 ,*, 彭昆鹏信阳师范学院生命科学学院/大别山农业生物资源保护与利用研究院, 河南信阳 464000
,*, 彭昆鹏信阳师范学院生命科学学院/大别山农业生物资源保护与利用研究院, 河南信阳 464000Screening of NFR1α-interactive proteins in soybean using yeast two hybrid system
KE Dan-Xia ,*, PENG Kun-PengCollege of Life Sciences, Xinyang Normal University/Institute for Conservation and Utilization of Agro-bioresources in Dabie Mountains, Xinyang 464000, Henan, China
,*, PENG Kun-PengCollege of Life Sciences, Xinyang Normal University/Institute for Conservation and Utilization of Agro-bioresources in Dabie Mountains, Xinyang 464000, Henan, China通讯作者:
收稿日期:2019-03-7接受日期:2019-08-9网络出版日期:2019-09-02
| 基金资助: |
Received:2019-03-7Accepted:2019-08-9Online:2019-09-02
| Fund supported: |

摘要
关键词:
Abstract
Keywords:
PDF (3098KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
柯丹霞, 彭昆鹏. 利用酵母双杂交系统筛选大豆结瘤因子受体NFR1α的互作蛋白[J]. 作物学报, 2020, 46(1): 31-39. doi:10.3724/SP.J.1006.2020.94036
KE Dan-Xia, PENG Kun-Peng.
豆科植物与根瘤菌互惠共生, 在形成的特殊器官根瘤中, 大气中分子态的氮被转变为可被植物直接利用的氨。类黄酮化合物作为豆科植物分泌的信号分子, 诱导根瘤菌产生结瘤因子(nod factor, NF)。NF作为根瘤菌信号分子反过来作用于豆科植物, 启动一系列结瘤反应[1,2,3]。在模式豆科植物百脉根和苜蓿中, 结瘤因子受体蛋白分别为LjNFR1和LjNFR5[4,5]、MtLYK3/MtLYK4和MtNFP [6,7,8]。它们属于LysM类受体激酶(LysM-RLKs), 由膜内的丝/苏氨酸蛋白激酶结构域、中部跨膜结构域和膜外2~3个LysM结构域共同组成。百脉根LjNFR1和苜蓿MtLYK3均具有蛋白激酶活性, 而LjNFR5和MtNFP因为缺少激活环而不具备激酶活性。研究表明, 百脉根的2个结瘤因子受体蛋白可以形成异源二聚体[9], 高亲和性地直接结合NF [10,11], 并且LjNFR1能够体外磷酸化LjNFR5 [9], 这些证据表明结瘤因子受体蛋白可能以异源二聚体的形式与NF结合, 共同参与接收NF信号, 随后通过激酶间的磷酸化作用将NF信号传递下去。
近年的研究发现, 结瘤因子受体蛋白接收并传递NF信号的过程还需要其他蛋白共同参与。在苜蓿中筛选到1个MtLYK3的互作蛋白-E3泛素连接酶MtPUB1。MtLYK3的激酶结构域能够磷酸化MtPUB1, MtPUB1在结瘤过程的早期侵染阶段发挥作用[12]。在百脉根中也发现1个E3泛素连接酶LjPUB13能够专一地泛素化LjNFR5的激酶结构域, 从而正调控百脉根根瘤器官的发生[13]。此外, 苜蓿中的MtSYMREM1能够分别与MtLYK3和MtNFP互作, MtSYMREM1属于Remorin蛋白, 是一类植物特有的蛋白家族。MtSYMREM1在质膜通过调控结瘤因子受体蛋白参与早期侵染过程, 影响侵染线的极性生长以及根瘤菌的释放[14]。同源克隆百脉根LjSYMREM1发现, 它能够在体内与结瘤因子受体蛋白发生瞬时互作, 在体外能够被LjNFR1磷酸化, 参与调控根瘤菌侵染过程[15]。柯丹霞等[16,17]前期利用酵母双杂交技术, 在百脉根中筛选到LjNFR5的互作蛋白LjROP6, 并证实LjROP6正调控根瘤菌对豆科植物的侵染。最近张忠明研究团队又报道了1个新的LjNFR5相互作用蛋白-百脉根二氢黄酮醇还原酶LjDFL1, 并证实苜蓿中的同源蛋白MtNFP和MtDFL1之间也能够发生相互作用, 推测这2种蛋白之间的互作在不同豆科植物中保守存在[18]。以上证据表明, NFRs及其介导的结瘤信号转导途径在模式豆科植物百脉根和苜蓿中得到了较为广泛和深入的研究, 而大豆中关于这方面的研究报道较少。
大豆(Glycine max)是一种重要的蛋白质和油料作物。充分发挥大豆的根瘤共生固氮作用, 对提高产量, 改良品质意义重大。大豆中已经鉴定到4个结瘤因子受体蛋白, 即GmNFR1α/β和GmNFR5α/ β[19]。GmNFR1α和GmNFR1β分别位于大豆第2号、14号染色体上, 二者之间有着相似的外显子-内含子结构, 碱基和氨基酸序列同源性分别为92%和89%。GmNFR1α和GmNFR1β在DNA序列和外显子-内含子结构上与LjNFR1高度相似, 与LjNFR1和MtLYK3的氨基酸序列相似性分别为79%和75%。生物学功能研究表明, 超表达GmNFR1α能够增加每株大豆的结瘤数, 而GmNFR1β的突变体对结瘤没有影响, GmNFR1β在根瘤菌滴度较低情况下不能感知NF, 但是可以诱导皮层细胞分裂。GmNFR5α/β的突变体不能引起结瘤过程的任何形态学变化。GmNFR1β单独与GmNFR5α/β结合, 在表皮和根毛细胞中不能有效识别NF, 根毛变形、卷曲以及侵染线的形成也被抑制。相反, GmNFR1α与GmNFR5α/β结合能够实现完整的有效结瘤过程[19]。以上结果说明, GmNFR1β的功能是冗余的, GmNFR1α与LjNFR1功能相似, 对结瘤至关重要。但是目前关于GmNFR1α 在共生互作中的具体功能和信号传递机制尚不清楚, 因此, 筛选GmNFR1α相互作用蛋白, 阐明互作蛋白之间结瘤信号的传递方式, 对于揭示GmNFR1α在结瘤信号转导途径中的调控机制具有重要意义。
本研究以大豆结瘤因子受体蛋白GmNFR1α为诱饵, 利用酵母双杂交技术, 筛选大豆根瘤AD-cDNA文库, 钓取GmNFR1α的互作蛋白, 通过互作蛋白的生物信息学分析及其在共生结瘤信号途径中的生物学功能鉴定, 补充和完善GmNFR1α介导的结瘤信号传递途径的认知, 为大豆与根瘤菌共生互作机制提供新的分子证据。
1 材料与方法
1.1 试验材料
大豆根瘤cDNA文库、酵母菌株Y2Hgold、Y187由中国农业科学院油料作物研究所大豆研究室提供; 大豆品种Willimas 82由中国科学院东北地理与农业生态研究所孔凡江研究员提供; 酵母质粒抽提试剂盒、ABA、X-α-Gal等购自Clontech公司; pMD18-T载体、T4 DNA连接酶、限制性内切酶、RNA提取及RT-PCR试剂盒等购自TaKaRa公司; 常规的克隆载体、大肠杆菌菌株等均为本实验室保存。1.2 植物材料处理
在通风厨中令大豆种子在生成的Cl2中消毒过夜[20]。将无菌种子种脐朝下均匀平铺于无菌水润湿的滤纸上, 以封口膜密封培养皿, 22°C暗培养。大豆幼苗根长2~3 cm时收集根部组织, 液氮速冻后保存于-80°C冰箱。1.3 诱饵质粒的构建
根据NCBI网站公布的GmNFR1α序列(GenBank登录号为DQ219805)设计NFR1α-pk (943~ 1755位碱基)引物。F-NFR1α-pk为5′-GGAATTC AGCTTGGAGAATAAAATTGG-3′ (下画线部分为EcoR I酶切位点), R-NFR1α-pk为5′-GCGTCGAC AAGTGTCATGAGAGCAAC-3′ (下画线部分为Sal I酶切位点)。提取大豆根部组织总RNA, 按照TaKaRa公司RT-PCR试剂盒说明书进行反转录, 扩增出NFR1α-pk片段, 其长度为813 bp。将测序正确的目的片段经EcoR I/Sal I双酶切后连接到pGBKT7上。酶切、测序检测无误后即获得诱饵质粒pGBKT7- GmNFR1α-pk。1.4 诱饵质粒自激活及毒性检测
采用LiAc法制备酵母菌株Y2HGold 的感受态细胞, 将测序正确的诱饵质粒与pGADT7空载体共转化Y2HGold, 以含有pGBKT7-53和pGADT7-T质粒的酵母菌为阳性对照, 以含有pGBKT7-lam和pGADT7-T 质粒的酵母菌为阴性对照。将转化后的菌液涂布于DDO/X (SD/-Leu/-Trp/X-α-Gal)平板, 置30°C培养箱暗培养3~5 d, 观察菌落的显色情况, 确定诱饵蛋白是否具有自激活活性。分别挑取SD/-Trp平皿上直径为2~3 mm的诱饵质粒和对照空载体pGBKT7单菌落接种于SD/-Trp/Kan (50 μg mL-1)的液体培养基中, 30°C摇床培养24 h, 测定OD600值以判定诱饵蛋白是否对酵母菌株产生毒性。1.5 筛选大豆根瘤AD-cDNA文库
参照Clontech公司Matchmaker Gold Yeast Two-Hybrid System User Manual所述方法, 接种新鲜的2~3 mm诱饵菌落到SD/-Trp液体培养基中, 30°C摇床培养过夜至OD600达到0.8。离心, 弃上清液, 用4~5 mL SD/-Trp液体重悬沉淀。室温融化1 mL文库菌株, 将文库菌株与诱饵菌株加入含有45 mL 2×YPDA/Kan (50 μg mL-1)液体培养基的2 L锥形瓶中, 30°C摇床低速振荡培养, 以防止细胞在锥形瓶中沉淀(剧烈摇动会降低交配效率, 但晃动过慢会使细胞沉淀, 也降低交配效率)。将杂交20~24 h后的混合液涂布在DDO/X/A (SD/-Leu/-Trp/X-α- Gal/AbA)选择培养基上, 30°C培养4~6 d, 挑取直径大于2 mm的蓝色菌落点种于QDO/X/A (SD/-Ade/- His/-Leu/-Trp/X-α-Gal/AbA)选择培养基上培养3~5 d, 观察并统计酵母的生长及显蓝情况。1.6 文库阳性克隆的鉴定
