎没有株系存活; 植株成活率同时受秋水仙素处理浓度和处理方式的影响, 随着处理浓度的增高, 植株成活率明显降低, 0.05%、0.10%浓度时植株成活率差异不大, 但浓度提高到0.25%时, 成活率明显降低, 0.5%浓度时, 很少有株系存活; 当处理材料以120转 min-1转动时, 植株成活率明显低于不转动(0转 min-1)的处理材料, 表明转动使马铃薯茎段与秋水仙素接触更充分, 对茎段生长影响更严重(图2)。图2
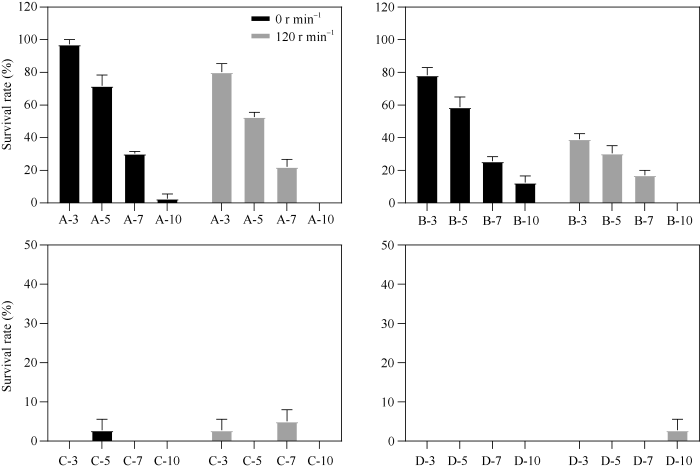 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2秋水仙素处理材料成活率统计
数值为平均值±SE (n=4)。A-3: A浓度(0.05%)处理3 d; B-3: B浓度(0.10%)处理3 d; C-3: C浓度(0.25%)处理3 d; D-3: D浓度(0.50%)处理3 d。
Fig. 2Statistics of survival rate of colchicin treated materials
Values are means ± standard errors (SE) (n=4). A-3: A concentration at 0.05% treatment for three days; B-3: B concentration at 0.10% treatment for three days; C-3: C concentration at 0.25% treatment for three days; D-3: D concentration at 0.50% treatment for three days.
2.3 加倍材料倍性检测及加倍率统计
大部分材料倍性没有改变, 与二倍体对照AC142一致, 峰值为50 (图3-C), 处理材料中出现峰值为100的四倍体材料(图3-D), 后期进一步进行染色体计数检测。同时在检测材料中出现一些三倍体材料(图3-E)以及嵌合体材料(图3-F, G)。对照处理FT073-4流式细胞仪检测和染色体计数结果一致, 均为二倍体(图3-H和图4-A)。图3
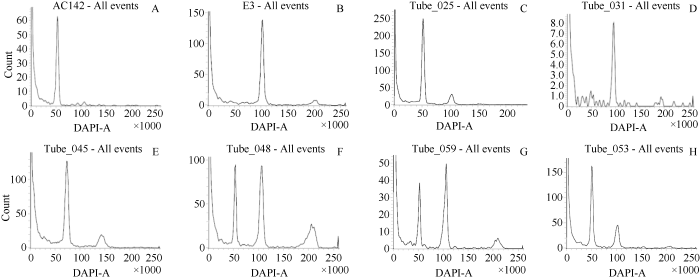 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3加倍处理材料倍性鉴定
A: AC142; B: E3; C: 未加倍材料; D: 四倍体; E: 三倍体; F, G: 嵌合体; H: 对照。
Fig. 3Identification of ploidy of treated materials
A: AC142; B: E3; C: no doubling materials; D: tetraploid; E: triploid; F, G: chimaera; H: control.
