 ,*中国农业科学院作物科学研究所 / 农业农村部谷物品质监督检验测试中心, 北京 100081
,*中国农业科学院作物科学研究所 / 农业农村部谷物品质监督检验测试中心, 北京 100081Evaluation of matrix reference material of Fumonisins FB1 in corn flour
NIU Xin-Ning, WANG Bu-Jun ,*Institute of Crop Sciences, Chinese Academy of Agricultural Sciences / Cereal Quality Supervision and Testing Center, Ministry of Agriculture, Beijing 100081, China
,*Institute of Crop Sciences, Chinese Academy of Agricultural Sciences / Cereal Quality Supervision and Testing Center, Ministry of Agriculture, Beijing 100081, China通讯作者:
收稿日期:2019-10-15接受日期:2020-03-24网络出版日期:2020-07-12
| 基金资助: |
Received:2019-10-15Accepted:2020-03-24Online:2020-07-12
| Fund supported: |
作者简介 About authors
E-mail:niuxinning123@126.com。

摘要
关键词:
Abstract
Keywords:
PDF (345KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
牛欣宁, 王步军. 玉米粉中伏马毒素FB1基体标准物质的评价[J]. 作物学报, 2020, 46(7): 1128-1133. doi:10.3724/SP.J.1006.2020.93056
NIU Xin-Ning, WANG Bu-Jun.
伏马毒素(Fumonisins), 是由串珠镰刀菌(Fusarium moniliforme)、轮状镰刀菌(Fusarium verticillioides)等在适宜的温度和湿度条件下产生的一种次级代谢产物。到目前为止, 已发现A、B、C和P四大类共28种伏马毒素, FB1、FB2、FB3三种最为常见, 其中FB1污染最多, 含量最高(约占60%)、毒性最强、对谷物的污染最为严重[1,2]。伏马毒素易溶于甲醇、乙腈、水等极性溶剂, 具有极强的热稳定性, 广泛存在于玉米、小麦、水稻等粮食作物及其加工制品中, 主要污染玉米及其制品, 可通过食物链进入人体及动物体内, 引起各种疾病, 包括神经毒性[3,4,5]、免疫毒性[6,7,8,9,10]、器官和组织毒性[11,12,13,14]、生殖毒性[15,16]、致癌性[17]等, 因此加强对粮食作物中伏马毒素的监测和控制格外重要。
标准物质具有准确的测量标准, 可对量值溯源性、实验室的质量保证能力及监测体系进行客观评价[18]。我国对标准物质的研制起步比较晚, 尤其在生物毒素基体标准物质研制方面与国外其他国家相比仍相对落后。生物毒素基体标准物质是指基体中有确定含量的生物毒素物质。基体标准物质可以避免基质中所存在的活性物质与生物毒素产生反应, 对测定结果产生干扰。与纯品标准物质相比, 基体标准物质为目标化合物和基体结合, 与真实检测样品更一致, 可以保障测试结果的准确性和质量控制的有效性[19]。
国外伏马毒素相关的标准物质价格昂贵, 且货运期长, 购买不便, 远远不能满足国内对于伏马毒素的检测和质量控制的需求。而我国“国家标准物质资源共享平台”上暂未查到有关伏马毒素FB1的基体标准物质, 本次试验根据JJF 1006-1994《一级标准物质技术规范》[20]、JJF 1343-2012《标准物质定值的通用原则及统计学原理》[21]以及ISO导则[22]研制了玉米粉中伏马毒素FB1基体标准物质, 对于实验室中玉米及其加工品中伏马毒素FB1的检测起到准确性、可溯源性等重要作用。
1 材料与方法
1.1 主要仪器与试剂
仪器包括: 超高效液相色谱-串联四级杆质谱(UPLC-XEVO TQ, 美国Waters公司); THZ-300恒温培养摇床(上海一恒科学仪器公司); 台式高速冷冻离心机(德国Sigma公司); MILLI-Q纯水机(美国Millipore公司); LGJ-10FD真空冷冻干燥机(北京松源华兴科技发展有限公司); V型混合机; 均质分离机; 涡旋混匀器; 氮吹仪; 天平。试剂包括: 甲醇、乙腈(色谱级, 美国Fisher公司); 甲酸(色谱级, 美国Sigma公司); 伏马毒素标准品(美国Romer公司)。玉米原料购买自市场。
1.2 仪器条件
1.2.1 色谱条件 色谱柱为C18柱(100 mm×2.1 mm, 粒径1.6 μm); 柱温40℃; 样品温度10℃; 进样体积为5 μL; 流动相A: 甲醇(色谱级), 流动相B: 0.1%的甲酸, 流速为0.2 mL min-1; FB1梯度洗脱程序0~5.5 min流动相A由2%增加至85%, 5.5~5.6 min流动相A降至2%, 平衡2.4 min。1.2.2 质谱条件 电喷雾离子源(electrospray ionization, ESI), 正离子模式; MRM多反应监测模式; 毛细管电压2.50 kV; 离子源温度110℃; 去溶剂气流量800 L h-1; 溶剂气温度为450℃。
