 ,3,*, 刘敏轩
,3,*, 刘敏轩 ,2,*
,2,*Screening and identification of Chinese sorghum landraces for salt tolerance at germination and seedling stages
BAO Li-Ge1,2, LU Ping2, SHI Meng-Sha2, XU Yue ,3,*, LIU Min-Xuan
,3,*, LIU Min-Xuan ,2,*
,2,*通讯作者:
收稿日期:2019-09-17接受日期:2020-01-15网络出版日期:2020-02-17
| 基金资助: |
Received:2019-09-17Accepted:2020-01-15Online:2020-02-17
| Fund supported: |
作者简介 About authors
E-mail:baolg17@mails.jlu.edu.cn。

摘要
关键词:
Abstract
Keywords:
PDF (327KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
宝力格, 陆平, 史梦莎, 许月, 刘敏轩. 中国高粱地方种质芽期苗期耐盐性筛选及鉴定[J]. 作物学报, 2020, 46(5): 734-744. doi:10.3724/SP.J.1006.2020.94138
BAO Li-Ge, LU Ping, SHI Meng-Sha, XU Yue, LIU Min-Xuan.
土壤盐渍化是危害我国乃至世界农业发展的一个重要因素[1]。据联合国教科文组织(UNESCO)和粮农组织(FAO)的不完全统计, 全世界各种盐渍土面积约10亿公顷, 广泛分布于100多个国家和地区[2], 其中我国盐渍化土地面积近1亿公顷[3]。此外, 由于工业污染、不合理灌溉以及化肥使用不当等人为因素的影响, 次生盐渍化土壤的面积仍然在快速增加[4]。高粱是禾本科C4植物, 作为世界五大谷类作物之一[5], 是世界上5亿最贫困人口的主食[6], 同时也是我国优质白酒酿造的主要原料[7], 在世界上干旱和半干旱地区广泛种植[8]。高粱起源于非洲贫瘠的环境条件, 在长期自然和人工选择作用下形成了对干旱、盐碱、低温等一系列的抗性, 特别是高粱的耐盐碱性, 在禾本科作物中表现非常突出。在不同生育阶段高粱的耐盐碱性是有所区别的, 其耐盐机理也有所不同。因此, 研究不同高粱品种在各个生育阶段的耐盐性, 筛选出一批综合耐盐能力突出的品种对开发利用盐渍化土地、增加粮食产量和维持农业可持续发展具有重要意义。
随着粮食需求和可耕种土地面积之间矛盾的日益突出, 国内外****对多种农作物, 如水稻[9,10]、玉米[11,12]、小麦[13]等进行了耐盐性研究, 取得了一定的进展。目前, 针对高粱不同生育时期使用不同筛选方法的耐盐鉴定研究有很多, Costa等[14]比较了饲用高粱耐盐品种和盐敏感品种的酶活性变化, 结果表明在盐胁迫下耐盐高粱的超氧化物歧化酶和过氧化氢酶活性增幅更大, 而盐敏感品种的过氧化物酶活性减小, 耐盐品种的过氧化物酶活性增加; 孙璐等[15]通过主成分分析法对42份高粱品种进行了萌发期筛选, 并提出了根部生长因子是对盐胁迫的主要响应因子; 张士超等[16]使用了光化学效率以及幼苗和根的Na+、K+含量等指标作为高粱苗期耐盐筛选的参考指标; 崔江慧等[17]使用了侧根数盐害率、叶片萎蔫数、叶片萎蔫时间等指标。这些指标的使用虽然使耐盐评价更加准确, 但并不适合大规模的耐盐筛选。任富莉等[18]使用多个指标以及不同的分析方法对田间苗期和室内苗期进行了耐盐分析, 说明了隶属函数法和主成分分析法在分析结果上差异很小; 王春语等[19]采用不断提高盐浓度的方法从396份高粱品种中筛选出芽期强耐盐材料, 但由于盐胁迫浓度远大于高粱的半致死胁迫浓度, 并不能将该方法应用于其他生育期; 吕建澎等[20]用NaCl和Na2CO3进行了混合盐胁迫筛选, 相比于单独NaCl胁迫, 筛选的结果更为客观。
目前, 大多数针对高粱耐盐性筛选的研究要么选定的生育时期相对比较单一, 要么筛选材料群体规模较小、生态区域代表性不够, 很少有研究对我国不同生态区的主要高粱地方品种进行多生育期、多筛选指标的耐盐性鉴定。在此背景下, 本研究以涵盖我国主要生态区的110份高粱品种为试验材料, 针对高粱在盐胁迫下最为重要的2个生长发育时期进行多筛选指标综合分析, 拟建立更加系统、全面、快速的高粱耐盐评价体系, 发掘一批芽期和苗期耐盐能力都比较优异的高粱种质资源以供耐盐机理研究、耐盐育种利用和盐渍地推广种植。
1 材料与方法
1.1 试验材料
110份试验材料包括来自全国16个省(自治区)的高粱代表性地方品种(附表1), 均由中国农业科学院作物科学研究所种质资源中心小宗作物课题组提供。Supplementary table 1
附表1
附表1参试高粱品种及来源
Supplementary table 1
| 统一编号 Code | 品种名称 Landrace | 来源地 Source |
|---|---|---|
| 00001067 | 北平五号 Beipingwuhao | 北京 Beijing |
| 00001075 | 北平黑壳白 Beipingheikebai | 北京 Beijing |
| 00001076 | 北平南郊白 Beipingnanjiaobai | 北京 Beijing |
| 00001079 | 白抢高粱 Baiqianggaoliang | 北京 Beijing |
| 00001080 | 白娥粘 Baienian | 北京 Beijing |
| 00001081 | 白鞑子帽 Baidazimao | 北京 Beijing |
| 00001082 | 白粘高粱 Bainiangaoliang | 北京 Beijing |
| 00001108 | 黄粘高粱 Huangniangaoliang | 北京 Beijing |
| 00001112 | 粘高粱 Niangaoliang | 北京 Beijing |
| 00001121 | 矬腿汗 Cuotuihan | 北京 Beijing |
| 00001122 | 矬粘高梁 Cuoniangaoliang | 北京 Beijing |
| 00001124 | 锦州白 Jinzhoubai | 北京 Beijing |
| 00007606 | 大岑 Dacen | 北京 Beijing |
| 00007611 | 白仁高粱 Bairengaoliang | 北京 Beijing |
| 00001185 | 八尺秆 Bachigan | 河北 Hebei |
| 00001256 | 大红高粱 Dahonggaoliang | 河北 Hebei |
| 00001283 | 大青高粱 Daqinggaoliang | 河北 Hebei |
| 00001310 | 大散码 Dasanma | 河北 Hebei |
| 00001315 | 万人迷 Wanrenmi | 河北 Hebei |
| 00001316 | 山西白 Shanxibai | 河北 Hebei |
| 00001381 | 东北地丁 Dongbeididing | 河北 Hebei |
| 00001504 | 多穗高梁 Duosuigaoliang | 河北 Hebei |
| 00001534 | 保八斗 Baobadou | 河北 Hebei |
| 00001804 | 八叶青 Bayeqing | 山西 Shanxi |
| 00001808 | 八月齐 Bayueqi | 山西 Shanxi |
| 00001810 | 八叶齐 Bayueqi | 山西 Shanxi |
| 00001812 | 八叶春齐 Bayechunqi | 山西 Shanxi |
| 00001817 | 大娥黄 Daehuang | 山西 Shanxi |
| 00001855 | 大软茭 Daruanjiao | 山西 Shanxi |
| 00001864 | 大山东 Dashandong | 山西 Shanxi |
| 00002144 | 没狼窝 Molangwo | 山西 Shanxi |
| 00002333 | 鸽子眼 Geziyan | 山西 Shanxi |
| 00002361 | 短三尺 Duansanchi | 山西 Shanxi |
| 00002401 | 锣锤穗 Luochuisui | 山西 Shanxi |
| 00007828 | 八月齐 Bayueqi | 山西 Shanxi |
| 00007901 | 斗穗高粱 Dousuigaoliang | 山西 Shanxi |
| 00008015 | 低秆红 Diganhong | 山西 Shanxi |
| 00008186 | 黑眼睛高粱 Heiyanjinggaoliang | 山西 Shanxi |
| 00002018 | 东腰黄高粱 Dongyaohuanggaoliang | 内蒙古 Inner Mongolia |
| 00002443 | 八叶齐 Bayeqi | 内蒙古 Inner Mongolia |
| 00002446 | 八叶齐 Bayeqi | 内蒙古 Inner Mongolia |
| 00002500 | 小红高粱 Xiaohonggaoliang | 内蒙古 Inner Mongolia |
| 00002555 | 大白色 Dabaise | 内蒙古 Inner Mongolia |
| 00002591 | 大高粱 Dagaoliang | 内蒙古 Inner Mongolia |
| 00002592 | 大高粱 Dagaoliang | 内蒙古 Inner Mongolia |
| 00002596 | 大红粮 Dahongliang | 内蒙古 Inner Mongolia |
| 00002699 | 白棒锤 Baibangchui | 内蒙古 Inner Mongolia |
| 00003011 | 朝阳棒槌 Chaoyangbangchui | 内蒙古 Inner Mongolia |
| 00008219 | 小白高粱 Xiaobaigaoliang | 内蒙古 Inner Mongolia |
| 00008230 | 大白色 Dabaise | 内蒙古 Inner Mongolia |
| 00008263 | 白壳白 Baikebai | 内蒙古 Inner Mongolia |
| 00003043 | 八大头 Badatou | 辽宁 Liaoning |
| 00003044 | 八大叶 Badaye | 辽宁 Liaoning |
| 00003092 | 大二户高粱 Daerhugaoliang | 辽宁 Liaoning |
| 00003126 | 小关东白 Xiaoguandongbai | 辽宁 Liaoning |
| 00003217 | 白大秆 Baidagan | 辽宁 Liaoning |
| 00003260 | 打锣锤 Daluochui | 辽宁 Liaoning |
| 00003435 | 高秆大白色 Gaogandabaise | 辽宁 Liaoning |
| 00003436 | 高秆关东青 Gaoganguandongqing | 辽宁 Liaoning |
| 00003634 | 矮青壳 Aiqingke | 辽宁 Liaoning |
| 00003659 | 九三一五 Jiusanyiwu | 吉林 Jilin |
| 00004539 | 黑壳棒 Heikebang | 黑龙江 Heilongjiang |
| 00004551 | 黑壳棒子 Heikebangzi | 黑龙江 Heilongjiang |
| 00004595 | 短棒子 Duanbangzi | 黑龙江 Heilongjiang |
| 00008566 | 顶头红 Dingtouhong | 黑龙江 Heilongjiang |
| 00008675 | 黑壳棒 Heikebang | 黑龙江 Heilongjiang |
| 00005015 | 八叶高粱 Bayegaoliang | 山东 Shandong |
| 00005035 | 小红萼 Xiaohonge | 山东 Shandong |
| 00005038 | 小老头矮高梁 Xiaolaotouaigaoliang | 山东 Shandong |
| 00005059 | 大气厦尾 Daqishawei | 山东 Shandong |
| 00005061 | 大毛衣 Damaoyi | 山东 Shandong |
| 00005091 | 大青壳 Daqingke | 山东 Shandong |
| 00005092 | 大青壳栗母鸡 Daqingkelimuji | 山东 Shandong |
| 00005103 | 大粒红 Dalihong | 山东 Shandong |
| 00005110 | 大黑柳 Daheiliu | 山东 Shandong |