挑取QDO/X/A平板上生长良好且显蓝的菌落, 参考Easy Yeast Plasmid Isolation Kit说明书提取质粒。pGADT7载体通用引物(F-AD: 5′-CTATTCGA TGATGAAGATACCCCACCAAACCC-3′; R-AD: 5′- GTGAACTTGCGGGGTTTTTCAGTATCTACGATT-3′)进行菌落PCR, 根据扩增的PCR片段大小分析pGADT7载体中插入的DNA片段长度, 并排除大小一致的重复克隆, 初步确定阳性克隆子。1.7 阳性克隆的测序及酵母回转验证
将上述阳性克隆子转化大肠杆菌DH5α, 涂布于LB/Amp (100 μg mL-1)培养基上用于分离文库质粒。抽提文库质粒, 送交公司测序。根据测序结果将获得的文库质粒逐一与pGBKT7-GmNFR1α-pk诱饵质粒共转化至Y2HGold酵母菌株中, 并在QDO/X/A选择性培养基上培养筛选, 回转酵母后再次显蓝的文库质粒可以保种用于今后的研究。1.8 烟草体内BiFC实验
根据NCBI网站公布的GmNFR1α和GmLbc2序列分别构建BiFC载体; 将测序正确的pSCYNE- NFR1α和pSCYCE(R)-GmLbc2荧光互补载体分别转化农杆菌GV3101, 倒置, 28°C培养2~3 d; 挑取单菌落于5 mL LBK液体培养基中, 28°C, 振荡培养1~2 d, 待OD600达到0.6左右, 离心收集菌体, 用农杆菌转化缓冲液分别将农杆菌的OD600调至0.5, 等体积混合两个荧光互补载体的农杆菌悬浮液。室温放置1 h活化农杆菌; 去掉1 mL注射器的针头, 吸取农杆菌混合液, 注射至烟草叶片的下表皮; 将转化后的烟草黑暗培养1~2 d; 然后制片, 用激光共聚焦显微镜观察荧光信号并照相。1.9 阳性克隆的生物信息学分析
根据测序结果分析文库载体中插入的DNA序列, 利用NCBI网站的Blast功能预测目的基因开放读码框长度以及编码蛋白的二级、三级结构特征, 用DNAMAN软件进行序列同源性比对分析, 利用MEGA 4.0构建系统进化树。1.10 转GmLbc2基因超表达复合体植株的共生表型鉴定
将上述扩增的GmLbc2基因全长序列连入植物表达载体pU1301中, 构建超表达载体pU 1301- GmLbc2, 并转入发根农杆菌LBA1334菌株中。利用百脉根(Lotus japonicus)毛根转化方法获得转GmLbc2基因的超表达复合体植株。百脉根MG20种子经表面灭菌、萌发后, 剪去下胚轴, 将幼苗子叶部分放入携带重组质粒的农杆菌菌悬液中侵染30 min, 随后转移到MS平板上暗培养5 d, 再移至含250 mg L-1羧卞的MS培养基上, 直至毛根长出。取毛根根尖处1 cm根段, 用GUS染液鉴定转基因阳性毛根, 然后提取阳性毛根总RNA, 以RT-PCR检测GmLbc2基因的表达, 百脉根多聚泛素(polyubiquitin) UBI基因作为内参, 空载体(pU1301)阳性毛根作为阴性对照。将RT-PCR检测为阳性的复合体百脉根移入无菌沙盆中炼苗5 d, 接种根瘤菌MAFF303099, 每天浇灌无菌Fahraeus无氮营养液。接种30 d后, 对结瘤表型进行统计并拍照, 根据单株结瘤数和样本量(n = 20), 计算单株(CK和GmLbc2-OX)平均结瘤数, 试验重复3次, 采用Microsoft Excel 2007中的t检验分析数据, 并绘制图表。2 结果与分析
2.1 诱饵质粒的构建和自激活及毒性检测
经PCR扩增得到的GmNFR1α膜内激酶结构域GmNFR1α-pk, 大小为813 bp, 与预期长度一致。通过EcoR I/Sal I双酶切, 将GmNFR1α-pk构建至诱饵载体pGBKT7上。重组质粒经双酶切可获得7300 bp的载体片段和813 bp 的插入片段(图1-A)。测序结果表明, 插入片段与NCBI数据库中公布的GmNFR1α-pk基因序列完全一致。说明已成功克隆了GmNFR1α-pk基因并构建了酵母诱饵质粒pGBKT7-GmNFR1α-pk。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1诱饵质粒pGBKT7-GmNFR1α-pk的构建及自激活检测
(A) M: trans 2K Plus II DNA marker; 1: GmNFR1α-pk目的片段扩增; 2: pGBKT7-GmNFR1α-pk诱饵质粒双酶切; 3: pGBKT7空载体双酶切。(B)阳性对照(pGBKT7-53和pGADT7-T)。(C)阴性对照(pGBKT7-lam和pGADT7-T)。(D) pGBKT7-GmNFR1α-pk与pGADT7空载体。
Fig. 1Construction and auto-activation test of the bait plasmid pGBKT7-GmNFR1α-pk
(A) M: trans 2K Plus II DNA marker; 1: isolation of GmNFR1α-pk cDNA; 2: pGBKT7-GmNFR1α-pk digested by EcoR I and Sal I; 3: pGBKT7 digested by EcoR I and Sal I. (B) positive control (pGBKT7-53 and pGADT7-T). (C) negative control (pGBKT7-lam and pGADT7-T). (D) pGBKT7-GmNFR1α-pk and pGADT7.
共转化诱饵质粒pGBKT7-GmNFR1α-pk与pGADT7空载体, 以含有pGBKT7-53和pGADT7-T质粒的酵母菌为阳性对照, 以含有pGBKT7-lam和pGADT7-T质粒的酵母菌为阴性对照。结果显示只有阳性对照在DDO/X平板上出现蓝色菌落, 诱饵质粒和pGADT7空载体组合与阴性对照一样, 只出现白色菌落(图1-B~D), 说明表达的GmNFR1α-pk诱饵蛋白不具有自激活活性。含有诱饵质粒和对照空载体的2种酵母菌在相同条件下培养24 h后生长情况相似, OD600值均大于0.8, 说明诱饵蛋白长势良好, 对酵母菌株无毒性, 可以直接用于后续酵母双杂交文库的筛选。
2.2 筛选与GmNFR1α-pk互作的蛋白
以pGBKT7-GmNFR1α-pk为诱饵筛选大豆根瘤cDNA文库, 将含有诱饵质粒的酵母菌株Y2HGold与含有文库的酵母菌株Y187进行有性杂交, 将杂交后的混合液涂布于DDO/X/A平板上, 30°C培养4~6 d, 挑取直径大于2 mm的蓝色菌落点种于QDO/X/A平板上培养3~5 d, 统计菌落生长及显蓝情况。结果共有71个生长状况良好且显蓝的菌落, 部分结果见图2-A。图2
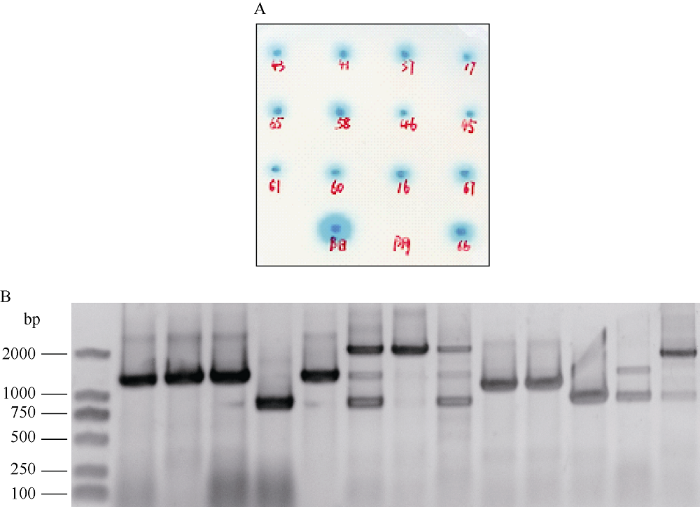 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2酵母双杂交 cDNA 文库的筛选
(A) QDO/X/A平板上的阳性克隆; (B) PCR 检测酵母双杂交cDNA文库插入片段大小。
Fig. 2Screening of yeast two-hybrid cDNA library
(A) positive clones on QDO/X/A plate; (B) PCR detection of inserts from yeast two-hybrid cDNA library.
挑取QDO/X/A平板上的阳性菌落, 扩繁并提取酵母质粒, 以PCR鉴定cDNA文库插入片段的大小, 舍弃500 bp 以下的小片段(图2-B)。将剩余的阳性酵母质粒转化大肠杆菌DH5α, 菌液送交公司进行核苷酸序列测定, 测序结果与GenBank数据库进行同源性比较, 将载体的阅读框与目的基因不同的克隆排除掉, 最终筛选到12种与GmNFR1α-pk互作的蛋白, 包括钙离子结合手性蛋白、豆血红蛋白、结瘤素Nod44、肌醇-磷酸合酶、甲酰转移酶、氨基酸转移酶、NAD激酶、分子伴侣样蛋白HYPK、抗增殖蛋白prohibitin-2等。
2.3 蛋白相互作用的验证
为进一步验证GmNFR1α-pk与靶蛋白的相互作用, 本研究以筛选到的其中一个互作蛋白-大豆血红蛋白GmLbc2 (Leghemoglobin C2)为例, 分别将GmLbc2/GmNFR1α-pk、阴性对照及阳性对照质粒转入酵母菌株Y2HGold, 涂在QDO/X/A培养基上, 30°C培养3~5 d。观察发现, GmNFR1α-pk与靶蛋白GmLbc2共转化时, 在QDO/X/A培养基上菌落呈蓝色(图3-A), 表明二者在酵母体内能够发生相互作用, 酵母回转实验进一步验证了阳性克隆的正确性, 排除了假阳性的干扰。将GmNFR1α和GmLbc2基因分别构建到BiFC中配对的2个载体上, 然后通过瞬时表达系统共转化烟草, 在激光共聚焦显微镜下观察荧光发生情况, 证实2个蛋白在烟草体内也存在相互作用, 且互作的位置在烟草表皮细胞的细胞膜(图3-B)。图3
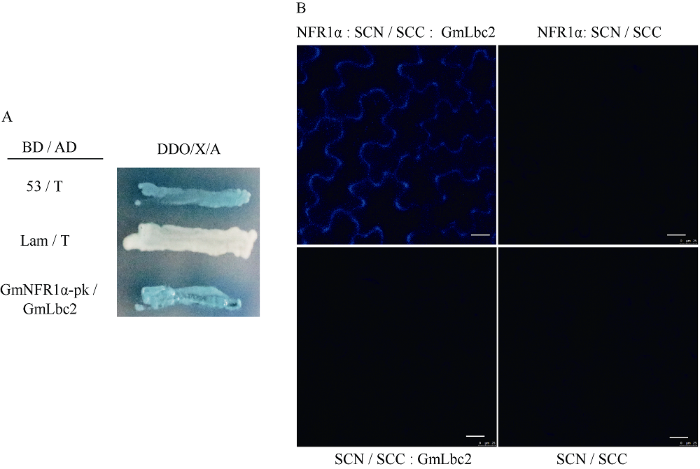 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3GmNFR1α与GmLbc2相互作用的验证
(A)酵母双杂交系统验证GmNFR1α-pk与GmLbc2的相互作用。1: 阳性对照(pGBKT7-53和pGADT7-T); 2: 阴性对照(pGBKT7-lam和pGADT7-T); 3: pGBKT7-GmNFR1α-pk与pGADT7-GmLbc2。(B)双分子荧光互补技术验证GmNFR1α全长与GmLbc2的相互作用。
Fig. 3Interaction between GmNFR1α and GmLbc2