1.3 标准物质的研制
将所购玉米颗粒进行伏马毒素FB1毒素含量初检, 符合要求后按照一定比例添加一定浓度伏马毒素FB1标准溶液, 经浸泡使之达到预期含量, 之后将样品冷冻干燥、磨细粉, 过40目筛, 充分混匀并分装, 抽真空密封保存。1.4 均匀性检验
均匀性是标准物质最基本的属性, 是标准物质研制能顺利进行的基础。按照JJG 1006-1994[20]和JJF 1343-2012[21]中的相关规定, 从制得的200袋样品中随机抽取15袋, 进行单元间均匀性检测, 每袋平行测定3次, 进行单元内均匀性检测。样品最小取样量为2.0 g, 用超高液相色谱串联质谱法检测, 对检测结果进行单因素方差(F检验法)分析, 评价样品的均匀性。1.5 稳定性检验
依据JJG 1006-1994[20]和JJF 1343-2012[21]的规定, 按照先密后疏的原则进行样品运输过程中的短期稳定性考察和储存过程中的长期稳定性考察。在44℃条件下, 于0、1、4、7、14 d检测样品中的FB1毒素含量, 考察样品的短期稳定性; 在4℃和-18℃条件下, 于0、1、2、4、6月分别检测样品的FB1毒素含量, 考察样品的长期稳定性。每次检测随机取3袋标准物质样品, 每一袋样品平行测定2次, 取2次数据的平均值作为最终检测结果。所用检测方法与均匀性检验保持一致。对测定结果采用趋势分析法进行分析, 以此来考察线性关系的斜率随样品稳定性变化的变化值, 用该变化值对标准物质的稳定性进行评价,拟合直线Y=b0+b1X, X表示稳定性考察时间, Y表示标准物质的特性值, b0、b1表示回归系数, 若|b1|<t0.95, n-2?s(b1), 说明斜率无显著性变化, 该标准物质在监测时间内能够保持稳定。该拟合直线的斜率计算公式为:
b1=$\frac{\sum\nolimits_{i=1}^{n}{({{x}_{i}}-\bar{x})({{Y}_{i}}-\bar{Y})}}{\sum\nolimits_{i=1}^{n}{({{x}_{i}}-\bar{x})}}$
截距计算公式为:
b0=Y - b1 X
直线标准偏差计算公式为:
s2=$\frac{\sum\nolimits_{i=1}^{n}{{{({{Y}_{i}}-{{b}_{0}}-{{b}_{1}}{{x}_{i}})}^{2}}}}{n-2}$
斜率的不确定度计算公式为:
s(b1)= $\frac{s}{\sqrt{\sum\nolimits_{i=1}^{n}{{{({{x}_{i}}-\overline{x})}^{2}}}}}$
1.6 标准物质定值和不确定度
标准物质定值依据《标准样品定值的一般原则和统计方法》[23]的要求, 本次标准物质的定值采取6~8家实验室联合测定的方法, 所选定的实验室均为国内权威检测机构且具有伏马毒素检测资质的实验室。各实验室均按照GB 5009.240-2016《食品安全国家标准食品中伏马毒素的测定》进行检测。将各实验室的组间数据经格拉布斯(Grubbs)检验进行可疑值剔除, 科克伦(Cochran)法检验实验室间数据是否为等精度数据, 狄克逊(Dixon)法检验实验室间数据是否有离群值。计算出符合条件的实验室数据的平均值, 该平均值即为样品的定值结果。依据《不确定度评估指南》[24]和GB/T 15000.3-2008 [25]的方法, 标准物质的不确定度主要由3个方面组成, 即均匀性引起的不确定度ubb、稳定性引起的不确定度us、定值过程中引入的不确定度uchar。其中稳定性不确定度us包括短期稳定性不确定度usts和长期稳定性不确定度uIts。
标准物质相对扩展不确定度 $U=k\sqrt{{{u}_{\text{bb}}}^{2}+{{u}_{s}}^{2}+{{u}_{char}}^{2}}$ (k=2)。
2 结果与分析
2.1 均匀性检验结果
采用单因素方差分析法进行与单元内的均匀性检验, F为单元间方差(S12)与单元内方差(S22)的比值, 与在95%置信水平下的临界值Fα比较大小, 若F值小于Fα, 则认为样品间无显著性的差异, 均匀性好。本文Fα为F0.05(14,30) = 2.04, 检验结果由表1可知, F < F0.05(14,30), 说明玉米粉中FB1样品均匀, 能满足标准物质均匀性要求。Table 1
表1
表1玉米粉中伏马毒素FB1均匀性检验结果
Table 1
| 编号 No. | 测定值Estimated value | 平均值 Mean | ||
|---|---|---|---|---|
| 样品1 Sample 1 | 样品2 Sample 2 | 样品3 Sample 3 | ||
| 1 | 1526.94 | 1515.99 | 1548.48 | 1530.47 |
| 2 | 1475.93 | 1561.67 | 1466.99 | 1501.53 |
| 3 | 1541.65 | 1578.75 | 1586.70 | 1569.03 |
| 4 | 1642.69 | 1523.99 | 1477.12 | 1547.93 |