| 00005197 | 打字帽高粱 Dazimaogaoliang | 山东 Shandong |
| 00005349 | 红白粘秫秫 Hongbainianshushu | 山东 Shandong |
| 00005487 | 麦皮子 Maipizi | 山东 Shandong |
| 00005624 | 爱国二号高梁 Aiguoerhaogaoliang | 山东 Shandong |
| 00007177 | 二节红 Erjiehong | 甘肃 Gansu |
| 00007178 | 二牛心 Erniuxin | 甘肃 Gansu |
| 00007182 | 小辈高梁 Xiaobeigaoliang | 甘肃 Gansu |
| 00007184 | 小蛇眼 Xiaosheyan | 甘肃 Gansu |
| 00007226 | 西藏白 Xizangbai | 甘肃 Gansu |
| 00007247 | 秤坨高梁 Chengtuogaoliang | 甘肃 Gansu |
| 00007249 | 黄高梁 Huanggaoliang | 甘肃 Gansu |
| 00007251 | 黑矮高梁 Heiaigaoliang | 甘肃 Gansu |
| 00007257 | 矬高粱 Cuogaoliang | 甘肃 Gansu |
| 00007265 | 矮秆红 Aiganhong | 甘肃 Gansu |
| 00007315 | 柯姆孜中高梁 Kemuzizhonggaoliang | 新疆 Xinjiang |
| 00006906 | 马尾高粱 Maweigaoliang | 湖北 Hubei |
| 00009037 | 大粒高粱 Daligaoliang | 湖北 Hubei |
| 00009065 | 红高粱 Honggaoliang | 湖北 Hubei |
| 00009079 | 独石矮 Dushiai | 湖北 Hubei |
| 00009111 | 矮高粱 Aigaoliang | 湖北 Hubei |
| 00007173 | 软高梁 Ruangaoliang | 陕西 Shaanxi |
| 00009822 | 疙瘩高粱 Gedagaoliang | 陕西 Shaanxi |
| 00009883 | 草高粱 Caogaoliang | 陕西 Shaanxi |
| 00009893 | 黑高粱 Heigaoliang | 陕西 Shaanxi |
| 00009903 | 矮高粱 Aigaoliang | 陕西 Shaanxi |
| 00009904 | 矮脚高粱 Aijiaogaoliang | 陕西 Shaanxi |
| 00009905 | 矮脚黄糯 Aijiaohuangnuo | 陕西 Shaanxi |
| 00009929 | 露仁高粱 Lurengaoliang | 陕西 Shaanxi |
| 00010016 | 达拉牛 Dalaniu | 云南 Yunnan |
| 00013028 | 鼓眼糯 Guyannuo | 四川 Sichuan |
| 00013029 | 鼓眼糯 Guyannuo | 四川 Sichuan |
| 00013035 | 上龙高粱 Shanglonggaoliang | 广西 Guangxi |
| 00013036 | 大粒高粱 Daligaoliang | 广西 Guangxi |
| 00013125 | 阿粱 Aliang | 贵州 Guizhou |
| 00013200 | 褐高粱 Hegaoliang | 贵州 Guizhou |
新窗口打开|下载CSV
1.2 试验方法
1.2.1 芽期耐盐性鉴定 随机选择九三一五(00003659)、二牛心(00007178)、大粒高粱(00009037) 3个高粱品种, 从中挑选籽粒饱满、大小一致的种子, 先用1%的次氯酸钠溶液消毒15 min, 再用自来水和蒸馏水各冲洗3次, 放入铺有双层滤纸的灭菌培养皿, 每培养皿放置30粒种子, 盐浓度梯度设置为0 (CK)、50、100、150、200、250、300 mmol L-1 NaCl, 每个处理3个重复。将培养皿放入光照培养箱(PER CIVAL-AR36L3)中, 在25℃恒温、昼夜光照周期为12 h/12 h、70%湿度、光照强度200 μmol m-2 s-1条件下, 培养至第4天更换新的灭菌培养皿和处理溶液并统计发芽数, 第7天统计发芽数, 以胚根长度达到0.2 mm作为发芽指标。通过测定不同盐胁迫浓度下3个品种的第4天发芽势, 第7天发芽率, 发现在200 mmol L-1 NaCl胁迫下品种间生长情况差异最明显, 将其作为芽期耐盐筛选的盐胁迫浓度。在200 mmol L-1的NaCl溶液或蒸馏水(对照)的条件下, 使用上述鉴定方法对所有参试高粱进行芽期耐盐鉴定。发芽势 = 第4天发芽种子数/供试种子数×100%
发芽率 = 第7天发芽种子数/供试种子数×100%
1.2.2 苗期耐盐性鉴定 苗期最适盐胁迫浓度筛选所用材料同芽期, 将蛭石装入育苗盘中, 并用蒸馏水对其完全浸润, 种子消毒处理后种入育苗盘, 播种深度约1 cm, 放入光照培养箱中, 在25℃恒温、昼夜光照周期为12 h/12 h、70%湿度、光照强度200 μmol m-2 s-1条件下培养, 约7 d种子发芽, 用1/2 Hoagland营养液浇灌[21], 待幼苗长至二叶一心期, 挑选长势一致的幼苗每孔定苗5株, 开始用含有NaCl的 Hoagland营养液处理幼苗, 设NaCl浓度梯度为0 (CK)、50、100、150、200 mmol L-1 NaCl, 每个处理3个重复, 长至四叶一心期时挑选每孔3株测定苗长、根长、苗鲜重、根鲜重、苗干重、根干重、叶绿素含量, 取平均值, 用SPAD仪(TYS-B)测定叶绿素含量。筛选发现在100 mmol L-1浓度胁迫下品种间生长差异最明显, 最终确定苗期耐盐筛选的NaCl浓度为100 mmol L-1 [22], 使用上述方法对所有材料进行苗期耐盐性鉴定。
1.3 统计方法
计算芽期、苗期各耐盐指标的相对值即盐胁迫下性状值占对照性状值的百分比。采用隶属函数值法[23], 计算芽期耐盐性指标相对值的隶属值, 若测定指标与耐盐性呈正相关, 使用公式(1)计算, 若为负相关, 则用公式(2)计算。式中, Xij 表示第i个品种第j指标的耐盐指数, Xmax 为最大值, Xmin是最小值, Uij是第i个品种第j指标(j = 1, 2, …, )的隶属函数值。按不同品种对各指标的隶属函数值进行平均计算, 得到隶属函数平均值。
对苗期耐盐性指标的相对值使用主成分分析法[15], 其中综合得分模型F值用公式(3)计算。
式中, wl代表权重, 即各主成分的相对方差贡献率, fl代表第l个因子的因子得分。
1.4 数据分析
用Microsoft Excel 2016统计各品种性状相对值并进行方差分析, 计算各处理平均数及各性状相对值, 用SPSS 24.0进行描述性统计分析、相关性分析、主成分分析及聚类分析。2 结果与分析
2.1 不同高粱品种对芽、苗期盐胁迫的响应
在200 mmol L-1 NaCl胁迫下, 110份高粱品种的相对发芽势和相对发芽率平均值分别为47.09%和77.13%, 其中相对发芽势的变异系数比较大, 说明110份高粱品种在该浓度盐胁迫下相对发芽势差异非常明显(表1)。在所有参试材料中, 来自山西的八叶青(00001804)、来自广西的上龙高粱(00013035)及大粒高粱(00013036)在盐胁迫下的相对发芽势均为0, 而来自内蒙古的朝阳棒槌(00003011)的相对发芽势在所有材料中最高, 达到98.89% (附表2)。相对发芽率测定结果显示, 来自陕西的露仁高粱(00009929)最低, 仅23.65%, 而河北的多穗高粱(00001504)最高, 达101.79%,山西的锣锤穗(00002401)、河北的大红高粱(00001256)、辽宁的白大秆(00003217)也很高, 超过100%。综合2个指标发现, 大毛衣(00005061)、锣锤穗(00002401)、朝阳棒槌(00003011)的相对发芽势和相对发芽率均比较高, 表明在盐胁迫下这些材料的种子发芽能力和活力均表现较高水平, 从而具有较好的芽期耐盐性。露仁高粱(00009929)、八叶青(00001804)等高粱品种芽期耐盐能力比较弱。Table 1
表1
表1在盐胁迫下110份高粱品种各耐盐指标的描述统计量及方差分析
Table 1
| 鉴定指标 Identification index | 平均值 Mean | 范围 Range of variation (%) | 标准差 Standard deviation | 变异系数 Coefficient of variation | F值 F-value | P值 P-value |
|---|---|---|---|---|---|---|
| 相对发芽势G1 | 47.09 | 0-98.89 | 29.02 | 61.61 | 17.77 | <0.001 |
| 相对发芽率G2 | 77.13 | 23.65-101.79 | 18.72 | 24.27 | 11.87 | <0.001 |
| 相对叶绿素含量S1 | 88.49 | 59.53-99.91 | 7.66 | 8.66 | 8.03 | <0.001 |
| 相对苗长S2 | 77.35 | 52.47-95.23 | 9.53 | 12.32 | 14.89 | <0.001 |
| 相对根长S3 | 80.26 | 47.87-100.14 | 12.00 | 14.95 | 7.18 | <0.001 |
| 相对苗鲜重S4 | 58.12 | 27.43-95.28 | 13.00 | 22.38 | 11.93 | <0.001 |
| 相对苗干重S5 | 70.74 | 30.48-98.26 | 13.75 | 19.43 | 7.87 | <0.001 |
| 相对根鲜重S6 | 65.36 | 21.62-100.34 | 16.85 | 25.78 | 9.49 | <0.001 |
| 相对根干重S7 | 69.45 | 31.46-102.13 | 14.53 | 20.93 | 5.68 | <0.001 |
新窗口打开|下载CSV
Supplementary table 2
附表2
附表2高粱芽,苗期盐胁迫下各性状的相对值
Supplementary table 2
| 品种统一编号 Code | G1 | G2 | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|---|---|
| 00001067 | 0.8444 | 0.9111 | 0.7852 | 0.8784 | 0.9507 | 0.7170 | 0.8772 | 0.5938 | 0.5791 |
| 00001075 | 0.1984 | 0.9144 | 0.9241 | 0.8709 | 0.8443 | 0.7208 | 0.7760 | 0.7752 | 0.7431 |
| 00001076 | 0.4286 | 0.5333 | 0.8536 | 0.5847 | 0.6121 | 0.3771 | 0.5418 | 0.6079 | 0.6094 |
| 00001079 | 0.6764 | 0.7787 | 0.9824 | 0.6924 | 0.7535 | 0.5135 | 0.5993 | 0.5402 | 0.4937 |
| 00001080 | 0.3221 | 0.7937 | 0.9434 | 0.9467 | 0.8792 | 0.8517 | 0.9804 | 0.8627 | 0.8102 |
| 00001081 | 0.8610 | 0.9095 | 0.9581 | 0.9117 | 0.7272 | 0.6937 | 0.7777 | 0.9688 | 1.0214 |
| 00001082 | 0.8536 | 0.8747 | 0.8641 | 0.7907 | 0.9129 | 0.6151 | 0.7532 | 0.5772 | 0.6315 |
| 00001108 | 0.4530 | 0.7588 | 0.9834 | 0.6988 | 0.8616 | 0.6992 | 0.8627 | 0.7423 | 0.7025 |
| 00001112 | 0.9550 | 0.9360 | 0.9372 | 0.7704 | 0.6803 | 0.6169 | 0.7440 | 0.4602 | 0.7053 |
| 00001121 | 0.7037 | 0.8027 | 0.8564 | 0.6724 | 0.8161 | 0.5955 | 0.7197 | 0.6016 | 0.7715 |
| 00001122 | 0.7929 | 0.8425 | 0.9087 | 0.7404 | 0.7598 | 0.5437 | 0.6504 | 0.5925 | 0.6459 |