(A) yeast two-hybrid analysis of interaction between GmNFR1α-pk and GmLbc2. 1: positive control (pGBKT7-53 and pGADT7-T); 2: negative control (pGBKT7-lam and pGADT7-T); 3: pGBKT7-GmNFR1α-pk and pGADT7-GmLbc2. (B) BiFC analysis of interaction between GmNFR1α and GmLbc2.
2.4 GmLbc2蛋白的生物信息学分析
已有研究表明, 大豆血红蛋白GmLb受根瘤菌诱导表达, 是存在于共生根瘤类菌体内的一种组织特异性蛋白。GmLb的含量和根瘤的固氮酶活性呈正相关性, 根瘤中GmLb未表达前, 根瘤是没有固氮能力的; 随着GmLb含量的增加, 固氮能力增强; 衰老的根瘤伴随着GmLb的消失, 固氮作用也丧失。GmLb由4个主要组分Lbc1、Lbc2、Lbc3、Lba构成。本研究中筛选到的GmNFR1α-pk互作蛋白即为其中的Lbc2蛋白。序列分析表明, 全长的大豆GmLbc2基因大小为438 bp, 编码蛋白包含145个氨基酸残基。预测的分子量大小为15.5 kD, 理论等电点为5.38。经过Blastp以及DNAMAN软件分析, 对大豆GmLbc2基因编码的氨基酸序列进行同源比对发现, GmLbc2与GmLbc3、野生大豆GsLbc1和GmLba同源性较高(图4)。同时, GmLbc2蛋白与上述不同豆科植物的豆血红蛋白的进化树分析表明, GmLbc2与GmLbc3处在同一进化分支上, 亲缘关系最近(图5)。图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4大豆GmLbc2与其他植物同源蛋白的序列比对分析
Gm: 栽培大豆, Glycine max; Gs: 野生大豆, Glycine soja; Cc: 木豆, Cajanus cajan; Va: 赤豆, Vigna angularis; Vr: 绿豆, Vigna radiata; Mt: 蒺藜苜蓿, Medicago truncatula; Lj: 百脉根, Lotus japonicus; As: 紫云英, Astragalus sinicus。
Fig. 4Amino acid sequence alignment analysis of GmLbc2 and its homologous proteins in some other plants
Gm: Glycine max; Gs: Glycine soja; Cc: Cajanus cajan; Va: Vigna angularis; Vr: Vigna radiata; Mt: Medicago truncatula; Lj: Lotus japonicus; As: Astragalus sinicus.
图5
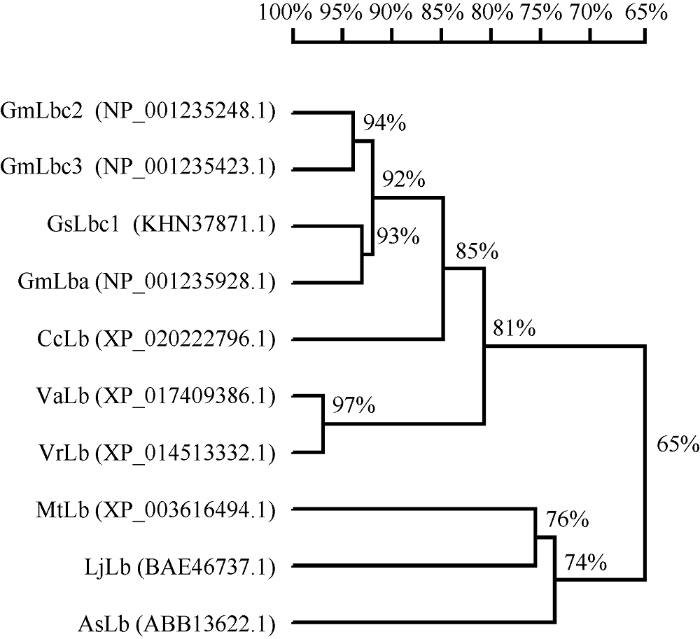 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5大豆GmLbc2蛋白与同系物的系统进化分析
标尺代表遗传相似性, 表明不同物种间同系物进化关系的远近。缩写同
Fig. 5Phylogenetic tree of GmLbc2 and its homologs
The scale represents genetic similarity, indicating the proximity relationships among species. Abbreviations are the same as those given in
2.5 过表达GmLbc2基因对百脉根阳性毛根共生结瘤的影响
为了鉴定GmLbc2基因在结瘤过程中的生物学功能, 根据基因序列信息, 构建超表达载体pU1301- GmLbc2, 通过毛根转化法导入模式豆科植物百脉根毛根中, 进行盆栽实验。接种根瘤菌30 d后, GmLbc2基因在百脉根毛根中的过量表达可以显著增加结瘤数目(图6-A~C)。qRT-PCR结果显示, 在超表达植株中GmLbc2基因的表达水平为对照的12倍, 说明百脉根复合体植株结瘤数目的增加是由于GmLbc2基因的过量表达引起的。以qRT-PCR进一步分析结瘤标志基因NIN、ENOD40-1和ENOD40-2在复合体植株毛根中的表达水平表明, GmLbc2基因的过量表达显著增加这3种结瘤标志基因的表达水平(图6-D)。图6
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6过表达GmLbc2对百脉根阳性毛根共生结瘤的影响
(A)空载体对照复合体植株的结瘤表型。(B)超表达GmLbc2 (GmLbc2-OX)复合体植株的结瘤表型; 接种根瘤菌30 d 后照相, Bars = 5 mm。(C)平均每个植株的结瘤数目, **表示P<0.01。(D) qRT-PCR检测GmLbc2、NIN、Enod40-1和Enod40-2 的表达水平。
Fig. 6Effect of GmLbc2 overexpression on nodulation in L. japonicus
(A) phenotype of hairy roots expressing p1301U (CK). (B) phenotype of hairy roots overexpressing GmLbc2 (GmLbc2-OX); photographs were taken at 30 d after inoculation, Bars = 5 mm. (C) mean number of nodules per plant with standard deviation (SD) of L. japonicus expressing empty vector pU1301 (CK) of GmLbc2-OX at 30 days after inoculation with M. loti, **P<0.01. (D) transcript levels of GmLbc2, NIN, Enod40-1, and Enod40-2 in CK and GmLbc2-OX hairy roots detected by qRT-PCR.
3 讨论
大豆在农业生产中发挥着举足轻重的作用, 大豆与谷物作物玉米、小麦等间作可促进氮的转移, 减少施肥量的同时增加谷物作物的产量, 并改善土壤氮营养状况, 对大豆共生信号调控网络的研究具有重要的现实意义。前人研究表明, 过量表达大豆结瘤因子受体激酶GmNFR1α可以使结瘤增加, 并增强大豆植株在酸性土壤中形成根瘤的能力。GmNFR1α能够直接参与调控大豆G蛋白信号通路。G蛋白亚基Gα与G蛋白活性调节因子RGS都能够直接与GmNFR1α发生相互作用, GmNFR1α通过磷酸化RGS, 调控Gα蛋白活性, 从而维持根瘤的正常发育[21,22]。目前关于GmNFR1α在共生结瘤固氮中的信号传递机制研究才刚刚起步, 其介导的下游信号传递途径仍有待进一步补充和完善。因此, 筛选新的GmNFR1α相互作用蛋白, 阐明互作蛋白之间结瘤信号的传递方式, 对于揭示GmNFR1α在结瘤信号转导途径中的调控机制具有重要意义, 该工作具有原创性。酵母双杂交技术可以在活体内验证蛋白之间的相互作用, 是一种高效发掘新基因、探索基因调控网络的方法[23]。本研究成功地利用该系统筛选到GmNFR1α的相互作用蛋白, 并对这些靶蛋白进行了进化及功能分析, 为揭示GmNFR1α在结瘤因子信号传递过程中的调控机制提供了新证据。利用BLAST数据库对得到的靶蛋白进行结构功能域和同源性分析, 筛选到12种与GmNFR1α-pk互作的蛋白, 包括钙离子结合手性蛋白、豆血红蛋白、结瘤素Nod44、肌醇-磷酸合酶、甲酰转移酶、氨基酸转移酶、NAD 激酶、分子伴侣样蛋白HYPK、抗增殖蛋白prohibitin-2等。其中, 重复克隆数最多的蛋白为大豆血红蛋白GmLbc2。大豆血红蛋白与固氮作用密切相关, 通过调节根瘤中游离O2的浓度, 保护类菌体产生的易受O2破坏的固氮酶[24]。根瘤中大豆血红蛋白的含量与固氮酶活性呈正相关性, 根瘤菌侵染大豆根系诱导大豆血红蛋白表达后, 根瘤才能够正常地进行固氮作用, 才能为宿主植物提供生长所需的氮源[25]。在大豆结瘤信号途径中,本研究首次将结瘤因子受体蛋白与豆血红蛋白联系起来, 证实二者在酵母体内的互作。推测GmNFR1α-pk与GmLbc2互作可能将早期结瘤因子信号感知和后期有效根瘤的形成有机整合起来, 但具体作用方式及机制还需进一步研究。下一步我们将通过细胞生化实验进一步研究这些靶蛋白与GmNFR1α的互作机制和互作功能区域, 通过靶标基因的超表达和敲除实验研究这些靶蛋白的生物学功能, 期待破解大豆结瘤因子受体蛋白GmNFR1α在共生信号转导途径中的具体功能及分子调控机制。
综上所述, 本研究从大豆根瘤AD-cDNA文库中获得多个与GmNFR1α相互作用的靶蛋白, 并以大豆血红蛋白GmLbc2为例, 对其进行同源蛋白比对及系统进化树分析, 推测二者互作共同调控结瘤过程, 研究结果为进一步阐明GmNFR1α的信号传递机制提供了理论依据。可以相信, 对GmNFR1α互作蛋白功能及作用机制的深入研究必将大大推动大豆共生信号调控网络的研究, 也将为实现非豆科植物的共生固氮提供重要的科学线索。
4 结论
利用酵母双杂交筛选大豆根瘤中GmNFR1α- pk的相互作用蛋白, 获得12种靶蛋白。以大豆豆血红蛋白GmLbc2为例, 对其进行同源蛋白比对、系统进化树及生物学功能分析, 为进一步阐明GmNFR1α的信号传递机制提供了理论依据。参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.7554/eLife.33506URLPMID:29957177 [本文引用: 1]