| 5 | 1547.76 | 1553.40 | 1320.62 | 1473.93 |
| 6 | 1550.76 | 1523.61 | 1429.61 | 1501.33 |
| 7 | 1409.22 | 1429.40 | 1519.65 | 1452.76 |
| 8 | 1564.07 | 1584.00 | 1582.17 | 1576.74 |
| 9 | 1515.83 | 1487.58 | 1465.17 | 1489.52 |
| 10 | 1550.90 | 1519.14 | 1513.76 | 1527.93 |
| 11 | 1498.30 | 1473.52 | 1516.96 | 1496.26 |
| 12 | 1425.48 | 1570.33 | 1425.64 | 1473.82 |
| 13 | 1455.89 | 1375.58 | 1479.23 | 1436.90 |
| 14 | 1461.25 | 1342.56 | 1568.66 | 1457.49 |
| 15 | 1446.23 | 1483.95 | 1393.13 | 1441.11 |
| 组间标准偏差的平方 S12 | 5915.88 | |||
| 组内标准偏差的平方 S22 | 4164.09 | |||
| 统计量F Statistic F | 1.42 | |||
| F0.05(14,30) | 2.04 | |||
| 判断 Judgment | 均匀 Inhomogeneous | |||
新窗口打开|下载CSV
2.2 稳定性检验结果
表2为玉米样品在44℃环境下保存14 d所测数据, 由表2可知, 玉米样品在14 d内所计算出|b1|<t0.95, n-2?s(b1), 且由图1所示, 直线拟合方程的斜率不显著, 因此该标准物质在44℃模拟运输环境下可以保持稳定。Table 2
表2
表2玉米粉中伏马毒素FB1短期稳定性检验结果
Table 2
| 时间 Day (d) | 测定值 Estimated value | 平均值 Mean | ||
|---|---|---|---|---|
| 样品1 Sample 1 | 样品2 Sample 2 | 样品3 Sample 3 | ||
| 0 | 1443.21 | 1527.20 | 1401.19 | 1457.20 |
| 1 | 1437.83 | 1505.11 | 1410.89 | 1451.28 |
| 4 | 1483.95 | 1512.63 | 1563.87 | 1520.15 |
| 7 | 1457.61 | 1471.37 | 1438.96 | 1455.98 |
| 14 | 1412.94 | 1400.17 | 1369.62 | 1394.24 |
| 斜率 b1 | -4.79 | |||
| 斜率不确定度 s(b1) | 3.64 | |||
| 临界值 t0.95, n-2×s(b1) | 11.58 | |||
| 结论Conclusion | 稳定Stabilization | |||
新窗口打开|下载CSV
图1
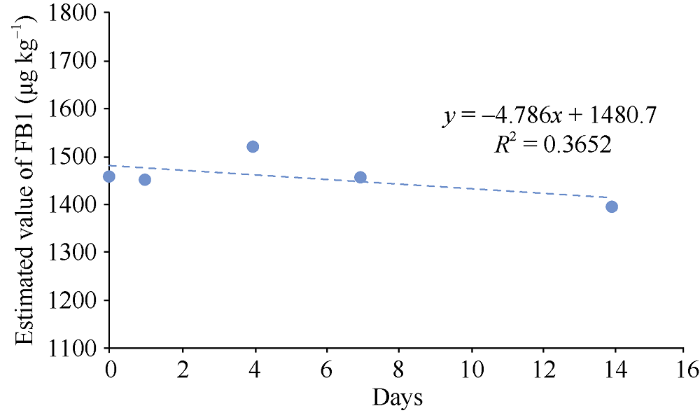 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1玉米粉中伏马毒素FB1短期稳定性趋势分析图
Fig. 1Analysis chart of short-term stability trend of Fumonisins FB1 in corn flour
表3为标准物质在4℃和-18℃条件下储存6个月, 分别在1、2、4、6个月时对样品用UPLC-MS/MS进行伏马毒素含量检测, 取样方法、数据处理与短期稳定性检验一致, 由表3可知, 该标准物质在6个月时间范围内|b1|<t0.95, n-2?s(b1), 且由图2所示, 该标准物质的拟合直线斜率不显著, 即玉米粉中伏马毒素FB1含量无明显增加或减少, 因此可认为该标准物质在4℃、-18℃温度下可以较好地储存6个月。
Table 3
表3
表3玉米粉中伏马毒素FB1长期稳定性检验结果
Table 3
| 温度 Temperature | 时间(月) Time (month) | 测定值Estimated value | 平均值 Mean | 斜率 b1 | 临界值 t0.95, n-2×s(b1) | 结论 Conclusion | ||
|---|---|---|---|---|---|---|---|---|
| 样品1 Sample 1 | 样品2 Sample 2 | 样品3 Sample 3 | ||||||
| 4℃ | 0 | 1443.21 | 1527.20 | 1401.19 | 1457.20 | -20.86 | 31.60 | 稳定 Stabilization |
| 1 | 1481.61 | 1527.86 | 1525.53 | 1511.67 | ||||
| 2 | 1368.36 | 1430.24 | 1428.94 | 1409.18 | ||||
| 4 | 1442.54 | 1422.36 | 1477.85 | 1447.58 | ||||
| 6 | 1389.45 | 1334.99 | 1283.45 | 1335.96 | ||||