图4
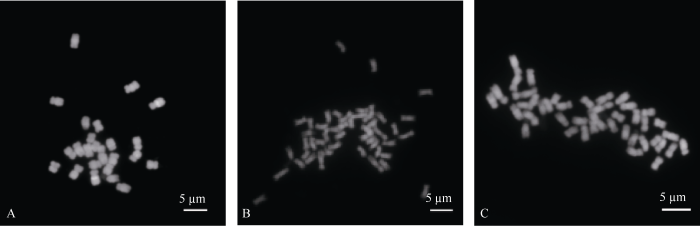 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4加倍材料染色体数目检测
A: 对照FT073-4; B, C: 加倍四倍体材料。
Fig. 4Detection of chromosome number for doubling materials
A: FT073-4 as the control; B, C: doubling tetraploid materials.
对流式细胞仪检测加倍为四倍体的株系进行染色体计数分析, 部分材料染色体计数视野如图4所示。加倍处理材料染色体数目确实由24条变为48条(1.00≤c2 5.77; df = 29, c20.05 = 42.56), 表明植株倍性发生变化, 由二倍体加倍到四倍体。
由图5可知, 在秋水仙素浓度为0.05%条件下, 7 d之内处理时间越长加倍率越高; 0.1%浓度条件下, 植株成活率降低, 但加倍株系数目和0.05%浓度大体一致, 因此加倍率高于0.05%浓度, 便于材料筛选, 减少工作量; 0.25%、0.50%浓度条件下, 材料成活率极低, 不易作为染色体加倍处理浓度, 加倍率统计结果不予列出。结合加倍率统计结果, 总体上转动能提高植株加倍效率。所有处理组合中3个处理组合加倍率达到30%以上, 但A-7-0和B-5-120两种处理组合嵌合体比例较高, 占加倍材料的40%。因此建议采用120转 min-1转速条件下以B-3的处理方式进行马铃薯加倍处理, 材料加倍率高达33%, 且四倍体比例占80%。
图5
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5秋水仙素处理材料加倍率统计
数值为平均值±SE (n=4)。A-3-0: A浓度(0.05%)处理3 d, 转速为0转 min-1; A-3-120: A浓度(0.05%)处理3 d, 转速为120转 min-1; B-3-0: B浓度(0.10%)处理3 d, 转速为0转 min-1; B-3-120: B浓度(0.10%)处理3 d, 转速为120转 min-1。
Fig. 5Statistics of doubling rate of colchicin treated materials
Values are means ± standard errors (SE) (n=4). A-3-0: A concentration at 0.05% treatment for three days with 0 r min-1; A-3-120: A concentration at 0.05%) treatment for three days with 120 r min-1; B-3-0: B concentration at 0.10% treatment for three days with 0 r min-1; B-3-120: B concentration at 0.10% treatment for three days with 120 r min-1.
2.4 加倍株系农艺性状统计
将加倍株系种植到植物生长室, 观察植株表型变化发现, 加倍株系株高明显增高, 茎粗变粗, 叶茸毛增多, 但在花药形状、柱头长度等方面没有明显变化。在荧光显微镜下观察花粉粒大小, 并选取不少于20个视野进行花粉活力统计, 表明加倍后四倍体花粉粒大小显著增大, 花粉活力与加倍前大致相当(图6)。图6
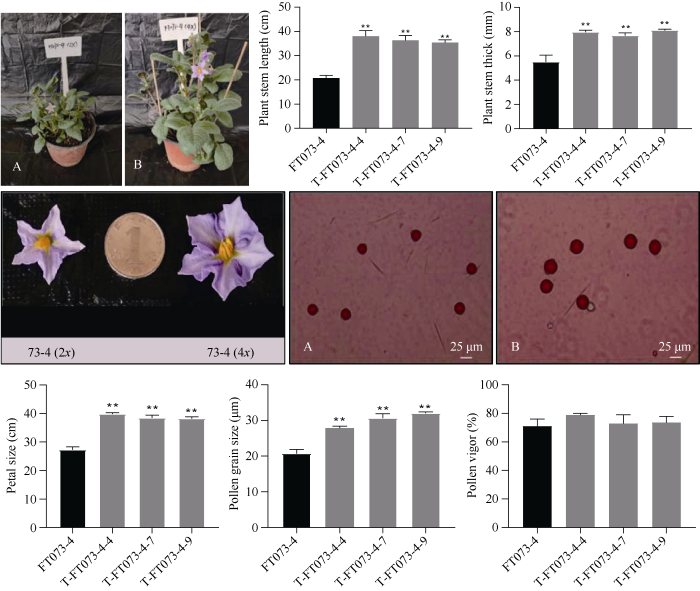 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6部分加倍株系植株表型
A: FT073-4; B: T-FT073-4-7。数值为平均值±SE (n ≥ 5)。* 表示在0.05水平上显著相关, ** 表示在0.01水平上显著相关。
Fig. 6Agronomic traits of some doubling strains
A: FT073-4; B: T-FT073-4-7. Values are means ± standard errors (SE) (n ≥ 5). * and ** mean significant difference at the 0.05 and 0.01 probability levels, respectively.