| 00001124 | 0.8095 | 0.8183 | 0.8614 | 0.8481 | 0.8785 | 0.7007 | 0.8953 | 0.6894 | 0.7484 |
| 00007606 | 0.0714 | 0.8395 | 0.8284 | 0.8371 | 0.8214 | 0.9370 | 0.7908 | 0.7409 | 0.5269 |
| 00007611 | 0.5690 | 0.9828 | 0.6260 | 0.9221 | 0.6656 | 0.5489 | 0.7130 | 0.8633 | 0.9388 |
| 00001185 | 0.1178 | 0.2874 | 0.9103 | 0.6492 | 0.8046 | 0.5580 | 0.8031 | 0.7476 | 0.7588 |
| 00001256 | 0.4667 | 1.0044 | 0.9787 | 0.8866 | 0.9340 | 0.6485 | 0.9111 | 0.8146 | 0.9458 |
| 00001283 | 0.7113 | 0.6727 | 0.9167 | 0.7473 | 0.8994 | 0.4842 | 0.5978 | 0.7041 | 0.8667 |
| 00001310 | 0.5751 | 0.8862 | 0.6422 | 0.7815 | 0.5649 | 0.5499 | 0.6563 | 0.7902 | 0.6331 |
| 00001315 | 0.2749 | 0.6983 | 0.6854 | 0.7513 | 0.6775 | 0.4899 | 0.5038 | 0.4573 | 0.5699 |
| 00001316 | 0.3743 | 0.8476 | 0.8769 | 0.8327 | 0.9912 | 0.6224 | 0.8076 | 0.6442 | 0.6383 |
| 00001381 | 0.7568 | 0.9266 | 0.8607 | 0.6128 | 0.7925 | 0.3455 | 0.5410 | 0.5432 | 0.7388 |
| 00001504 | 0.0222 | 1.0179 | 0.8468 | 0.5954 | 0.9631 | 0.3261 | 0.4376 | 0.6013 | 0.7437 |
| 00001534 | 0.6210 | 0.7262 | 0.8210 | 0.7107 | 0.8217 | 0.4313 | 0.5857 | 0.6031 | 0.8029 |
| 00001804 | 0.0000 | 0.2652 | 0.9329 | 0.8076 | 0.7960 | 0.5868 | 0.6861 | 0.6597 | 0.7406 |
| 00001808 | 0.8167 | 0.9661 | 0.9341 | 0.7659 | 0.9313 | 0.4220 | 0.4844 | 0.8130 | 0.7374 |
| 00001810 | 0.3859 | 0.8969 | 0.9119 | 0.8320 | 0.9148 | 0.6254 | 0.9668 | 0.7396 | 0.8419 |
| 00001812 | 0.9084 | 0.9556 | 0.8529 | 0.8422 | 0.8375 | 0.6726 | 0.8462 | 0.7564 | 0.8028 |
| 00001817 | 0.3103 | 0.8172 | 0.9433 | 0.7025 | 0.8408 | 0.5889 | 0.6950 | 0.6628 | 0.7225 |
| 00001855 | 0.4664 | 0.5525 | 0.9625 | 0.7658 | 1.0014 | 0.5370 | 0.7023 | 0.7207 | 0.8186 |
| 00001864 | 0.3793 | 0.8793 | 0.8704 | 0.9449 | 0.9436 | 0.7413 | 0.8945 | 0.5668 | 0.6801 |
| 00002144 | 0.0444 | 0.3667 | 0.9648 | 0.8056 | 0.8729 | 0.6085 | 0.6054 | 0.4830 | 0.5513 |
| 00002333 | 0.0333 | 0.6938 | 0.8468 | 0.8419 | 0.9985 | 0.7580 | 0.8897 | 0.6273 | 0.7081 |
| 00002361 | 0.8496 | 0.9663 | 0.9470 | 0.7637 | 0.7366 | 0.5551 | 0.7770 | 0.8768 | 0.9089 |
| 00002401 | 0.9513 | 1.0018 | 0.8916 | 0.7824 | 0.7491 | 0.4106 | 0.5710 | 0.7996 | 0.5940 |
| 00007828 | 0.6689 | 0.6109 | 0.7804 | 0.8454 | 0.6964 | 0.6178 | 0.6704 | 0.4056 | 0.4135 |
| 00007901 | 0.8367 | 0.7845 | 0.9200 | 0.6148 | 0.6227 | 0.4413 | 0.5795 | 0.6704 | 0.6696 |
| 00008015 | 0.2582 | 0.6484 | 0.8332 | 0.7524 | 0.8639 | 0.4745 | 0.6469 | 0.4768 | 0.5099 |
| 00008186 | 0.6688 | 0.7262 | 0.8541 | 0.5961 | 0.7977 | 0.4014 | 0.5465 | 0.5876 | 0.6306 |
| 00002018 | 0.2989 | 0.8889 | 0.9694 | 0.8455 | 0.8176 | 0.7095 | 0.9827 | 0.7296 | 0.7932 |
| 00002443 | 0.8820 | 0.9092 | 0.9356 | 0.8548 | 0.8764 | 0.6049 | 0.9454 | 0.5172 | 0.6281 |
| 00002446 | 0.0357 | 0.9259 | 0.9898 | 0.8194 | 0.9190 | 0.6544 | 0.8692 | 0.8573 | 0.7859 |
| 00002500 | 0.4388 | 0.6617 | 0.9323 | 0.6566 | 0.6477 | 0.5074 | 0.6626 | 0.9147 | 0.8046 |
| 00002555 | 0.4563 | 0.5450 | 0.8860 | 0.8150 | 0.7970 | 0.5430 | 0.7345 | 0.8654 | 0.8380 |
| 00002591 | 0.2900 | 0.6121 | 0.9028 | 0.8445 | 0.8458 | 0.5804 | 0.7993 | 0.5669 | 0.8586 |
| 00002592 | 0.4212 | 0.8453 | 0.9297 | 0.8924 | 0.8485 | 0.5635 | 0.6943 | 0.4375 | 0.6447 |
| 00002596 | 0.4978 | 0.7310 | 0.8097 | 0.7607 | 0.9279 | 0.4126 | 0.5465 | 0.4437 | 0.4845 |
| 00002699 | 0.1563 | 0.4889 | 0.8739 | 0.7587 | 0.8158 | 0.6696 | 0.6614 | 0.6055 | 0.4966 |
| 00003011 | 0.9889 | 0.9540 | 0.8363 | 0.8229 | 0.9757 | 0.7144 | 0.8730 | 0.8242 | 0.8209 |
| 00008219 | 0.0222 | 0.8719 | 0.9392 | 0.7648 | 0.9797 | 0.6093 | 0.8374 | 0.5428 | 0.8165 |
| 00008230 | 0.8703 | 0.9175 | 0.8980 | 0.9043 | 0.9106 | 0.7710 | 0.6897 | 0.8348 | 0.6842 |
| 00008263 | 0.8851 | 0.8556 | 0.8733 | 0.7700 | 0.8291 | 0.7747 | 0.6659 | 0.7492 | 0.6844 |
| 00003043 | 0.6628 | 0.8539 | 0.8653 | 0.7506 | 0.8138 | 0.4351 | 0.5351 | 0.7603 | 0.6698 |
| 00003044 | 0.8385 | 0.9060 | 0.8207 | 0.6079 | 0.7173 | 0.3908 | 0.4925 | 0.5808 | 0.5511 |
| 00003092 | 0.6695 | 0.7460 | 0.9671 | 0.6891 | 0.7581 | 0.6483 | 0.6609 | 0.6485 | 0.6885 |
| 00003126 | 0.6429 | 0.7550 | 0.9471 | 0.7830 | 0.8505 | 0.6885 | 0.8756 | 0.7592 | 0.7607 |
| 00003217 | 0.6717 | 1.0023 | 0.8532 | 0.8128 | 0.9974 | 0.6743 | 0.7580 | 0.9468 | 0.8861 |
| 00003260 | 0.5517 | 0.8667 | 0.9536 | 0.7408 | 0.7312 | 0.5084 | 0.7521 | 0.8114 | 0.6940 |
| 00003435 | 0.2327 | 0.8519 | 0.9923 | 0.8683 | 0.7986 | 0.7132 | 0.7142 | 0.7680 | 0.9434 |
| 00003436 | 0.1962 | 0.8569 | 0.8810 | 0.6303 | 0.6371 | 0.5112 | 0.6475 | 0.4761 | 0.5144 |
| 00003634 | 0.2059 | 0.7783 | 0.8903 | 0.7740 | 0.9288 | 0.4618 | 0.5910 | 0.5395 | 0.6721 |
| 00003659 | 0.2649 | 0.4910 | 0.9322 | 0.7647 | 0.9088 | 0.3904 | 0.5061 | 0.4079 | 0.5855 |
| 00004539 | 0.0936 | 0.5345 | 0.9992 | 0.7059 | 0.8361 | 0.4554 | 0.7001 | 0.9909 | 0.8150 |
| 00004551 | 0.7035 | 0.8731 | 0.9158 | 0.8147 | 0.5621 | 0.6015 | 0.7850 | 0.8785 | 0.9328 |
| 00004595 | 0.7990 | 0.8914 | 0.9333 | 0.7343 | 0.8868 | 0.5741 | 0.6535 | 0.8379 | 0.9554 |
| 00008566 | 0.7963 | 0.9259 | 0.8368 | 0.7881 | 0.7031 | 0.7274 | 0.8474 | 0.9358 | 0.8115 |
| 00008675 | 0.8693 | 0.9310 | 0.9151 | 0.8560 | 0.7602 | 0.5493 | 0.7149 | 0.6657 | 0.5439 |
| 00005015 | 0.9000 | 0.9815 | 0.9508 | 0.8794 | 0.8329 | 0.4962 | 0.5030 | 0.7096 | 0.5684 |
| 00005035 | 0.8071 | 0.8814 | 0.8731 | 0.7642 | 0.7503 | 0.6372 | 0.6281 | 0.4698 | 0.6938 |
| 00005038 | 0.4127 | 0.7273 | 0.7668 | 0.5666 | 0.8018 | 0.4944 | 0.5621 | 0.3306 | 0.4429 |
| 00005059 | 0.4522 | 0.9437 | 0.8428 | 0.7767 | 0.7513 | 0.6854 | 0.7416 | 0.7382 | 0.6894 |
| 00005061 | 0.9881 | 1.0000 | 0.8135 | 0.8100 | 0.8259 | 0.7485 | 0.8690 | 0.6183 | 0.6254 |