| -18℃ | 1 | 1468.15 | 1382.05 | 1450.64 | 1433.62 | -10.27 | 31.40 | 稳定Stabilization |
| 2 | 1498.44 | 1526.57 | 1531.25 | 1518.75 | ||||
| 4 | 1407.69 | 1372.59 | 1405.98 | 1395.42 | ||||
| 6 | 1457.35 | 1381.64 | 1398.07 | 1412.35 | ||||
新窗口打开|下载CSV
图2
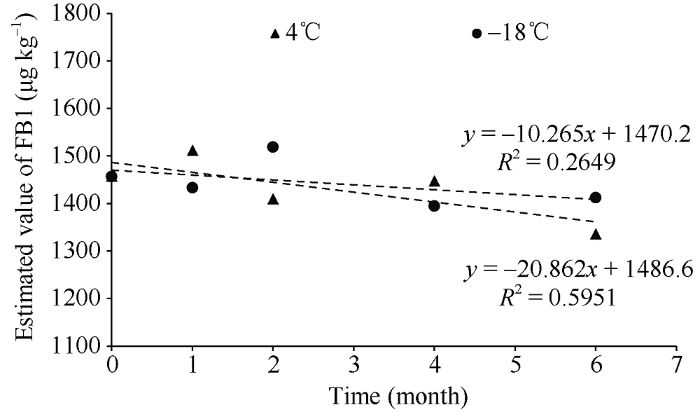 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2玉米粉中伏马毒素FB1长期稳定性趋势分析图
Fig. 2Analysis chart of long-term stability trend of Fumonisins FB1 in corn flour
2.3 定值和不确定度分析
本批标准物质由多家实验室采用指定检测方法进行联合定值, 为每家实验室随机分发两袋样品, 对每袋样品至少平行测定3次, 最终每家实验室提供6组数据。将所有实验室所测数据汇总并分析, 每个实验室的组内数据经狄克逊准则检验是否有异常值, 各实验室的定值数据经格拉布斯检验进行统计分析, 采用科克伦准则判断数据有无可疑值, 最终有6家实验室的数据被采用(表4), 将所保留的最终数据计算出平均值, 即为最终的定值结果。Table 4
表4
表4各实验室联合定值测定结果
Table 4
| 实验室编号 Laboratory number | 测定值Estimated value | |||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| A | 930 | 940 | 940 | 950 | 960 | 1100 |
| B | 1400 | 1300 | 1500 | 1500 | 1300 | 1300 |
| C | 1800 | 1500 | 1300 | 1600 | 1400 | 1400 |
| D | 1800 | 2200 | 1900 | 2200 | 2100 | 2300 |
| E | 1400 | 1400 | 1200 | 1300 | 1400 | 1400 |
| F | 1500 | 1500 | 1500 | 1600 | 1600 | 1700 |
| 平均值Mean | 1475.56 | |||||
新窗口打开|下载CSV
2.4 标准物质的不确定度
2.4.1 均匀性不确定度Sbb2 =$\frac{1}{n}(S_{1}^{2}-S_{2}^{2})$=$\frac{1}{3}$(5915.88-4164.09)=583.93 μg kg-1
ubb=Sbb=24.165 μg kg-1
2.4.2 稳定性引入的不确定度
短期稳定性不确定度 usts= s(b1)?t=3.6429×14=51 μg kg-1
长期稳定性不确定度ults = 59.61 μg kg-1
2.4.3 定值引入的不确定度 标准物质定值过程中带来的A类不确定度, 主要为联合定值过程中引入的不确定度, B类不确定度主要为对测量产生影响的因素所产生的不确定度。
联合定值引入的不确定度uA =10.05%
测量过程中不确定度uB=1.0%
合成不确定度uchar= uchar,rel $\cdot \bar{X}$=$\sqrt{{{u}_{\text{A}}}^{2}+{{u}_{B}}^{2}}\times $ X=148.85 μg kg-1
2.4.4 扩展不确定度
${{U}_{\text{CRM}}}=k\sqrt{{{u}_{\text{bb}}}^{2}+{{u}_{\text{sts}}}^{2}+{{u}_{\text{lts}}}^{2}+{{u}_{\text{char}}}^{2}}$=169.98 μg kg-1
3 讨论
本研究通过对JJF 1343-2012《标准物质定值的通用原则及统计学原理》[21]以及ISO导则[22]的学习和研究, 制备了玉米粉中伏马毒素FB1基体标准物质, 将购买的玉米样品经筛选, 加伏马毒素FB1标准溶液浸泡, 冷冻干燥, 磨粉, 混匀后抽真空包装制得标准物质。按照JJG 1006-1994[20]与JJF1343-2012[21]中的有关规定, 对标准物质进行均匀性检验和为期半年的稳定性检验的研究。根据《标准样品定值的一般原则和统计方法》[23]中的要求, 选取了多家具有伏马毒素检测资质的实验室进行定值, 因毒素检测较为复杂, 且不同实验室操作环境及不同操作人员难以保证检测结果在误差范围内, 最终只有6家实验室结果予以采用, 最后计算平均值即为标准物质的定值结果。实验中对标准物质中的伏马毒素FB1用超高效液相色谱-串联质谱法进行了均匀性和稳定性检验, 均匀性检验F< F0.05(14,30), 可以证明所研制的标准物质达到了均匀性的要求, 稳定性检验分为短期稳定性和长期稳定性检验, 检验结果在置信水平p=0.95% (95%置信水平)下, 检验拟合直线斜率b1小于t0.95, n-2?s(b1), 斜率无显著性差异, 因此在6个月时间范围内, 伏马毒素FB1稳定性良好, 符合标准物质要求。最后经联合定值得出该标准物质的特征值为1475.56 μg kg-1, 不确定度为169.98 μg kg-1。