Recognition of Nod factors by LysM receptors is crucial for nitrogen-fixing symbiosis in most legumes. The large families of LysM receptors in legumes suggest concerted functions, yet only NFR1 and NFR5 and their closest homologs are known to be required. Here we show that an epidermal LysM receptor (NFRe), ensures robust signalling in L. japonicus. Mutants of Nfre react to Nod factors with increased calcium spiking interval, reduced transcriptional response and fewer nodules in the presence of rhizobia. NFRe has an active kinase capable of phosphorylating NFR5, which in turn, controls NFRe downstream signalling. Our findings provide evidence for a more complex Nod factor signalling mechanism than previously anticipated. The spatio-temporal interplay between Nfre and Nfr1, and their divergent signalling through distinct kinases suggests the presence of an NFRe-mediated idling state keeping the epidermal cells of the expanding root system attuned to rhizobia.
DOI:10.1146/annurev.arplant.59.032607.092839URLPMID:18444906 [本文引用: 1]

The formation of nitrogen-fixing nodules on legumes requires an integration of infection by rhizobia at the root epidermis and the initiation of cell division in the cortex, several cell layers away from the sites of infection. Several recent developments have added to our understanding of the signaling events in the epidermis associated with the perception of rhizobial nodulation factors and the role of plant hormones in the activation of cell division leading to nodule morphogenesis. This review focuses on the tissue-specific nature of the developmental processes associated with nodulation and the mechanisms by which these processes are coordinated during the formation of a nodule.
DOI:10.1038/nrmicro2990URL [本文引用: 1]