由图7可知, 加倍前后材料在抗寒能力方面没有明显差异, 但较栽培种AC142、E3抗寒能力均显著提高。
图7
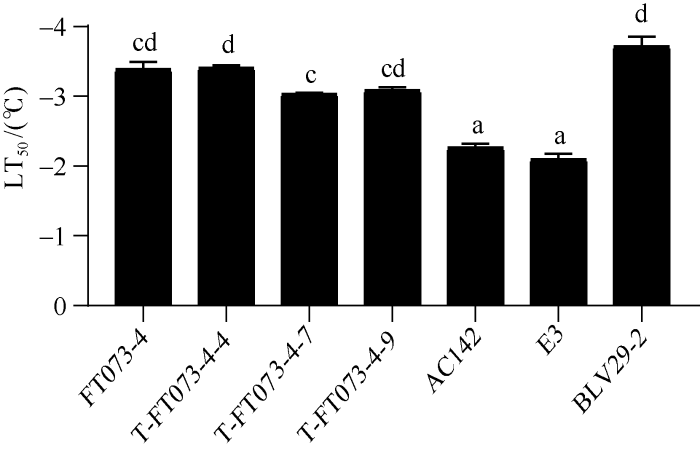 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图7加倍材料抗寒能力检测
数值为平均值±SE (n=3)。柱子上方的字母表示在P < 0.05水平上的差异显著性。
Fig. 7Detection of freezing tolerance for doubling materials
Values are means ± standard errors (SE) (n=3). Different letters indicate significant difference at P < 0.05.
3 讨论
多倍体化在动植物进化过程中普遍发生, 植物多倍体化过程中, 细胞核内染色体数目成倍增加, 植物体形态及营养器官普遍增大, 糖、蛋白质、氨基酸和维生素等营养物质含量升高, 植物对环境的适应能力及逆境的抵抗能力等大幅提高[14,15,16,17]。De Maine等[18]利用秋水仙素处理马铃薯双单倍体材料, 成功获得加倍后的四倍体材料, 但其中大部分为嵌合体材料, 这些材料的L1、L2和L3胚层中四倍体所占比例各不相同。在生长和繁殖过程中, 倍性变化依赖于2x细胞和4x细胞的竞争关系, 嵌合体在育种过程中的倍性分离会给遗传育种研究带来不利影响[19]。本试验在前人研究基础上对马铃薯茎段加倍处理期间, 利用120转 min-1转速转动处理, 加倍率得到提高, 且处理所需时间缩短, 有效提高了工作效率, 同时加倍材料中嵌合体较少, 四倍体比列高达80%, 表明成活率随秋水仙素处理浓度增高, 处理时间延长, 成活率下降, 这与前人研究结果一致[20,21]。加倍处理期间利用摇床转动处理茎段能提高加倍率的可能原因是, 转动使秋水仙素与处理茎段接触更充分, 导致成活率下降的原因是由于秋水仙素接触越充分, 其本身对茎段的毒害作用越明显, 使得处理材料成活率降低。对加倍后株系农艺学性状统计结果表明, 加倍株系植株均生长正常, 且在株高、茎粗、花瓣大小和花粉粒大小等方面较原始二倍体株系显著增强, 没有生长发育不良和畸形植株的产生, 且加倍材料花粉活力都在70%以上, 说明该方法是一个较为稳定有效的资源创制方法。马铃薯栽培种中缺乏抗寒资源, 研究人员通过多种方法包括体细胞融合和染色体加倍等技术, 将不能和栽培种直接杂交的野生种的抗寒性导入栽培种中,这些育种方法的应用极大丰富了栽培种的抗寒性资源。Preiszner等[22]通过细胞融合技术获得了马铃薯四倍体栽培种与二倍体野生种(S. brevidens)叶肉原生质体融合材料, 材料中出现具有中等抗寒能力的体细胞融合杂种。Cardi等[23]利用体细胞融合的方法, 将二倍体S. commersonii的抗寒性状成功导入二倍体栽培种中, 融合后代出现倍性分离, 70%的四倍体和30%的六倍体, 体细胞杂种植株表现出很强的直接抗寒能力和驯化能力。邹莹[24]利用S. malmeanum 和栽培种二倍体为材料, 通过体细胞融合的方法, 获得了抗寒性明显增强的融合后代。徐志君[25]利用秋水仙素成功加倍二倍体马铃薯野生种S. chacoense, 通过种间杂交, 将S. chacoense遗传资源导入栽培种。本试验室在前期二倍体野生种S. boliviense抗寒性鉴定的基础上, 将其与栽培种杂交, 获得了二倍体种间杂种FT073-4, 通过染色体加倍的方法, 得到了四倍体T-FT073-4-X的多个株系, 获得具有S. boliviense血缘的马铃薯四倍体种间杂种材料, 杂种材料在植株长势、花粉育性等方面表现优良, 且抗寒能力较普通栽培种明显增强, 这为抗寒育种提供了良好的基础材料。同时, 野生种S. boliviense具有抗根结线虫、镰刀菌、茎软腐病等多种潜在利用价值[26,27,28], 通过进一步的性状鉴定, 也可为这些性状的遗传改良提供重要的基因资源。
4 结论
本研究通过探索不同处理条件下马铃薯试管苗茎段加倍效率, 获得了适合马铃薯染色体加倍的技术方法, 同时通过染色体加倍创制了一批具有野生种S. boliviense遗传背景的新抗寒种质资源, 为马铃薯抗寒育种提供了丰富的基础材料。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1007/BF02874375URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/s11540-006-9002-5URL [本文引用: 1]

The genetic resources available for the improvement of the cultivated potato (Solanum tuberosum) are reviewed along with progress in their utilisation. The conclusions are as follows. The wild and cultivated species of potato have been utilised in potato breeding to good effect, but only a very small sample of the available biodiversity has been exploited. New knowledge and technology will open possibilities for much