因目前国内对于生物毒素基体标准物质研制较少, 尤其基体标准物质起步更晚, 基体标准物质研制过程较为复杂, 但其与待检测样品有较高相似性, 能够避免干扰检测结果, 因此研制基体标准物质是很有必要的, 对于我国的生物毒素检测技术的提升具有重要意义。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URLPMID:26342287 [本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.neuro.2004.10.001URLPMID:15713342 [本文引用: 1]

Fumonisin B1 (FB1), a mycotoxin produced by Fusarium verticillioides, causes equine leukoencephalomalacia, a condition not reproduced in any other species. We hypothesized that direct exposure of murine brain to FB1 will result in neurotoxicity, characterized by biochemical and pathological alterations. The present study compared the toxicity of FB1 in mouse brain after an intracerebroventricular (icv) or subcutaneous (sc) infusion. Female BALB/c mice (5/group) were infused (0.5 microl/h) with total doses of 0, 10 or 100 microg FB1 in saline over 7 days via osmotic pumps implanted either via icv cannulation of the ventricle or via the sc route. One day after the last day of treatment, brains were dissected either fresh or after intracardiac paraformaldehyde fixation. In mice given 100 microg of FB1 icv, FluoroJade B staining revealed neurodegeneration in the cortex, and anti-glial fibrillary acidic protein staining detected activated astrocytes in the hippocampus. High performance liquid chromatography indicated accumulation of free sphinganine in animals given FB1 icv in all brain regions and increased free sphingosine after the 100 microg FB1 in the cortex. The concentration of cortical sphingomyelin and complex sphingolipids remained unchanged. The icv administration of FB1 induced expression of tumor necrosis factor alpha, interleukin-1beta, interleukin-6 and interferon gamma after both doses, assayed by the real-time polymerase chain reaction. The sc administration of 100 microg FB1 caused slight sphinganine accumulation and increased IL-1beta expression in cortex only. Results indicated that icv injection of FB1 caused neurodegeneration with simultaneous inhibition of de novo ceramide synthesis, stimulation of astrocytes, and upregulation of pro-inflammatory cytokines in the murine brain. A relative lack of FB1 availability into the brain could be responsible for the absence of its neurotoxicity in mouse.