Plants associate with a wide range of microorganisms, with both detrimental and beneficial outcomes. Central to plant survival is the ability to recognize invading microorganisms and either limit their intrusion, in the case of pathogens, or promote the association, in the case of symbionts. To aid in this recognition process, elaborate communication and counter-communication systems have been established that determine the degree of ingress of the microorganism into the host plant. In this Review, I describe the common signalling processes used by plants during mutualistic interactions with microorganisms as diverse as arbuscular mycorrhizal fungi and rhizobial bacteria.
DOI:10.1038/425569aURLPMID:14534570 [本文引用: 1]
DOI:10.1038/nature02045URLPMID:14534591 [本文引用: 1]

Plants belonging to the legume family develop nitrogen-fixing root nodules in symbiosis with bacteria commonly known as rhizobia. The legume host encodes all of the functions necessary to build the specialized symbiotic organ, the nodule, but the process is elicited by the bacteria. Molecular communication initiates the interaction, and signals, usually flavones, secreted by the legume root induce the bacteria to produce a lipochitin-oligosaccharide signal molecule (Nod-factor), which in turn triggers the plant organogenic process. An important determinant of bacterial host specificity is the structure of the Nod-factor, suggesting that a plant receptor is involved in signal perception and signal transduction initiating the plant developmental response. Here we describe the cloning of a putative Nod-factor receptor kinase gene (NFR5) from Lotus japonicus. NFR5 is essential for Nod-factor perception and encodes an unusual transmembrane serine/threonine receptor-like kinase required for the earliest detectable plant responses to bacteria and Nod-factor. The extracellular domain of the putative receptor has three modules with similarity to LysM domains known from peptidoglycan-binding proteins and chitinases. Together with an atypical kinase domain structure this characterizes an unusual receptor-like kinase.
DOI:10.1126/science.1090074URLPMID:12947035 [本文引用: 1]

The rhizobial infection of legumes has the most stringent demand toward Nod factor structure of all host responses, and therefore a specific Nod factor entry receptor has been proposed. The SYM2 gene identified in certain ecotypes of pea (Pisum sativum) is a good candidate for such an entry receptor. We exploited the close phylogenetic relationship of pea and the model legume Medicago truncatula to identify genes specifically involved in rhizobial infection. The SYM2 orthologous region of M. truncatula contains 15 putative receptor-like genes, of which 7 are LysM domain-containing receptor-like kinases (LYKs). Using reverse genetics in M. truncatula, we show that two LYK genes are specifically involved in infection thread formation. This, as well as the properties of the LysM domains, strongly suggests that they are Nod factor entry receptors.
.
DOI:10.1104/pp.106.084657URLPMID:16844829 [本文引用: 1]