greater use of these genetic resources in breeding. The strategy for utilising the cultivars native to Latin America will either be the introgression of desirable genes or the direct use of parents from improved populations, depending on how far modern S. tuberosum cultivars have genetically diverged from them and the extent to which S. tuberosum cultivars have been improved in the process. Molecular marker-assisted selection will be used for faster introgression of desirable genes from wild species, and the possibility exists of moving genes directly from wild species to cultivated potato with transgenic methods. New cultivars will continue to come from crosses between pairs of parents with complementary features but adapted to local growing conditions. However, increasingly these parents will possess desirable genes which have been introgressed from wild species and may also be from complementary groups of cultivated germplasm to exploit hybrid vigour. Successful cultivars may be genetically modified, if consumers see benefits in the use of the technology, to introduce genes not present in cultivated potatoes and their wild relatives to achieve novel biochemistry and further desirable improvements.]]>
DOI:10.1007/s12229-014-9146-yURL [本文引用: 1]

The common potato, Solanum tuberosum L., is the third most important food crop and is grown and consumed worldwide. Indigenous cultivated (landrace) potatoes and wild potato species, all classified as Solanum section Petota, are widely used for potato improvement. Members of section Petota are broadly distributed in the Americas from the southwestern United States to the Southern Cone of South America. The latest comprehensive taxonomic treatment of section Petota was published by John (Jack) Hawkes in 1990; it recognized seven cultivated species and 228 wild species, divided into 21 taxonomic series. Since 1990, intensive field collections from throughout the range of the group, coupled with morphological and molecular studies, have halved the number of species and elucidated new ingroup and outgroup relationships. The recent sequencing of the potato genome has greatly accelerated investigation of all aspects of potato biology and allows us to address new questions not conceivable before. The purpose of this review is to provide a historical overview and update since 1990 of the systematics, diversity, genetics, domestication, evolution, and breeding of Solanum section Petota that will serve as a reference for the next generation of studies in the potato.