[本文引用: 1]
DOI:10.1002/mnfr.201000402URLPMID:21259430 [本文引用: 1]

SCOPE: Deoxynivalenol (DON) and fumonisins (FB) are the most frequently encountered mycotoxins produced by Fusarium species and most commonly co-occur in animal diets. These mycotoxins were studied for their toxicity in piglets on several parameters including plasma biochemistry, organ histopathology and immune response. METHODS AND RESULTS: Twenty-four 5-wk-old animals were randomly assigned to four different groups, receiving separate diets for 5 wk, a control diet, a diet contaminated with either DON (3 mg/kg) or FB (6 mg/kg) or both toxins. At days 4 and 16 of the trial, the animals were subcutaneously immunized with ovalbumin to assess their specific immune response. The different diets did not affect animal performance and had minimal effect on hematological and biochemical blood parameters. By contrast, DON and FB induced histopathological lesions in the liver, the lungs and the kidneys of exposed animals. The liver was significantly more affected when the two mycotoxins were present simultaneously. The contaminated diets also altered the specific immune response upon vaccination as measured by reduced anti-ovalbumin IgG level in the plasma and reduced lymphocyte proliferation upon antigenic stimulation. Because cytokines play a key role in immunity, the expression levels of IL-8, IL-1beta, IL-6 and macrophage inflammatory protein-1beta were measured by RT-PCR at the end of the experiment. The expression of these four cytokines was significantly decreased in the spleen of piglets exposed to multi-contaminated diet. CONCLUSION: Taken together, our data indicate that ingestion of multi-contaminated diet induces greater histopathological lesions and higher immune suppression than ingestion of mono-contaminated diets.
DOI:10.1016/j.intimp.2017.03.031URLPMID:28432936 [本文引用: 1]

Fumonisin B1 (FB1) is one kind of mycotoxins that has the neurotoxicity, carcinogenicity, hepatotoxicity and immunotoxicity produced by the fungus Fusarium verticillioides, which commonly infects corn and other crops and is harmful to animal and human health upon consumption of FB1-contaminated feed or food. However, the mechanism of immunotoxicity, especially the immunosuppression induced by FB1 is still unclear. The most pivotal cells in the induction of immune responses are dendritic cells (DCs). In this study, we used murine bone marrow-derived dendritic cells (BMDCs) as a model system to elucidate the effect of FB1 on the function of BMDCs through biological methods. We found that FB1 reversed the morphological changes and enhanced the endocytosis of FITC-dextran in LPS-treated BMDCs. At the same time, FB1 decreased the LPS-induced expressions of MHC II, C[1]D80 and CD86 molecules in BMDCs (p<0.05), as well as the T-cell stimulatory capacity of BMDCs (p<0.01). Moreover, the secretions of IL-6, IL-10 and IL-12, but not TNF-alpha induced by LPS exposure were suppressed by FB1 in a dose dependent (p<0.01). It was considered that the immunosuppressive effects of FB1 were mainly caused by changing the morphology and interfering with the process of antigen uptake, processing and presentation. The results highlighted that FB1 had the capacity to modulate the immune responses of BMDCs.