Rhizobial Nod factors are key symbiotic signals responsible for starting the nodulation process in host legume plants. Of the six Medicago truncatula genes controlling a Nod factor signaling pathway, Nod Factor Perception (NFP) was reported as a candidate Nod factor receptor gene. Here, we provide further evidence for this by showing that NFP is a lysin [corrected] motif (LysM)-receptor-like kinase (RLK). NFP was shown both to be expressed in association with infection thread development and to be involved in the infection process. Consistent with deviations from conserved kinase domain sequences, NFP did not show autophosphorylation activity, suggesting that NFP needs to associate with an active kinase or has unusual functional characteristics different from classical kinases. Identification of nine new M. truncatula LysM-RLK genes revealed a larger family than in the nonlegumes Arabidopsis (Arabidopsis thaliana) or rice (Oryza sativa) of at least 17 members that can be divided into three subfamilies. Three LysM domains could be structurally predicted for all M. truncatula LysM-RLK proteins, whereas one subfamily, which includes NFP, was characterized by deviations from conserved kinase sequences. Most of the newly identified genes were found to be expressed in roots and nodules, suggesting this class of receptors may be more extensively involved in nodulation than was previously known.
DOI:10.1104/pp.107.100495URLPMID:17586690 [本文引用: 1]

Rhizobia secrete nodulation (Nod) factors, which set in motion the formation of nitrogen-fixing root nodules on legume host plants. Nod factors induce several cellular responses in root hair cells within minutes, but also are essential for the formation of infection threads by which rhizobia enter the root. Based on studies using bacterial mutants, a two-receptor model was proposed, a signaling receptor that induces early responses with low requirements toward Nod factor structure and an entry receptor that controls infection with more stringent demands. Recently, putative Nod factor receptors were shown to be LysM domain receptor kinases. However, mutants in these receptors, in both Lotus japonicus (nfr1 and nfr5) and Medicago truncatula (Medicago; nfp), do not support the two-receptor model because they lack all Nod factor-induced responses. LYK3, the putative Medicago ortholog of NFR1, has only been studied by RNA interference, showing a role in infection thread formation. Medicago hair curling (hcl) mutants are unable to form curled root hairs, a step preceding infection thread formation. We identified the weak hcl-4 allele that is blocked during infection thread growth. We show that HCL encodes LYK3 and, thus, that this receptor, besides infection, also controls root hair curling. By using rhizobial mutants, we also show that HCL controls infection thread formation in a Nod factor structure-dependent manner. Therefore, LYK3 functions as the proposed entry receptor, specifically controlling infection. Finally, we show that LYK3, which regulates a subset of Nod factor-induced genes, is not required for the induction of NODULE INCEPTION.
DOI:10.1111/j.1365-313X.2010.04431.xURL [本文引用: 2]

P>Soil-living rhizobia secrete lipochitin oligosaccharides known as Nod factors, which in Lotus japonicus are perceived by at least two Nod-factor receptors, NFR1 and NFR5. Despite progress in identifying molecular components critical for initial legume host recognition of the microsymbiont and cloning of downstream components, little is known about the activation and signalling mechanisms of the Nod-factor receptors themselves. Here we show that both receptor proteins localize to the plasma membrane, and present evidence for heterocomplex formation initiating downstream signalling. Expression of NFR1 and NFR5 in Nicotiana benthamiana and Allium ampeloprasum (leek) cells caused a rapid cell-death response. The signalling leading to cell death was abrogated using a kinase-inactive variant of NFR1. In these surviving cells, a clear interaction between NFR1 and NFR5 was detected in vivo through bimolecular fluorescence complementation (BiFC). To analyse the inter- and intramolecular phosphorylation events of the kinase complex, the cytoplasmic part of NFR1 was assayed for in vitro kinase activity, and autophosphorylation on 24 amino acid residues, including three tyrosine residues, was found by mass spectrometry. Substitution of the phosphorylated amino acids of NFR1 identified a single phosphorylation site to be essential for NFR1 Nod-factor signalling in vivo and kinase activity in vitro. In contrast to NFR1, no in vitro kinase activity of the cytoplasmic domain of NFR5 was detected. This is further supported by the fact that a mutagenized NFR5 construct, substituting an amino acid essential for ATP binding, restored nodulation of nfr5 mutant roots.
DOI:10.1073/pnas.1205171109URLPMID:22859506 [本文引用: 1]

Lipochitin oligosaccharides called Nod factors function as primary rhizobial signal molecules triggering legumes to develop new plant organs: root nodules that host the bacteria as nitrogen-fixing bacteroids. Here, we show that the Lotus japonicus Nod factor receptor 5 (NFR5) and Nod factor receptor 1 (NFR1) bind Nod factor directly at high-affinity binding sites. Both receptor proteins were posttranslationally processed when expressed as fusion proteins and extracted from purified membrane fractions of Nicotiana benthamiana or Arabidopsis thaliana. The N-terminal signal peptides were cleaved, and NFR1 protein retained its in vitro kinase activity. Processing of NFR5 protein was characterized by determining the N-glycosylation patterns of the ectodomain. Two different glycan structures with identical composition, Man(3)XylFucGlcNAc(4), were identified by mass spectrometry and located at amino acid positions N68 and N198. Receptor-ligand interaction was measured by using ligands that were labeled or immobilized by application of chemoselective chemistry at the anomeric center. High-affinity ligand binding was demonstrated with both solid-phase and free solution techniques. The K(d) values obtained for Nod factor binding were in the nanomolar range and comparable to the concentration range sufficient for biological activity. Structure-dependent ligand specificity was shown by using chitin oligosaccharides. Taken together, our results suggest that ligand recognition through direct ligand binding is a key step in the receptor-mediated activation mechanism leading to root nodule development in legumes.
DOI:10.1002/cbic.201402125URL [本文引用: 1]

Recognition of carbohydrates by proteins is a ubiquitous biochemical process. In legume-rhizobium symbiosis, lipochitin oligosaccharides, also referred to as nodulation (nod) factors, function as primary rhizobial signal molecules to trigger root nodule development. Perception of these signal molecules is receptor mediated, and nod factor receptor 5 (NFR5) from the model legume Lotus japonicus is predicted to contain three LysM domain binding sites. Here we studied the interactions between nod factor and each of the three NFR5 LysM domains, which were chemically synthesized. LysM domain variants (up to 58 amino acids) designed to optimize solubility were chemically assembled by solid-phase peptide synthesis (SPPS) with microwave heating. Their interaction with nod factors and chitin oligosaccharides was studied by isothermal titration calorimetry and circular dichroism (CD) spectroscopy. LysM2 showed a change in folding upon nod factor binding, thus providing direct evidence that the LysM domain of NFR5 recognizes lipochitin oligosaccharides. These results clearly show that the L. japonicus LysM2 domain binds to the nod factor from Mesorhizobium loti, thereby causing a conformational change in the LysM2 domain. The preferential affinity for nod factors over chitin oligosaccharides was demonstrated by a newly developed glycan microarray. Besides the biological implications, our approach shows that carbohydrate binding to a small protein domain can be detected by CD spectroscopy.
DOI:10.1104/pp.15.01694URLPMID:26839127 [本文引用: 1]