| 00005091 | 0.6475 | 0.8446 | 0.9308 | 0.7733 | 0.6979 | 0.4666 | 0.6580 | 0.4696 | 0.6323 |
| 00005092 | 0.7619 | 0.8937 | 0.9297 | 0.8852 | 0.7669 | 0.9528 | 0.9107 | 0.8221 | 0.8219 |
| 00005103 | 0.4068 | 0.7672 | 0.9732 | 0.7967 | 0.9583 | 0.5423 | 0.6888 | 0.4084 | 0.6154 |
| 00005110 | 0.7411 | 0.8569 | 0.8504 | 0.7754 | 0.8076 | 0.5422 | 0.6033 | 0.4392 | 0.4674 |
| 00005197 | 0.4275 | 0.7454 | 0.5954 | 0.6524 | 0.9495 | 0.4170 | 0.4666 | 0.4274 | 0.4730 |
| 00005349 | 0.4532 | 0.9770 | 0.9224 | 0.6474 | 0.9303 | 0.5437 | 0.7454 | 0.5515 | 0.7546 |
| 00005487 | 0.4637 | 0.7652 | 0.8421 | 0.9388 | 0.8921 | 0.6990 | 0.6931 | 0.8080 | 0.7852 |
| 00005624 | 0.3418 | 0.9747 | 0.9676 | 0.8880 | 0.9287 | 0.5974 | 0.8623 | 0.3713 | 0.6054 |
| 00007177 | 0.6089 | 0.8358 | 0.9957 | 0.7863 | 0.7437 | 0.5885 | 0.6639 | 0.6179 | 0.7819 |
| 00007178 | 0.3318 | 0.5237 | 0.8737 | 0.5560 | 0.5161 | 0.2743 | 0.4227 | 0.2162 | 0.3425 |
| 00007182 | 0.6164 | 0.7628 | 0.8766 | 0.7123 | 0.6337 | 0.6602 | 0.7564 | 0.6219 | 0.6062 |
| 00007184 | 0.4506 | 0.9191 | 0.8799 | 0.6715 | 0.7296 | 0.5487 | 0.7963 | 0.6389 | 0.8932 |
| 00007226 | 0.6283 | 0.8908 | 0.9197 | 0.8255 | 0.8307 | 0.6160 | 0.6474 | 0.6334 | 0.5302 |
| 00007247 | 0.4042 | 0.7448 | 0.8720 | 0.9523 | 0.6839 | 0.6975 | 0.7918 | 1.0034 | 1.0001 |
| 00007249 | 0.7104 | 0.7231 | 0.9723 | 0.7760 | 0.8477 | 0.6280 | 0.7102 | 0.8121 | 0.8965 |
| 00007251 | 0.0345 | 0.5250 | 0.8534 | 0.7875 | 0.8607 | 0.8224 | 0.9001 | 0.6637 | 0.7497 |
| 00007257 | 0.5486 | 0.8497 | 0.9093 | 0.8304 | 0.8315 | 0.7241 | 0.9339 | 0.7398 | 0.8061 |
| 00007265 | 0.1636 | 0.5069 | 0.8296 | 0.8402 | 0.5297 | 0.5648 | 0.6329 | 0.7456 | 0.5391 |
| 00007315 | 0.4685 | 0.9112 | 0.8142 | 0.6726 | 0.5952 | 0.3827 | 0.5058 | 0.3687 | 0.4861 |
| 00006906 | 0.0423 | 0.5509 | 0.9141 | 0.8692 | 0.6327 | 0.4536 | 0.6303 | 0.3630 | 0.6325 |
| 00009037 | 0.5294 | 0.9545 | 0.9212 | 0.7787 | 0.8157 | 0.7016 | 0.7346 | 0.6854 | 0.6104 |
| 00009065 | 0.1331 | 0.4617 | 0.7491 | 0.6886 | 0.6909 | 0.5603 | 0.6696 | 0.8746 | 0.7179 |
| 00009079 | 0.2330 | 0.6190 | 0.9826 | 0.7433 | 0.9006 | 0.5401 | 0.7002 | 0.6863 | 0.7463 |
| 00009111 | 0.0303 | 0.3122 | 0.9177 | 0.6040 | 0.6542 | 0.4301 | 0.6384 | 0.6182 | 0.7359 |
| 00007173 | 0.0256 | 0.5460 | 0.8136 | 0.9196 | 0.8674 | 0.8293 | 0.9204 | 0.8069 | 0.6227 |
| 00009822 | 0.1800 | 0.9500 | 0.9000 | 0.7643 | 0.8971 | 0.5266 | 0.7937 | 0.7471 | 0.9474 |
| 00009883 | 0.5868 | 0.9470 | 0.7561 | 0.7675 | 0.5450 | 0.5730 | 0.7815 | 0.7693 | 0.7996 |
| 00009893 | 0.3472 | 0.9692 | 0.9417 | 0.7873 | 0.9383 | 0.5316 | 0.7244 | 0.6574 | 0.6426 |
| 00009903 | 0.2502 | 0.8301 | 0.8593 | 0.7116 | 0.6402 | 0.4467 | 0.5868 | 0.3926 | 0.4363 |
| 00009904 | 0.1895 | 0.7738 | 0.8771 | 0.8812 | 0.6934 | 0.7650 | 0.9759 | 0.5882 | 0.7048 |
| 00009905 | 0.7384 | 0.8247 | 0.9149 | 0.7439 | 0.6216 | 0.5873 | 0.7563 | 0.5256 | 0.5252 |
| 00009929 | 0.0760 | 0.2365 | 0.9278 | 0.8687 | 0.7359 | 0.5641 | 0.6741 | 0.6407 | 0.7282 |
| 00010016 | 0.1191 | 0.6089 | 0.8045 | 0.9486 | 0.9129 | 0.7329 | 0.8539 | 0.9782 | 0.8572 |
| 00013028 | 0.6356 | 0.8741 | 0.8838 | 0.7678 | 0.9230 | 0.5957 | 0.6631 | 0.7897 | 0.8372 |
| 00013029 | 0.2568 | 0.3344 | 0.9774 | 0.8006 | 0.9438 | 0.5522 | 0.6434 | 0.5604 | 0.5790 |
| 00013035 | 0.0000 | 0.4216 | 0.8703 | 0.6068 | 0.7941 | 0.3864 | 0.5123 | 0.4658 | 0.5258 |
| 00013036 | 0.0000 | 0.5810 | 0.8158 | 0.7155 | 0.7624 | 0.5382 | 0.6495 | 0.3777 | 0.6834 |
| 00013125 | 0.1174 | 0.6889 | 0.9574 | 0.7991 | 0.8477 | 0.4651 | 0.6194 | 0.5350 | 0.6207 |
| 00013200 | 0.0617 | 0.4420 | 0.7235 | 0.5248 | 0.4788 | 0.3457 | 0.3048 | 0.3484 | 0.3146 |
新窗口打开|下载CSV
在100 mmol L-1 NaCl胁迫下, 110份高粱品种苗期的相对叶绿素含量、相对苗长、相对根长、相对苗鲜重、相对苗干重、相对根鲜重和相对根干重的平均值和变化范围见表1, 其中相对叶绿素含量的变异系数最低, 表明在该浓度盐胁迫下不同高粱品种中相对叶绿素含量变异程度较低。黑龙江的黑壳棒(00004539)相对叶绿素含量、相对根鲜重分别为99.92%和99.09%, 其相对根长为83.61%, 根部受盐胁迫影响较小, 其地上部分受胁迫影响明显, 相对苗鲜重仅为45.54%, 耐盐能力表现平平; 而甘肃的二牛心(00007178)和贵州的褐高粱(00013200)的各项相对指标均比较低, 苗期耐盐能力较差; 北京的白娥粘(00001080)和河北的大红高粱(00001256)的各项相对指标都较高, 表现出了较好的苗期耐盐能力。
2.2 盐胁迫下高粱各指标的相关性分析
分别对盐胁迫下芽期的2个指标和苗期的7个指标进行了相关性分析, 结果见表2, 多数指标间相关性都达到了显著或极显著水平, 其中相对苗鲜重(S4)与相对苗干重(S5)相关性最高, 相关系数为0.798, 相对根鲜重(S6)与相对根干重(S7)的相关性也比较高为0.726, 相对苗长(S2)与相对苗鲜重(S4)和相对苗干重(S5)的相关性分别为0.671和0.616, 相对发芽势(G1)和相对发芽率(G2)的相关性达到0.593, 以上指标相关性极显著(P<0.01)。另外, 相对叶绿素含量与相对苗长、相对苗鲜重及相对根鲜重的相关性均比较低。Table 2
表2
表2盐胁迫下各指标的相关系数
Table 2
| 指标Index | G1 | G2 | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|---|---|
| G1 | 1 | ||||||||
| G2 | 0.593** | 1 | |||||||
| S1 | 1 | ||||||||
| S2 | 0.136 | 1 | |||||||
| S3 | 0.257** | 0.314** | 1 | ||||||
| S4 | 0.115 | 0.671** | 0.263** | 1 | |||||
| S5 | 0.241* | 0.616** | 0.299** | 0.798** | 1 | ||||
| S6 | 0.141 | 0.386** | 0.119 | 0.454** | 0.440** | 1 | |||
| S7 | 0.281** | 0.349** | 0.233* | 0.342** | 0.501** | 0.726** | 1 |
新窗口打开|下载CSV
2.3 110份高粱的芽期耐盐性分析
因芽期耐盐性指标与芽期耐盐性呈正相关, 所以根据公式(1)分别计算110份高粱品种的相对发芽势和相对发芽率2个指标的隶属函数值及其平均隶属函数值(附表3)。根据平均隶属函数值的大小, 使用基于欧式平方距离的组间连接法对110份高粱进行聚类分析(图1), 在欧式平方距离介于3和4之间时, 供试高粱被分为4类, 第一类群包括22个品种, 占供试材料的20%, 隶属函数均值范围在0.806~0.988之间, 这些材料盐胁迫下各性状相对数值很高, 可被认定为芽期高度耐盐品种; 第二类群, 共32个品种, 占供试材料的29.1%, 隶属函数均值范围在0.639~0.789之间, 各性状相对数值较高, 为芽期耐盐品种; 第三类群, 共37个品种, 占供试材料的33.6%, 隶属函数均值范围在0.349~0.628之间, 各性状相对数值较低, 属于芽期盐敏感材料, 第四类群, 共19个品种, 占供试材料的17.3%, 隶属函数均值范围在0.018~0.310之间, 各性状相对数值很低, 是芽期高度盐敏感品种。图1
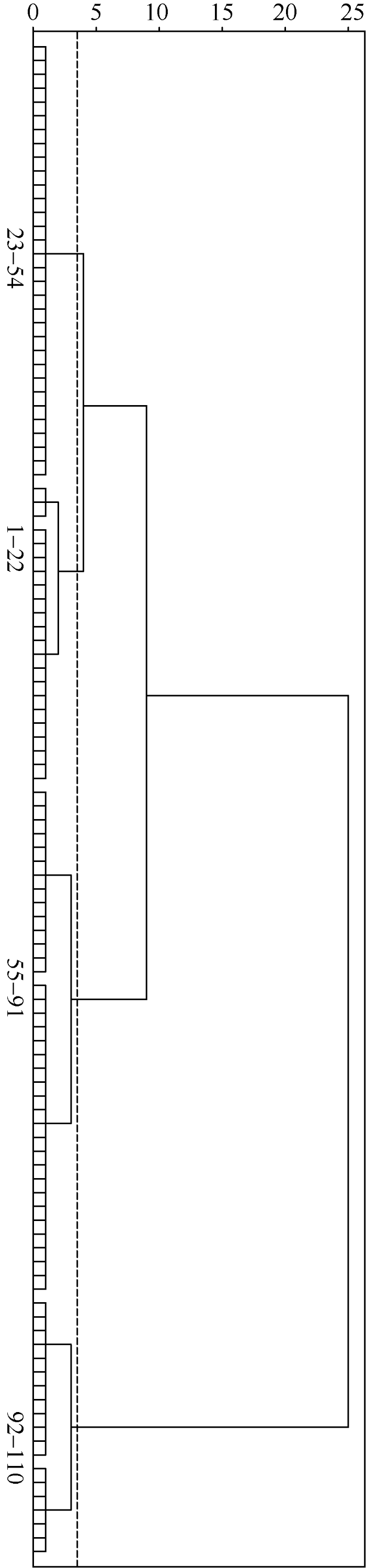 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1110份高粱品种的芽期耐盐性聚类图
图中数字范围为各品种耐盐性排序(平均隶属函数值)范围。
Fig. 1Cluster analysis of salt tolerance of 110 sorghum landraces at germination stage
The numerical range in figure is the range of the salt tolerance (based on average membership function value) of various landraces.