DOI:011.02/ijaai.165173URLPMID:22761190 [本文引用: 1]

Fumonisins, a family of mycotoxins, are mainly toxic and carcinogenic. The present study was carried out to evaluate fumonisin B1 (FB1) effects on the production of inflammatory cytokines by gastric and colon cell lines. The study was performed on two cell lines under in vitro condition, including gastric epithelial cell line (AGS) and human colon adenocarcinoma cell line (SW742). Lipopolysaccharide (LPS) was used for inflammatory cytokine induction. The culture medium was supplemented with 4.5-72 mg/l of FB1 for 72 h before cell induction. The supernatants were harvested 24 h after the induction and measured for cytokines by using enzyme-linked immunosorbent assay.FB1 induced a dose-dependent increase in the production of tumor necrosis factor-alpha and interleukin-1beta in both AGS and SW742 cell lines. This increase was statistically significant with concentration of FB1 between 9 and 72 mg/l (P < 0.05). FB1 also induced a dose-dependent decrease in interleukin-8 production. This decrease was seen in both cell lines and showed a statistical significance with FB1 concentration (P < 0.05).The results show that FB1 increases inflammatory cytokines production by various gastric and intestinal cells. This effect in the long run can possibly be the basis for the occurrence or development of inflammation and subsequent atrophy in the above-mentioned tissues.
[本文引用: 1]
DOI:10.1016/j.fct.2017.10.054URLPMID:29097114 [本文引用: 1]

Although pathological characteristics of fumonisin B1 are known to induce hepatic injury over prolonged periods, the cellular defense mechanisms against the detrimental effects of FB1 are still unknown. The underlying mechanisms of FB1 toxicity are thought to be related with the inhibition of ceramide synthase, causing an accumulation of sphingoid bases, which in turn cause development of oxidative stress. Herein, we investigated whether autophagy, a cellular defense mechanism, protects liver cells from FB1 exposure. To accomplish this, we utilized HepG2 cells and a mouse model to study the effects of FB1 in the autophagy pathway. FB1 was capable of inducing autophagy via the generation of ROS, induction of endoplasmic reticulum stress, phosphorylation of JNK, suppression of mTOR and activation of LC3I/II in HepG2 cells and mice livers. Treatment of HepG2 cells with the ROS scavenger N-acetyl-l-cysteine alleviated ER stress stimulation and induced HepG2 cell death. Moreover, suppression of autophagy with 3-Methyladenine enhanced HepG2 cells apoptosis. Concurrently, four consecutive days exposure of mice livers to FB1 altered the levels of sphingoid bases, hepatic enzymes and induced histopathological changes. Moreover, the expression levels of major ER stress and autophagy-related markers such as PERK, IRE1-alpha, and LC3I/II also increased. Autophagy activation protected HepG2 cells and mice livers from the lethal effects of FB1. Hence, these findings specify that, the compounds that modify autophagy might be useful therapeutic agents for treatment of patients with FB1 induced liver ailments.
DOI:10.1007/s00204-014-1323-6URLPMID:25155190 [本文引用: 1]

Fumonisin B1 (FB1) is a well-known inhibitor of de novo sphingolipid biosynthesis, due to its ability to inhibit ceramide synthases (CerS) activity. In mammals, this toxin triggers broad clinical symptoms with multi-organ dysfunction such as hepatotoxicity or pulmonary edema. The molecular mechanism of CerS inhibition by FB1 remains unknown. Due to the existence of six mammalian CerS isoforms with a tissue-specific expression pattern, we postulated that the organ-specific adverse effects of FB1 might be due to different CerS isoforms. The sphingolipid contents of lung and liver were compared in normal and FB1-exposed piglets (gavage with 1.5 mg FB1/kg body weight daily for 9 days). The effect of the toxin on each CerS was deduced from the analysis of its effects on individual ceramide (Cer) and sphingomyelin (SM) species. As expected, the total Cer content decreased by half in the lungs of FB1-exposed piglets, while in contrast, total Cer increased 3.5-fold in the livers of FB1-exposed animals. Our data also indicated that FB1 is more prone to bind to CerS4 and CerS2 to deplete lung and to enrich liver in d18:1/C20:0 and d18:1/C22:0 ceramides. It also interact with CerS1 to enrich liver in d18:1/C18:0 ceramides. Cer levels were counterbalanced by those of SM. In conclusion, these results demonstrate that the specificity of the effects of FB1 on tissues and organs is due to the effects of the toxin on CerS4, CerS2, and CerS1.