DOI:10.1111/aab.2005.146.issue-1URL [本文引用: 1]
DOI:10.1007/BF02861918URL [本文引用: 1]
DOI:10.1007/BF00043089URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/BF02907241URL [本文引用: 1]
[本文引用: 1]
DOI:10.1002/fedr.v103:5/6URL [本文引用: 1]
[本文引用: 1]
DOI:10.1086/284115URL [本文引用: 1]
DOI:10.1007/BF02357667URL [本文引用: 1]
URLPMID:26715561 [本文引用: 1]
DOI:10.3390/f9110728URL [本文引用: 1]
DOI:10.1007/BF02356150URL [本文引用: 1]
URL [本文引用: 1]

体细胞染色体加倍是"分解-综合育种"方案的重要环节之一,随着科学技术的发展,染色体加倍技术已由秋水仙素加倍法、组织培养加倍法发展到细胞融合加倍法.本文简述了各种染色体加倍方法及影响因素、存在的问题和发展前景.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
PMID:24190212 [本文引用: 1]
DOI:10.1007/BF00223764URLPMID:24190212 [本文引用: 1]

Somatic fusion of mesophyll protoplasts was used to produce hybrids between the frost-tolerant species Solanum commersonii (2n=2x=24) and dihaploid S. tuberosum (2n=2x=24). This is a sexually incompatible combination due to the difference in EBN (Endosperm Balance Number, Johnston et al. 1980). Species with different EBNs as a rule are sexually incompatible. Fifty-seven hybrids were analysed for variation in chromosome number, morphological traits, fertility and frost tolerance. About 70% of the hybrids were tetraploid, and 30% hexaploid. Chloroplast counts in stomatal guard cells revealed a low frequency of cytochimeras. The frequency of aneuploids was relatively higher at the hexaploid level (hypohexaploids) than at the tetraploid level (hypotetraploids). The somatic hybrids were much more vigorous than the parents, and showed an intermediate phenotype for several morphological traits and moderate to profuse flowering. Hexaploid hybrid clones were less vigorous and had a lower degree of flowering than the tetraploid hybrid clones. All of the hybrids were female fertile but male sterile except for one, which was fully fertile and self-compatible. Many seeds were produced on the latter clone by selfing and on the male-sterile clones by crossing. The somatic hybrid plants showed an introgression of genes for frost tolerance and an adaptability to cold from S. commersonii. Therefore, the use of these somatic hybrids in breeding for and in genetic esearch on frost tolerance and cold-hardening is suggested.
[本文引用: 1]
.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/BF02893240URL [本文引用: 1]
DOI:10.1007/BF02360262URL [本文引用: 1]
DOI:10.1007/BF02853578URL [本文引用: 1]