PUB1, an E3 ubiquitin ligase, which interacts with and is phosphorylated by the LYK3 symbiotic receptor kinase, negatively regulates rhizobial infection and nodulation during the nitrogen-fixing root nodule symbiosis in Medicago truncatula In this study, we show that PUB1 also interacts with and is phosphorylated by DOES NOT MAKE INFECTIONS 2, the key symbiotic receptor kinase of the common symbiosis signaling pathway, required for both the rhizobial and the arbuscular mycorrhizal (AM) endosymbioses. We also show here that PUB1 expression is activated during successive stages of root colonization by Rhizophagus irregularis that is compatible with its interaction with DOES NOT MAKE INFECTIONS 2. Through characterization of a mutant, pub1-1, affected by the E3 ubiquitin ligase activity of PUB1, we have shown that the ubiquitination activity of PUB1 is required to negatively modulate successive stages of infection and development of rhizobial and AM symbioses. In conclusion, PUB1 represents, to our knowledge, a novel common component of symbiotic signaling integrating signal perception through interaction with and phosphorylation by two key symbiotic receptor kinases, and downstream signaling via its ubiquitination activity to fine-tune both rhizobial and AM root endosymbioses.
DOI:10.1186/s12870-018-1425-zURLPMID:30285618 [本文引用: 1]

Post-translational modification of receptor proteins is involved in activation and de-activation of signalling systems in plants. Both ubiquitination and deubiquitination have been implicated in plant interactions with pathogens and symbionts.
DOI:10.1073/pnas.0913320107URLPMID:20133878 [本文引用: 1]

Remorin proteins have been hypothesized to play important roles during cellular signal transduction processes. Induction of some members of this multigene family has been reported during biotic interactions. However, no roles during host-bacteria interactions have been assigned to remorin proteins until now. We used root nodule symbiosis between Medicago truncatula and Sinorhizobium meliloti to study the roles of a remorin that is specifically induced during nodulation. Here we show that this oligomeric remorin protein attaches to the host plasma membrane surrounding the bacteria and controls infection and release of rhizobia into the host cytoplasm. It interacts with the core set of symbiotic receptors that are essential for perception of bacterial signaling molecules, and thus might represent a plant-specific scaffolding protein.
DOI:10.1371/journal.pone.0030817URLPMID:22292047 [本文引用: 1]

In legumes rhizobial infection during root nodule symbiosis (RNS) is controlled by a conserved set of receptor proteins and downstream components. MtSYMREM1, a protein of the Remorin family in Medicago truncatula, was shown to interact with at least three receptor-like kinases (RLKs) that are essential for RNS. Remorins are comprised of a conserved C-terminal domain and a variable N-terminal region that defines the six different Remorin groups. While both N- and C-terminal regions of Remorins belonging to the same phylogenetic group are similar to each other throughout the plant kingdom, the N-terminal domains of legume-specific group 2 Remorins show exceptional high degrees of sequence divergence suggesting evolutionary specialization of this protein within this clade. We therefore identified and characterized the MtSYMREM1 ortholog from Lotus japonicus (LjSYMREM1), a model legume that forms determinate root nodules. Here, we resolved its spatio-temporal regulation and showed that over-expression of LjSYMREM1 increases nodulation on transgenic roots. Using a structure-function approach we show that protein interactions including Remorin oligomerization are mainly mediated and stabilized by the Remorin C-terminal region with its coiled-coil domain while the RLK kinase domains transiently interact in vivo and phosphorylate a residue in the N-terminal region of the LjSYMREM1 protein in vitro. These data provide novel insights into the mechanism of this putative molecular scaffold protein and underline its importance during rhizobial infection.
DOI:10.1104/pp.112.197269URL [本文引用: 1]

Nod Factor Receptor5 (NFR5) is an atypical receptor-like kinase, having no activation loop in the protein kinase domain. It forms a heterodimer with NFR1 and is required for the early plant responses to Rhizobium infection. A Rho-like small GTPase from Lotus japonicus was identified as an NFR5-interacting protein. The amino acid sequence of this Rho-like GTPase is closest to the Arabidopsis (Arabidopsis thaliana) ROP6 and Medicago truncatula ROP6 and was designated as LjROP6. The interaction between Rop6 and NFR5 occurred both in vitro and in planta. No interaction between Rop6 and NFR1 was observed. Green fluorescent protein-tagged ROP6 was localized at the plasma membrane and cytoplasm. The interaction between ROP6 and NFR5 appeared to take place at the plasma membrane. The expression of the ROP6 gene could be detected in vascular tissues of Lotus roots. After inoculation with Mesorhizobium loti, elevated levels of ROP6 expression were found in the root hairs, root tips, vascular bundles of roots, nodule primordia, and young nodules. In transgenic hairy roots expressing ROP6 RNA interference constructs, Rhizobium entry into the root hairs did not appear to be affected, but infection thread growth through the root cortex were severely inhibited, resulting in the development of fewer nodules per plant. These data demonstrate a role of ROP6 as a positive regulator of infection thread formation and nodulation in L. japonicus.
[本文引用: 1]
[本文引用: 1]
DOI:10.1094/MPMI-04-18-0104-RURLPMID:30295579 [本文引用: 1]

In almost all symbiotic interactions between rhizobia and leguminous plants, host flavonoid-induced synthesis of Nod factors in rhizobia is required to initiate symbiotic response in plants. In this study, we found that Lotus japonicus Nod factor receptor 5 (LjNFR5) might directly regulate flavonoid biosynthesis during symbiotic interaction with rhizobia. A yeast two-hybrid analysis revealed that a dihydroflavonol-4-reductase-like protein (LjDFL1) interacts with LjNFR5. The interaction between MtDFL1 and MtNFP, two Medicago truncatula proteins with homology to LjDFL1 and LjNFR5, respectively, was also shown, suggesting that interaction between these two proteins might be conserved in different legumes. LjDFL1 was highly expressed in root hairs and epidermal cells of root tips. Lotus ljdfl1 mutants and Medicago mtdfl1 mutants produced significantly fewer infection threads (ITs) than the wild-type control plants following rhizobial treatment. Furthermore, the roots of stable transgenic L. japonicus plants overexpressing LjDFL1 formed more ITs than control roots after exposure to rhizobia. These data indicated that LjDFL1 is a positive regulator of symbiotic signaling. However, the expression of LjDFL1 was suppressed by rhizobial treatment, suggesting that a negative feedback loop might be involved in regulation of the symbiotic response in L. japonicus.
DOI:10.1111/j.1365-313X.2010.04398.xURL [本文引用: 2]

P>Two allelic non-nodulating mutants, nod49 and rj1, were characterized using map-based cloning and candidate gene approaches, and genetic complementation. From our results we propose two highly related lipo-oligochitin LysM-type receptor kinase genes (GmNFR1 alpha and GmNFR1 beta) as putative Nod factor receptor components in soybean. Both mutants contained frameshift mutations in GmNFR1 alpha that would yield protein truncations. Both mutants contained a seemingly functional GmNFR1 beta homeologue, characterized by a 374-bp deletion in intron 6 and 20-100 times lower transcript levels than GmNFR1 alpha, yet both mutants were unable to form nodules. Mutations in GmNFR1 beta within other genotypes had no defects in nodulation, showing that GmNFR1 beta was redundant. Transgenic overexpression of GmNFR1 alpha, but not of GmNFR1 beta, increased nodule number per plant, plant nitrogen content and the ability to form nodules with restrictive, ultra-low Bradyrhizobium japonicum titres in transgenic roots of both nod49 and rj1. GmNFR1 alpha overexpressing roots also formed nodules in nodulation-restrictive acid soil (pH 4.7). Our results show that: (i) NFR1 alpha expression controls nodule number in soybean, and (ii) acid soil tolerance for nodulation and suppression of nodulation deficiency at low titre can be achieved by overexpression of GmNFR1 alpha.
[本文引用: 1]
[本文引用: 1]
DOI:10.1104/pp.113.215400URL [本文引用: 1]