DOI:10.1093/toxsci/kfj198URLPMID:16613836 [本文引用: 1]

Fumonisins (FBs) are mycotoxins in maize and are inhibitors of ceramide synthase (CS), the most likely proximate cause of FB toxicity. In liver and kidney, the primary target organs in FB-fed rats, inhibition of CS results in a marked increase in the ceramide precursor sphinganine (Sa). This study was conducted to investigate the differential time- and dose-dependent changes in Sa, sphingosine (So), sphinganine 1-phosphate (Sa-1-P), and sphingosine 1-phosphate (So-1-P) in kidney, liver, serum, and heart of male Sprague-Dawley rats (3-4 weeks old) fed diets containing 1.1, 13.5, and 88.6 mug/g of total FB for 10 days. The tissues were microscopically examined for the presence and severity of lesions consistent with FB exposure. There was a time- and dose-dependent increase in Sa in both liver and kidney, which was closely correlated with the tissue concentration of fumonisin B(1) (FB(1)) and histopathologic findings. However, the Sa alone greatly underestimated the degree of disruption of sphingolipid metabolism since accumulated Sa and So were quickly metabolized to Sa-1-P and So-1-P as evidenced by large increases in these metabolites in kidney but not in liver. The concentration of FB(1) in liver and kidney that first elicited an increase in Sa was similar in both tissues, however, over time, the kidney accumulated significantly more FB(1) (10x) and total Sa (Sa plus Sa-1-P) compared to liver. Thus, the relative sensitivity of male Sprague-Dawley rat kidney and liver is most likely a consequence of differences in the mechanisms responsible for both FB(1) uptake/clearance and Sa metabolism.
DOI:10.1002/mnfr.200600266URLPMID:17642075 [本文引用: 1]

Fumonisins constitute a family of toxic and carcinogenic mycotoxins produced by Fusarium verticillioides (formerly F. moniliforme), a common fungal contaminant of corn. Contamination with fumonisin B(1) (FB(1)) is of concern as this mycotoxin causes various animal diseases. The gastrointestinal tract represents the first barrier against ingested chemicals, food contaminants, and natural toxins. Following ingestion of fumonisin-contaminated food or feed, intestinal epithelial cells could be exposed to a high concentration of toxin. In this review, we have summarized the data dealing with the impact of FB(1) on the intestine. Although FB(1 )is poorly absorbed and metabolized in the intestine, it induces intestinal disturbances (abdominal pain or diarrhea) and causes extra-intestinal organ pathologies (pulmonary edema, leukoencephalomalacia, or neural tube defects). The main toxicological effect of FB(1) reported in vivo and in vitro is the accumulation of sphingoid bases associated with the depletion of complex sphingolipids. This disturbance of the sphingolipid biosynthesis pathway could explain the other observed toxicological effects such as an alteration in intestinal epithelial cell viability and proliferation, a modification of cytokine production, and a modulation of intestinal physical barrier function.
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.fct.2012.07.024URLPMID:22841953 [本文引用: 1]

The fungal toxin fumonisin B1 (FB1) is a potential human carcinogen based on evidence of renal carcinogenicity in rats and hepatocarcinogenicity in mice. The toxicity and carcinogenicity of FB1 is linked to ceramide synthase inhibition. Based on this mechanism of action and on lack of evidence of genotoxicity, FB1 is considered a non-genotoxic carcinogen. The p53 heterozygous (p53+/-) mouse is a cancer-prone model used for carcinogenesis. The effects of chronic dietary FB1 exposure were characterized in p53+/- mice to confirm non-genotoxicity using a model which is more sensitive to genotoxic than non-genotoxic carcinogens and to clarify the relationship between p53 expression, altered sphingolipid metabolism, and FB1-induced carcinogenesis. Responses to FB1 were similar in p53+/- and p53+/+ mice after 26 weeks exposure to 0, 5, 50 or 150 mg FB1/kg diet, supporting a non-genotoxic mechanism of action. Hepatic adenomas and cholangiomas were observed in mice exposed to 150 mg/kg FB1. For a 10% increase in hepatic megalocytosis, the estimated 95% lower confidence limit of the benchmark dose (BMDL10) ranged from 0.15 and 1.11 mg FB1/kg bw/day. Based on similar responses in p53+/- and p53+/+ mice, p53 and related pathways play a secondary role in responses to FB1 toxicity and carcinogenesis.
URLPMID:25399074 [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 4]
[本文引用: 4]
[本文引用: 5]
[本文引用: 5]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