2.4 110份高粱品种苗期耐盐性分析
相关性分析结果显示苗期耐盐性指标间相关性较好, 但不同指标间相关性存在差异, 为了使评价性状体系简洁有效, 就需要避免性状反映信息重复, 因此使用主成分分析法对指标进行降维处理, 前3个主成分贡献率分别为47.9%、15.4%、15.0%, 累积贡献率达到78.2%, 结果见表3, 符合主成分分析要求(累计贡献率大于70%), 能够解释该组数据的变化趋势, 因此取前3个主成分作为数据的有效成分。各因子在主成分中的载荷可以代表主成分与各指标的相关系数见表4。第I主成分中, 相对苗干重的相关系数最大(0.859), 相对苗鲜重次之(0.820), 相对苗长、相对根鲜重和相对根干重这3个性状的相关系数也达到了0.7以上, 反映了高粱幼苗生长状况可作为判断其是否耐盐的主要因素; 第II主成分中, 相对叶绿素含量的相关系数最大, 说明幼苗的光合作用能力与高粱的耐盐强弱有密切关系; 第III主成分中, 相对根鲜重的相关系数最大, 相对根长和相对根干重也较大, 说明了根部生长情况反映了高粱的耐盐能力。Table 3
表3
表33个主成分的特征值及贡献率
Table 3
| 主成分Principal component | 特征值Eigen value | 贡献率Contribution (%) | 累积贡献率Cumulative contribution (%) |
|---|---|---|---|
| I | 3.350 | 47.9 | 47.9 |
| II | 1.076 | 15.4 | 63.3 |
| III | 1.049 | 15.0 | 78.2 |
新窗口打开|下载CSV
Table 4
表4
表4各因子载荷矩阵
Table 4
| 主成分Principal component | S1 | S2 | S3 | S4 | S5 | S6 | S7 |
|---|---|---|---|---|---|---|---|
| I | 0.351 | 0.764 | 0.450 | 0.820 | 0.859 | 0.712 | 0.723 |
| II | 0.780 | -0.273 | 0.401 | -0.366 | -0.172 | 0.018 | 0.262 |
| III | 0.133 | 0.264 | 0.538 | 0.209 | 0.147 | -0.586 | -0.514 |
新窗口打开|下载CSV
综上分析, 3个主成分足以反映盐胁迫下高粱苗期耐盐的信息, 因此相对苗干重、相对叶绿素含量和相对根鲜重可作为鉴定高粱苗期耐盐性的重要指标。
主成分载荷与其相对应特征值所开的平方根的比值[24], 可得到每个性状的成分得分系数, 据此可获得如下3个主成分得分公式。
F1=0.192S1+0.417S2+0.246S3+0.448S4+0.470S5+0.389S6+0.395S7
F2=0.752S1-0.264S2+0.386S3-0.352S4-0.165S5+0.018S6+0.253S7
F3=0.130S1+0.258S2+0.526S3+0.204S4+0.144S5-0.572S6-0.501S7
根据F1、F2和F3的值, 各品种的综合得分根据下列公式计算。
$F=\sum^{k(n)}_{l=1}w_{l}f_{l}$=0.47857 F1+0.15367 F2+0.14986 F3
根据上述公式计算获得110个高粱品种的苗期耐盐性综合得分(F)及耐盐性排序结果, 见附表3, 并利用系统聚类中基于欧式平方距离的组间连接法, 对110份高粱品种进行聚类分析, 见图2, 在欧式平方距离介于2和3之间时, 供试高粱被分为4类。第一类群包括37个品种, 占供试材料的33.6%, F值在1.0655~1.2184之间, 各相对性状数值很高, 受盐胁迫影响最小, 因此可认定为苗期高度耐盐品种; 第二类群, 共41个品种, 占供试材料的37.3%, F值在0.9465~1.0616之间, 各相对性状数值较高, 可认定为苗期耐盐品种; 第三类群, 共30个品种, 占供试材料的27.3%, F值在0.7673~0.9379之间, 各相对性状数值比较低, 可认定为苗期盐敏感品种; 第四类群, 共2个品种, 占供试材料的1.8%, F值在0.6079~0.6607之间, 各相对性状数值很低, 受盐胁迫影响最大, 是苗期高度盐敏感品种。
Supplementary table 3
附表3
附表3各品种芽期和苗期耐盐性排序
Supplementary table 3
| 统一编号 Code | 隶属值 Membership value | 隶属值排序 Rank | F值 F-value | F值排名 Rank |
|---|---|---|---|---|
| 00001067 | 0.859 | 13 | 1.0757 | 32 |
| 00001075 | 0.534 | 68 | 1.1006 | 21 |
| 00001076 | 0.407 | 86 | 0.8136 | 105 |
| 00001079 | 0.689 | 41 | 0.9143 | 86 |
| 00001080 | 0.519 | 72 | 1.2184 | 1 |
| 00001081 | 0.866 | 12 | 1.1442 | 7 |
| 00001082 | 0.840 | 17 | 1.0260 | 48 |
| 00001108 | 0.563 | 64 | 1.0900 | 25 |
| 00001112 | 0.931 | 5 | 0.9766 | 69 |
| 00001121 | 0.718 | 36 | 0.9889 | 65 |
| 00001122 | 0.789 | 23 | 0.9544 | 76 |
| 00001124 | 0.782 | 24 | 1.1051 | 20 |
| 00007606 | 0.422 | 84 | 1.0743 | 33 |
| 00007611 | 0.765 | 27 | 0.9897 | 64 |
| 00001185 | 0.092 | 107 | 1.0180 | 50 |
| 00001256 | 0.727 | 34 | 1.1902 | 2 |
| 00001283 | 0.639 | 54 | 1.0156 | 52 |
| 00001310 | 0.707 | 38 | 0.8732 | 98 |
| 00001315 | 0.435 | 82 | 0.8166 | 104 |
| 00001316 | 0.580 | 59 | 1.0786 | 31 |
| 00001381 | 0.824 | 19 | 0.8738 | 96 |
| 00001504 | 0.511 | 74 | 0.8910 | 94 |
| 00001534 | 0.627 | 55 | 0.9338 | 81 |
| 00001804 | 0.018 | 110 | 1.0200 | 49 |
| 00001808 | 0.880 | 9 | 0.9859 | 67 |
| 00001810 | 0.618 | 56 | 1.1436 | 8 |
| 00001812 | 0.919 | 6 | 1.0906 | 24 |
| 00001817 | 0.529 | 70 | 1.0130 | 57 |
| 00001855 | 0.438 | 81 | 1.0833 | 27 |
| 00001864 | 0.603 | 57 | 1.1275 | 14 |
| 00002144 | 0.106 | 106 | 0.9856 | 68 |
| 00002333 | 0.310 | 92 | 1.1285 | 12 |
| 00002361 | 0.897 | 7 | 1.0616 | 38 |
| 00002401 | 0.971 | 2 | 0.9266 | 83 |
| 00007828 | 0.578 | 60 | 0.8936 | 93 |
| 00007901 | 0.774 | 25 | 0.8735 | 97 |
| 00008015 | 0.394 | 87 | 0.9196 | 85 |
| 00008186 | 0.652 | 50 | 0.8703 | 99 |
| 00002018 | 0.569 | 62 | 1.1456 | 6 |
| 00002443 | 0.876 | 10 | 1.0794 | 30 |
| 00002446 | 0.459 | 78 | 1.1474 | 5 |
| 00002500 | 0.494 | 77 | 0.9678 | 74 |
| 00002555 | 0.428 | 83 | 1.0495 | 41 |
| 00002591 | 0.387 | 88 | 1.0655 | 37 |
| 00002592 | 0.603 | 58 | 1.0093 | 58 |
| 00002596 | 0.568 | 63 | 0.8910 | 95 |
| 00002699 | 0.241 | 96 | 0.9695 | 71 |
| 00003011 | 0.959 | 3 | 1.1421 | 9 |
| 00008219 | 0.418 | 85 | 1.0973 | 22 |
| 00008230 | 0.876 | 11 | 1.1059 | 19 |
| 00008263 | 0.844 | 16 | 1.0396 | 44 |
| 00003043 | 0.730 | 32 | 0.9352 | 80 |
| 00003044 | 0.852 | 14 | 0.8176 | 103 |
| 00003092 | 0.665 | 48 | 0.9917 | 62 |
| 00003126 | 0.657 | 49 | 1.1070 | 18 |
| 00003217 | 0.830 | 18 | 1.1390 | 10 |
| 00003260 | 0.682 | 42 | 1.0031 | 60 |
| 00003435 | 0.511 | 73 | 1.1187 | 16 |
| 00003436 | 0.496 | 76 | 0.8561 | 100 |
| 00003634 | 0.451 | 79 | 0.9699 | 70 |
| 00003659 | 0.297 | 94 | 0.9132 | 87 |
| 00004539 | 0.238 | 97 | 1.0488 | 42 |
| 00004551 | 0.763 | 28 | 1.0343 | 46 |
| 00004595 | 0.823 | 20 | 1.0704 | 35 |
| 00008566 | 0.844 | 15 | 1.0724 | 34 |
| 00008675 | 0.884 | 8 | 0.9862 | 66 |
| 00005015 | 0.932 | 4 | 0.9687 | 72 |
| 00005035 | 0.821 | 21 | 0.9562 | 75 |
| 00005038 | 0.523 | 71 | 0.8116 | 106 |
| 00005059 | 0.681 | 43 | 1.0134 | 56 |
| 00005061 | 0.988 | 1 | 1.0505 | 40 |
| 00005091 | 0.717 | 37 | 0.9224 | 84 |
| 00005092 | 0.806 | 22 | 1.1759 | 3 |
| 00005103 | 0.545 | 66 | 1.0156 | 53 |
| 00005110 | 0.772 | 26 | 0.9066 | 89 |
| 00005197 | 0.542 | 67 | 0.8062 | 107 |
| 00005349 | 0.703 | 39 | 1.0162 | 51 |
| 00005487 | 0.573 | 61 | 1.0950 | 23 |
| 00005624 | 0.645 | 52 | 1.0683 | 36 |
| 00007177 | 0.691 | 40 | 1.0141 | 55 |
| 00007178 | 0.352 | 90 | 0.6607 | 109 |