Heterotrimeric G proteins comprising G alpha, G beta, and G gamma subunits regulate many fundamental growth and development processes in all eukaryotes. Plants possess a relatively limited number of G-protein components compared with mammalian systems, and their detailed functional characterization has been performed mostly in Arabidopsis (Arabidopsis thaliana) and rice (Oryza sativa). However, the presence of single G alpha and G beta proteins in both these species has significantly undermined the complexity and specificity of response regulation in plant G-protein signaling. There is ample pharmacological evidence for the role of G proteins in regulation of legume-specific processes such as nodulation, but the lack of genetic data from a leguminous species has restricted its direct assessment. Our recent identification and characterization of an elaborate G-protein family in soybean (Glycine max) and the availability of appropriate molecular-genetic resources have allowed us to directly evaluate the role of G-protein subunits during nodulation. We demonstrate that all G-protein genes are expressed in nodules and exhibit significant changes in their expression in response to Bradyrhizobium japonicum infection and in representative supernodulating and nonnodulating soybean mutants. RNA interference suppression and overexpression of specific G-protein components results in lower and higher nodule numbers, respectively, validating their roles as positive regulators of nodule formation. Our data further show preferential usage of distinct G-protein subunits in the presence of an additional signal during nodulation. Interestingly, the G alpha proteins directly interact with the soybean nodulation factor receptors NFR1 alpha and NFR1 beta, suggesting that the plant G proteins may couple with receptors other than the canonical heptahelical receptors common in metazoans to modulate signaling.
DOI:10.1105/tpc.15.00517URLPMID:26498905 [本文引用: 1]

Signaling pathways mediated by heterotrimeric G-protein complexes comprising Gα, Gβ, and Gγ subunits and their regulatory RGS (Regulator of G-protein Signaling) protein are conserved in all eukaryotes. We have shown that the specific Gβ and Gγ proteins of a soybean (Glycine max) heterotrimeric G-protein complex are involved in regulation of nodulation. We now demonstrate the role of Nod factor receptor 1 (NFR1)-mediated phosphorylation in regulation of the G-protein cycle during nodulation in soybean. We also show that during nodulation, the G-protein cycle is regulated by the activity of RGS proteins. Lower or higher expression of RGS proteins results in fewer or more nodules, respectively. NFR1 interacts with RGS proteins and phosphorylates them. Analysis of phosphorylated RGS protein identifies specific amino acids that, when phosphorylated, result in significantly higher GTPase accelerating activity. These data point to phosphorylation-based regulation of G-protein signaling during nodule development. We propose that active NFR1 receptors phosphorylate and activate RGS proteins, which help maintain the Gα proteins in their inactive, trimeric conformation, resulting in successful nodule development. Alternatively, RGS proteins might also have a direct role in regulating nodulation because overexpression of their phospho-mimic version leads to partial restoration of nodule formation in nod49 mutants.
DOI:10.1016/j.cell.2004.11.044URLPMID:15680330 [本文引用: 1]

Brassinosteroids (BRs) signal through a plasma membrane-localized receptor kinase to regulate plant growth and development. We showed previously that a novel protein, BES1, accumulates in the nucleus in response to BRs, where it plays a role in BR-regulated gene expression; however, the mechanism by which BES1 regulates gene expression is unknown. In this study, we dissect BES1 subdomains and establish that BES1 is a transcription factor that binds to and activates BR target gene promoters both in vitro and in vivo. BES1 interacts with a basic helix-loop-helix protein, BIM1, to synergistically bind to E box (CANNTG) sequences present in many BR-induced promoters. Loss-of-function and gain-of-function mutants of BIM1 and its close family members display BR response phenotypes. Thus, BES1 defines a new class of plant-specific transcription factors that cooperate with transcription factors such as BIM1 to regulate BR-induced genes.
DOI:10.1073/pnas.1116559109URLPMID:22308405 [本文引用: 1]

Globins constitute a superfamily of proteins widespread in all kingdoms of life, where they fulfill multiple functions, such as efficient O(2) transport and modulation of nitric oxide bioactivity. In plants, the most abundant Hbs are the symbiotic leghemoglobins (Lbs) that scavenge O(2) and facilitate its diffusion to the N(2)-fixing bacteroids in nodules. The biosynthesis of Lbs during nodule formation has been studied in detail, whereas little is known about the green derivatives of Lbs generated during nodule senescence. Here we characterize modified forms of Lbs, termed Lba(m), Lbc(m), and Lbd(m), of soybean nodules. These green Lbs have identical globins to the parent red Lbs but their hemes are nitrated. By combining UV-visible, MS, NMR, and resonance Raman spectroscopies with reconstitution experiments of the apoprotein with protoheme or mesoheme, we show that the nitro group is on the 4-vinyl. In vitro nitration of Lba with excess nitrite produced several isomers of nitrated heme, one of which is identical to those found in vivo. The use of antioxidants, metal chelators, and heme ligands reveals that nitration is contingent upon the binding of nitrite to heme Fe, and that the reactive nitrogen species involved derives from nitrous acid and is most probably the nitronium cation. The identification of these green Lbs provides conclusive evidence that highly oxidizing and nitrating species are produced in nodules leading to nitrosative stress. These findings are consistent with a previous report showing that the modified Lbs are more abundant in senescing nodules and have aberrant O(2) binding.
DOI:10.1111/tpj.12762URLPMID:25603991 [本文引用: 1]

Protein tyrosine (Tyr) nitration is a post-translational modification yielding 3-nitrotyrosine (NO2 -Tyr). Formation of NO2 -Tyr is generally considered as a marker of nitro-oxidative stress and is involved in some human pathophysiological disorders, but has been poorly studied in plants. Leghemoglobin (Lb) is an abundant hemeprotein of legume nodules that plays an essential role as an O2 transporter. Liquid chromatography coupled to tandem mass spectrometry was used for a targeted search and quantification of NO2 -Tyr in Lb. For all Lbs examined, Tyr30, located in the distal heme pocket, is the major target of nitration. Lower amounts were found for NO2 -Tyr25 and NO2 -Tyr133. Nitrated Lb and other as yet unidentified nitrated proteins were also detected in nodules of plants not receiving NO3- and were found to decrease during senescence. This demonstrates formation of nitric oxide (˙NO) and NO2- by alternative means to nitrate reductase, probably via a ˙NO synthase-like enzyme, and strongly suggests that nitrated proteins perform biological functions and are not merely metabolic byproducts. In vitro assays with purified Lb revealed that Tyr nitration requires NO2-?+?H2 O2 and that peroxynitrite is not an efficient inducer of nitration, probably because Lb isomerizes it to NO3-. Nitrated Lb is formed via oxoferryl Lb, which generates nitrogen dioxide and tyrosyl radicals. This mechanism is distinctly different from that involved in heme nitration. Formation of NO2 -Tyr in Lb is a consequence of active metabolism in functional nodules, where Lb may act as a sink of toxic peroxynitrite and may play a protective role in the symbiosis.