| 00007182 | 0.649 | 51 | 0.9520 | 77 |
| 00007184 | 0.665 | 47 | 1.0024 | 61 |
| 00007226 | 0.736 | 31 | 0.9916 | 63 |
| 00007247 | 0.530 | 69 | 1.1258 | 15 |
| 00007249 | 0.671 | 44 | 1.0887 | 26 |
| 00007251 | 0.202 | 102 | 1.1087 | 17 |
| 00007257 | 0.670 | 45 | 1.1275 | 13 |
| 00007265 | 0.256 | 95 | 0.8970 | 91 |
| 00007315 | 0.669 | 46 | 0.7673 | 108 |
| 00006906 | 0.223 | 98 | 0.9012 | 90 |
| 00009037 | 0.727 | 35 | 1.0321 | 47 |
| 00009065 | 0.211 | 100 | 0.9379 | 79 |
| 00009079 | 0.363 | 89 | 1.0432 | 43 |
| 00009111 | 0.064 | 108 | 0.8946 | 92 |
| 00007173 | 0.211 | 101 | 1.1286 | 11 |
| 00009822 | 0.548 | 65 | 1.0828 | 28 |
| 00009883 | 0.751 | 29 | 0.9465 | 78 |
| 00009893 | 0.644 | 53 | 1.0371 | 45 |
| 00009903 | 0.506 | 75 | 0.8217 | 102 |
| 00009904 | 0.440 | 80 | 1.0809 | 29 |
| 00009905 | 0.750 | 30 | 0.9276 | 82 |
| 00009929 | 0.039 | 109 | 1.0047 | 59 |
| 00010016 | 0.299 | 93 | 1.1644 | 4 |
| 00013028 | 0.729 | 33 | 1.0583 | 39 |
| 00013029 | 0.193 | 103 | 1.0156 | 54 |
| 00013035 | 0.118 | 105 | 0.8361 | 101 |
| 00013036 | 0.220 | 99 | 0.9114 | 88 |
| 00013125 | 0.349 | 91 | 0.9680 | 73 |
| 00013200 | 0.163 | 104 | 0.6079 | 110 |
新窗口打开|下载CSV
图2
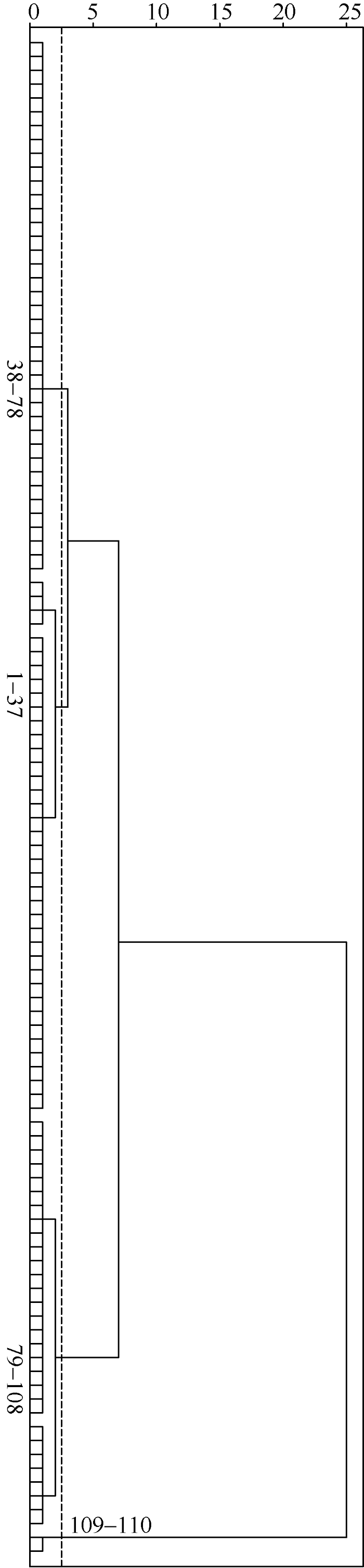 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2110份高粱品种的苗期耐盐性聚类图
图中数字范围为各品种耐盐性排序(F值)范围。
Fig. 2Cluster analysis of salt tolerance of 110 sorghum landraces at seedling stage
The numerical range in figure is the range of the salt tolerance (based on F-value) of various landraces.
2.5 芽期苗期耐盐性综合分析
根据芽期和苗期的耐盐性聚类分析, 110份高粱耐盐性分类清单见表5, 其中, 来自内蒙古的朝阳棒槌(00003011)、大白色(00008230)、八叶齐(00002443)、北京的白鞑子帽(00001081)、北平五号(00001067)、山东的大青壳栗母鸡(00005092)、辽宁的白大秆(00003217)、山西的八叶春齐(00001812)、黑龙江的顶头红(00008566)、短棒子(00004595)10个品种在芽期和苗期均高度耐盐, 其中朝阳棒槌在芽期耐盐性排序中为第3位, 在苗期耐盐性排序中为第9位, 白鞑子帽在芽期耐盐性排序中为第12位, 苗期耐盐性排序中为第7位, 2个品种在盐胁迫下各性状值都很高, 综合耐盐性表现最好; 广西的上龙高粱(00013035)和贵州的褐高粱(00013200) 2个品种在芽期和苗期耐盐性表现都比较差, 上龙高粱在芽期耐盐性排序为105位, 苗期耐盐性排序中为101位, 褐高粱在芽期耐盐性排序中为104位, 在苗期耐盐性排序中为110位, 这2个品种在盐胁迫下各性状受影响非常大, 综合耐盐性表现最差。Table 5
表5
表5110份高粱品种的芽苗期耐盐性分类
Table 5
| 分类 Classification | 分类名称 Group | 芽期品种编号 The code of landraces at germination stage | 苗期品种编号 The code of landraces at seedling stage |
|---|---|---|---|
| I | 高度耐盐品种 Highly salt-tolerant landraces | 00005061, 00002401, 00003011, 00005015, 00001112, 00001812, 00002361, 00008675, 00001808, 00002443, 00008230, 00001081, 00001067, 00003044, 00008566, 00008263, 00001082, 00003217, 00001381, 00004595, 00005035, 00005092. | 00001080, 00001256, 00005092, 00010016, 00002446, 00002018, 00001081, 00001810, 00003011, 00003217, 00007173, 00002333, 00007257, 00001864, 00007247, 00003435, 00007251, 00003126, 00008230, 00001124, 00001075, 00008219, 00005487, 00001812, 00001108, 00007249, 00001855, 00009822, 00009904, 00002443, 00001316, 00001067, 00007606, 00008566, 00004595, 00005624, 00002591. |
| II | 耐盐品种 Salt-tolerant landraces | 00001122, 00001124, 00007901, 00005110, 00007611, 00004551, 00009883, 00009905, 00007226, 00003043, 00013028, 00001256, 00009037, 00001121, 00005091, 00001310, 00005349, 00007177, 00001079, 00003260, 00005059, 00007249, 00007257, 00007315, 00007184, 00003092, 00003126, 00008186, 00007182, 00005624, 00009893, 00001283. | 00002361, 00013028, 00005061, 00002555, 00004539, 00009079, 00008263, 00009893, 00004551, 00009037, 00001082, 00001804, 00001185, 00005349, 00001283, 00005103, 00013029, 00007177, 00005059, 00001817, 00002592, 00009929, 00003260, 00007184, 00003092, 00007226, 00007611, 00001121, 00008675, 00001808, 00002144, 00001112, 00003634, 00002699, 00005015, 00013125, 00002500, 00005035, 00001122, 00007182, 00009883. |
| III | 盐敏感品种 Salt-sensitive landraces | 00001534, 00001810, 00001864, 00002592, 00001316, 00007828, 00005487, 00002018, 00002596, 00001108, 00009822, 00005103, 00005197, 00001075, 00007247, 00001817, 00005038, 00001080, 00003435, 00001504, 00009903, 00003436, 00002500, 00002446, 00003634, 00009904, 00001855, 00001315, 00002555, 00007606, 00008219, 00001076, 00008015, 00002591, 00009079, 00007178, 00013125. | 00009065, 00003043, 00001534, 00009905, 00002401, 00005091, 00008015, 00001079, 00003659, 00013036, 00005110, 00006906, 00007265, 00009111, 00007828, 00001504, 00002596, 00001381, 00007901, 00001310, 00008186, 00003436, 00013035, 00009903, 00003044, 00001315, 00001076, 00005038, 00005197, 00007315. |
| Ⅳ | 高度盐敏感品种 Highly salt-sensitive landraces | 00002333, 00010016, 00003659, 00007265, 00002699, 00004539, 00006906, 00013036, 00009065, 00007173, 00007251, 00013029, 00013200, 00013035, 00002144, 00001185, 00009111, 00009929, 00001804. | 00007178, 00013200. |
新窗口打开|下载CSV
对芽期隶属函数值排名以及苗期F值排名进行相关性分析显示, 其相关系数为0.123 (P=0.202), 说明高粱芽期耐盐性与苗期耐盐性是不相关的。很多高粱品种在芽期和苗期2个时期的耐盐性差异较大, 如芽期耐盐性排在第2位的锣锤穗(00002401), 苗期耐盐性排序仅在83位, 而在苗期耐盐性排序中第11位的软高粱(00007173), 在芽期耐盐性排序中为101位。
3 讨论
高粱作为耐盐能力较强的作物之一, 其耐盐机制尚未得到明确的解释, 对其进行耐盐性评价, 筛选鉴定出耐盐性较强的品种对于开展耐盐分子机制研究、耐盐育种以及充分利用盐渍化土地具有十分重要的意义。在前人的研究当中, 高粱的耐盐性鉴定主要集中在育成品种、杂交种、品系等材料上, 如孙璐等[15]采用人工气候箱内培养皿培养对42份高粱杂交种进行胁迫处理, 任富莉等[18]选取了60份选育品种和4份地方品种进行室内萌发期耐盐性评定, 周福平等[22]以20份高粱品系为材料, 利用隶属函数值法和聚类分析法进行耐盐性分析。对高粱地方品种开展耐盐性鉴定的研究也有一些, 如穆志新等[5]以山西省8个县收集到的49份高粱种质资源研究了芽期盐胁迫对不同高粱材料的影响, 王春语等[19]选取粒用高粱、甜高粱、草用高粱等189份地方品种进行了耐盐性研究, 但总体而言, 选用的材料数量相对较少且生态区覆盖不全面。育成品种是育种家根据各地区的育种目标, 利用原有品种基础, 通过一定的科学方法和育种技术, 培育出适于该地区生产发展需要的优良品种, 而地方品种是经过长期的自然选择和人工种植, 形成的能够适应当地自然生长环境的品种, 地方品种的遗传多样性更为丰富, 往往蕴含着优异的抗逆、抗病等基因资源, 因此对地方品种开展抗逆性鉴定对于抗逆优异资源挖掘和基因鉴定利用具有更为重要的意义。本研究选取我国16个省(自治区)的110份高粱地方品种进行了芽、苗期的耐盐性鉴定, 这些省份大部分位于干旱和半干旱地区的高粱主产地, 同时涵盖中国7大盐渍土分布区的绝大部分地区。根据试验结果, 材料间耐盐性存在较大差异, 其中苗期耐盐性达到耐盐级别以上的品种超过半数, 这表明了大量的地方品种具有很大的耐盐潜力, 可以为选育新的耐盐品种提供优良亲本。
鉴定指标的选择需要根据耐盐鉴定的时期和材料数量的多少来确定, 顾骁等[25]选取30份水稻品种使用可溶性蛋白、可溶性糖、过氧化物酶及多种生理生化指标进行了苗期耐盐性鉴定, 在材料数量较少时选取多个较为复杂的指标可以提高鉴定的准确度。而孙现军等[26]选取550份水稻品种使用有效分蘖数、主穗长度、主穗结实率、单株产量等农艺性状作为指标进行全生育期的大田耐盐鉴定, 说明在材料数量较多、生育期跨度较长的耐盐鉴定工作中更适合使用与产量直接相关的鉴定指标, 保证最大效率地筛选出所需要的耐盐品种。本研究使用发芽势和发芽率2个指标作为芽期耐盐性鉴定的指标, 发芽势和发芽率都能够检验种子在盐胁迫下的发芽能力, 其中发芽率主要检测种子发芽的多少, 而发芽势主要测试种子生命力的强弱, 比如盐胁迫下种子的发芽速度和发芽整齐度。在苗期耐盐性指标方面使用了相对叶绿素含量(SPAD), 盐胁迫下高粱相对叶绿素含量不同程度地低于对照组, 在主成分分析试验结果中, 第II主成分表明, 相对SPAD指标可以说明高粱品种的耐盐性, 已有大量的研究表明SPAD值与生化方法测定的叶绿素含量存在线性关系[27], 但因为各品种间相对SPAD变异程度不是特别明显, 所以相对SPAD并不适用于大量样本的筛选。此外, 苗鲜重、苗干重、根鲜重、根干重等指标能充分反映材料的耐盐性, 即生物量是表现植物生长情况的最直观证据[28]。
目前对于高粱的耐盐性研究多数针对单一生育时期, 也有一部分****对某2个生育期之间的过渡生育期进行研究, 孙璐等[15]和王龙海[29]的研究对高粱的芽期耐盐性筛选延长至第10天左右, 待种子发育成嫩苗后测定芽苗长、芽苗鲜重等指标。这种鉴定方法能在短时间内鉴定大量的材料, 效率较高, 但是鉴定结果可能与分别鉴定芽期和苗期耐盐性存在差异。本试验结果表明, 高粱芽期与苗期的耐盐能力没有明显的相关性, 这与陈亚萍[30]、姜奇彦等[31]研究结论相一致, 植物在芽期和苗期的耐盐能力都至关重要, 种子萌发是植物适应盐生环境并在该环境下成功建植的关键前提[32], 发芽期受到盐胁迫时, 种子因吸收水分的能力降低, 其发芽率会显著降低[33]。高粱苗期对盐胁迫最为敏感[34], 在苗期阶段盐胁迫对植物的危害包括渗透胁迫[35]、离子毒害[36,37]、电解质外渗率增加和自由基含量增加引起的质膜伤害[38,39]、光合作用和呼吸作用引起的生理代谢紊乱等[40,41], 耐盐性机制的不同可能导致植物不同生育时期的耐盐性差异。本研究对110份高粱品种同时进行芽期和苗期的多指标筛选, 鉴定出如朝阳棒槌和白鞑子帽等在2个生育时期耐盐能力都表现优异的高粱品种。
在农业生产中, 良好的出苗率和植株生长情况才是保证产量的基础, 对大量的高粱品种进行耐盐筛选时, 田间自然鉴定受外界影响较大, 尽量使用多个直观的形态指标在实验室条件下初步筛选, 可以有效提高筛选的效率, 同时关注各个生育时期的耐盐性, 保证筛选出的品种具有真正的耐盐应用价值。
4 结论
芽期与苗期耐盐性无显著相关性。苗干重和根鲜重可以作为大量高粱品种耐盐性评价指标。从110份高粱地方品种中筛选出朝阳棒槌(00003011)和白鞑子帽(00001081)等10个在芽期和苗期均表现为高度耐盐的品种可供后续耐盐基因发掘和耐盐品种选育等利用。附表 请见网络版: 1) 本刊网站http://zwxb. chinacrops.org/; 2) 中国知网http://www.cnki.net/; 3) 万方数据http://c.wanfangdata.com.cn/Periodical-zuo wxb.aspx。
参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1016/s1360-1385(00)01838-0URLPMID:11173290 [本文引用: 1]

Soil salinity is a major abiotic stress in plant agriculture worldwide. This has led to research into salt tolerance with the aim of improving crop plants. However, salt tolerance might have much wider implications because transgenic salt-tolerant plants often also tolerate other stresses including chilling, freezing, heat and drought. Unfortunately, suitable genetic model systems have been hard to find. A recently discovered halophytic plant species, Thellungiella halophila, now promises to help in the detection of new tolerance determinants and operating pathways in a model system that is not limited to Arabidopsis traits or ecotype variations.
URL [本文引用: 1]
URL [本文引用: 1]

棉花(Gossypum hirsutum L.)具有一定的耐盐能力,但盐分过量会对棉花造成离子毒害、渗透胁迫和氧化危害。棉花能够通过调控Na+/H+反向转运蛋白将盐离子排出胞质或区隔在液泡内,以减轻离子毒害;还能通过调节渗透相关基因合成脯氨酸、甜菜碱等小分子物质及LEA保护蛋白来进行渗透保护调节。棉花通过抗坏血酸-谷胱甘肽循环途径及提高体内超氧化物歧化酶、谷胱甘肽还原酶等的活性,清除活性氧物质。总结了棉花在耐盐和抗氧化机制方面的研究结果,探讨了棉花耐盐和抗氧化机制之间的相互关系,为提高棉花抗性水平的研究提供理论依据。
URL [本文引用: 1]

棉花(Gossypum hirsutum L.)具有一定的耐盐能力,但盐分过量会对棉花造成离子毒害、渗透胁迫和氧化危害。棉花能够通过调控Na+/H+反向转运蛋白将盐离子排出胞质或区隔在液泡内,以减轻离子毒害;还能通过调节渗透相关基因合成脯氨酸、甜菜碱等小分子物质及LEA保护蛋白来进行渗透保护调节。棉花通过抗坏血酸-谷胱甘肽循环途径及提高体内超氧化物歧化酶、谷胱甘肽还原酶等的活性,清除活性氧物质。总结了棉花在耐盐和抗氧化机制方面的研究结果,探讨了棉花耐盐和抗氧化机制之间的相互关系,为提高棉花抗性水平的研究提供理论依据。
URLPMID:12284747 [本文引用: 1]

Contamination is usually the 1st problem identified when the results of human actions on the environment are considered. However, 2 others are of concern: immoderate population growth and the perenially threatened production of foodstuffs. The miracle of the &quot;green revolution&quot; of the past decade has seen traditional importers of food convert themselves into exporters. The most striking case has been India, where as recently as 1943 a famine killed 1.5 million persons. The 2 pillars of the green revolution are the decreasing diversity of varieties cultivated, with only the most productive utilized, and the use of irrigation. But even with the green revolution, agricultural production between 1984-90 increased by only 1% annually, compared to almost 2% annually for population growth. Use of only a few varieties of basic foods has helped increase productivity because, in addition to being high-yield, they allow the same fertilizers and harvesting machinery to be used, facilitating planting of large areas. Such homogeneity, however, implies great vulnerability if a disease should strike, since the protection of crop diversity is gone. The Irish potato famine of the past century is an example or this type of occurrence. Nor is the use of irrigation without perils. Misuse of irrigation can lead in a relatively short time to salinization and alcalinization of the soil, so that future cultivation is impossible. Soils at risk of erosion are especially vulnerable. Per capita grain production in 1990 was below its past peak in virtually every part of the world. Nevertheless, the measures taken, risky though they have been, were prompted by the need to feed ever larger numbers of persons. Rapid population growth has been estimated to be the cause of 80% of the total loss of vegetable cover. Almost 1/5 of the world's cultivable land may be lost if it is not properly managed. Increased food production has not solved the problem of hunger in the world. It will be necessary for human beings to control their own growth in numbers in order to reduce pressures on the environment.
[本文引用: 2]
[本文引用: 2]
DOI:10.1038/ncomms3320URLPMID:23982223 [本文引用: 1]

Sorghum is a food and feed cereal crop adapted to heat and drought and a staple for 500 million of the world's poorest people. Its small diploid genome and phenotypic diversity make it an ideal C4 grass model as a complement to C3 rice. Here we present high coverage (16-45 × ) resequenced genomes of 44 sorghum lines representing the primary gene pool and spanning dimensions of geographic origin, end-use and taxonomic group. We also report the first resequenced genome of S. propinquum, identifying 8?M high-quality SNPs, 1.9?M indels and specific gene loss and gain events in S. bicolor. We observe strong racial structure and a complex domestication history involving at least two distinct domestication events. These assembled genomes enable the leveraging of existing cereal functional genomics data against the novel diversity available in sorghum, providing an unmatched resource for the genetic improvement of sorghum and other grass species.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1371/journal.pone.0213986URLPMID:31039145 [本文引用: 1]

Among cereal crops, salinity tolerance is rare and complex. Multiple genes control numerous pathways, which constitute plant's response to salinity. Cell cultures act as model system and are useful to investigate the salinity response which can possibly mimic a plant's response to stress. In the present study two indica rice varieties, KS-282 and Super Basmati which exhibited contrasting sodium chloride (NaCl) stress response were used to establish cell cultures. The cell cultures showed a contrasting response to salt stress at 100 mM NaCl. High level of intracellular hydrogen peroxide (H2O2) and nitric oxide (NO) were observed in sensitive cell culture for prolonged period as compared to the tolerant cells in which an extracellular H2O2 burst along with controlled intracellular H2O2 and NO signal was seen. To evaluate the role of NO in inducing cell death under salt stress, cell death percentage (CDP) was measured after 2-4-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) pre-treatment. CDP was reduced significantly in both tolerant and sensitive cell cultures emphasizing NO's possible role in programmed cell death. Expression analysis of apoplastic NADPH oxidase, i.e. OsRbohA and recently characterised OSCA family members i.e. OsOSCA 1.2 and OsOSCA 3.1 was done. Intracellular H2O2/NO levels displayed an interplay between Ca2+ influx and ROS/RNS signal. Detoxifying enzyme (i.e. ascorbate peroxidase and catalase) activity was considerably higher in tolerant KS-282 while the activity of superoxide dismutase was significantly prominent in the sensitive cells triggering greater oxidative damage owing to the prolonged presence of intracellular H2O2. Salt stress and ROS responsive TFs i.e. OsSERF1 and OsDREB2A were expressed exclusively in the tolerant cells. Similarly, the expression of genes involved in maintaining high [K+]/[Na+] ratio was considerably higher and earlier in the tolerant variety. Overall, we suggest that a control over ROS production, and an increase in the expression of genes important for potassium homeostasis play a dynamic role in salinity tolerance in rice cell cultures.
[本文引用: 1]
[本文引用: 1]
DOI:10.1111/ppl.2005.124.issue-3URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 4]
[本文引用: 4]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 2]
[本文引用: 2]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
URL [本文引用: 1]
URL [本文引用: 1]
DOI:10.3724/SP.J.1006.2019.92012URL [本文引用: 1]

对来自国内外不同地区的550份水稻资源进行全生育期耐盐性鉴定。设置淡水、0.3%和0.5%盐溶液浇灌3个处理, 插秧10 d后, 通过浇灌不同体积的淡水与海水调至设计浓度对水稻进行不同浓度的盐胁迫处理。分别调查了淡水及0.3%盐处理下水稻株高、有效分蘖数、主穗长度、主穗结实率、单株产量、抽穗期6项农艺性状和0.5%盐处理下的水稻耐盐表型。与淡水浇灌相比, 全生育期在0.3%盐溶液处理下, 550份水稻(100%)株高显著降低; 124份(22.55%)水稻有效分蘖数(90份增加, 34份减少)、414份(75.27%)水稻主穗长度(405份变短、9份增长)、145份(26.36%)水稻主穗结实率(84份减少, 61份增加)、375份(68.18%)水稻单株产量(343份减少, 32份增加)存在(极)显著差异; 水稻资源的抽穗期无显著差异。主成分分析表明, 主穗结实率、有效分蘖数及单株产量3项性状累计贡献77.25%的变异。根据产量耐盐系数筛选了121份耐盐水稻资源(产量耐盐系数≥0.8), 在0.5%盐胁迫持续处理42 d后, 筛选了78份耐盐水稻资源(耐盐表型为3级), 其中25份水稻资源全生育期在0.3%盐处理下单株产量耐盐系数≥0.8, 在0.5%盐胁迫持续处理42 d后的耐盐表型为3级。筛选的耐盐水稻资源为培育耐盐新品种及深入研究耐盐机制提供材料。
DOI:10.3724/SP.J.1006.2019.92012URL [本文引用: 1]

对来自国内外不同地区的550份水稻资源进行全生育期耐盐性鉴定。设置淡水、0.3%和0.5%盐溶液浇灌3个处理, 插秧10 d后, 通过浇灌不同体积的淡水与海水调至设计浓度对水稻进行不同浓度的盐胁迫处理。分别调查了淡水及0.3%盐处理下水稻株高、有效分蘖数、主穗长度、主穗结实率、单株产量、抽穗期6项农艺性状和0.5%盐处理下的水稻耐盐表型。与淡水浇灌相比, 全生育期在0.3%盐溶液处理下, 550份水稻(100%)株高显著降低; 124份(22.55%)水稻有效分蘖数(90份增加, 34份减少)、414份(75.27%)水稻主穗长度(405份变短、9份增长)、145份(26.36%)水稻主穗结实率(84份减少, 61份增加)、375份(68.18%)水稻单株产量(343份减少, 32份增加)存在(极)显著差异; 水稻资源的抽穗期无显著差异。主成分分析表明, 主穗结实率、有效分蘖数及单株产量3项性状累计贡献77.25%的变异。根据产量耐盐系数筛选了121份耐盐水稻资源(产量耐盐系数≥0.8), 在0.5%盐胁迫持续处理42 d后, 筛选了78份耐盐水稻资源(耐盐表型为3级), 其中25份水稻资源全生育期在0.3%盐处理下单株产量耐盐系数≥0.8, 在0.5%盐胁迫持续处理42 d后的耐盐表型为3级。筛选的耐盐水稻资源为培育耐盐新品种及深入研究耐盐机制提供材料。
[本文引用: 1]
[本文引用: 1]
DOI:10.1016/j.procbio.2011.12.016URL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.11686/cyxb2015233URL [本文引用: 1]

土壤盐碱化已经成为制约农作物生长及产量的重要因子之一,寻求将盐碱化对植物的危害降低到最小程度的策略势在必行。关于植物对盐逆境适应能力的研究已成为全球关注的热点, 如何提高植物的耐盐能力也已成为研究的重中之重。深入探究高等植物适应盐逆境的机制,有助于提高植物耐盐性,增加作物产量和保护生态环境。本文就高等植物适应盐逆境的重点研究进展,综述了盐胁迫对植物的危害;植物耐盐的生理机制,包括渗透调节、营养元素平衡和增强抗氧化胁迫等;植物耐盐相关基因研究进展,包括离子转运蛋白基因、渗透调节相关基因、信号传导相关基因和细胞抗氧化相关基因等;提高植物耐盐性的途径。最后针对今后植物适应盐逆境方面的研究方向进行了展望。
DOI:10.11686/cyxb2015233URL [本文引用: 1]

土壤盐碱化已经成为制约农作物生长及产量的重要因子之一,寻求将盐碱化对植物的危害降低到最小程度的策略势在必行。关于植物对盐逆境适应能力的研究已成为全球关注的热点, 如何提高植物的耐盐能力也已成为研究的重中之重。深入探究高等植物适应盐逆境的机制,有助于提高植物耐盐性,增加作物产量和保护生态环境。本文就高等植物适应盐逆境的重点研究进展,综述了盐胁迫对植物的危害;植物耐盐的生理机制,包括渗透调节、营养元素平衡和增强抗氧化胁迫等;植物耐盐相关基因研究进展,包括离子转运蛋白基因、渗透调节相关基因、信号传导相关基因和细胞抗氧化相关基因等;提高植物耐盐性的途径。最后针对今后植物适应盐逆境方面的研究方向进行了展望。
DOI:10.1093/aob/mcg058URLPMID:12646496 [本文引用: 1]

Tolerance to high soil [Na(+)] involves processes in many different parts of the plant, and is manifested in a wide range of specializations at disparate levels of organization, such as gross morphology, membrane transport, biochemistry and gene transcription. Multiple adaptations to high [Na(+)] operate concurrently within a particular plant, and mechanisms of tolerance show large taxonomic variation. These mechanisms can occur in all cells within the plant, or can occur in specific cell types, reflecting adaptations at two major levels of organization: those that confer tolerance to individual cells, and those that contribute to tolerance not of cells per se, but of the whole plant. Salt-tolerant cells can contribute to salt tolerance of plants; but we suggest that equally important in a wide range of conditions are processes involving the management of Na(+) movements within the plant. These require specific cell types in specific locations within the plant catalysing transport in a coordinated manner. For further understanding of whole plant tolerance, we require more knowledge of cell-specific transport processes and the consequences of manipulation of transporters and signalling elements in specific cell types.
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/s00425-011-1379-yURLPMID:21359959 [本文引用: 1]

Production of reactive oxygen species (hydroxyl radicals, superoxide radicals and hydrogen peroxide) was studied using EPR spin-trapping techniques and specific dyes in isolated plasma membranes from the growing and the non-growing zones of hypocotyls and roots of etiolated soybean seedlings as well as coleoptiles and roots of etiolated maize seedlings. NAD(P)H mediated the production of superoxide in all plasma membrane samples. Hydroxyl radicals were only produced by the membranes of the hypocotyl growing zone when a Fenton catalyst (FeEDTA) was present. By contrast, in membranes from other parts of the seedlings a low rate of spontaneous hydroxyl radical formation was observed due to the presence of small amounts of tightly bound peroxidase. It is concluded that apoplastic hydroxyl radical generation depends fully, or for the most part, on peroxidase localized in the cell wall. In soybean plasma membranes from the growing zone of the hypocotyl pharmacological tests showed that the superoxide production could potentially be attributed to the action of at least two enzymes, an NADPH oxidase and, in the presence of menadione, a quinone reductase.
[本文引用: 1]
DOI:10.1016/j.abb.2008.01.010URLPMID:18241665 [本文引用: 1]

As salt stress imposes a major environmental threat to agriculture, understanding the basic physiology and genetics of cell under salt stress is crucial for developing any transgenic strategy. Salt Overly Sensitive (SOS) genes (SOS1-SOS3) were isolated through positional cloning. Since sos mutants are hypersensitive to salt, their characterization resulted in the discovery of a novel pathway, which has helped in our understanding the mechanism of salt-stress tolerance in plants. Genetic analysis confirmed that SOS1-SOS3 function in a common pathway of salt tolerance. This pathway also emphasizes the significance of Ca2+ signal in reinstating cellular ion homeostasis. SOS3, a Ca2+ sensor, transduces the signal downstream after activating and interacting with SOS2 protein kinase. This SOS3-SOS2 complex activates the Na+/H+ antiporter activity of SOS1 thereby reestablish cellular ion homeostasis. Recently, SOS4 and SOS5 have also been characterized. SOS4 encodes a pyridoxal (PL) kinase that is involved in the biosynthesis of pyridoxal-5-phosphate (PLP), an active form of vitamin B6. SOS5 has been shown to be a putative cell surface adhesion protein that is required for normal cell expansion. Under salt stress, the normal growth and expansion of a plant cell becomes even more important and SOS5 helps in the maintenance of cell wall integrity and architecture. In this review we focus on the recent advances in salt stress and SOS signaling pathway. A broad coverage of the discovery of SOS mutants, structural aspect of these genes and the latest developments in the field of SOS1-SOS5 has been described.
