 ,1,2,*, 赵惠贤
,1,2,*, 赵惠贤 ,1,2,*
,1,2,*Development and validation of the functional marker of grain weight-related gene TaCYP78A5 in wheat (Triticum aestivum L.)
SI Wen-Jie1, WU Lin-Nan1, GUO Li-Jian1, ZHOU Meng-Die1, LIU Xiang-Li1,2, MA Meng ,1,2,*, ZHAO Hui-Xian
,1,2,*, ZHAO Hui-Xian ,1,2,*
,1,2,*通讯作者:
收稿日期:2019-02-17接受日期:2019-06-12网络出版日期:2019-07-08
| 基金资助: |
Received:2019-02-17Accepted:2019-06-12Online:2019-07-08
| Fund supported: |
作者简介 About authors
司文洁,E-mail:wenjiesi2016@126.com;dik725@163.com。

摘要
关键词:
Abstract
Keywords:
PDF (580KB)元数据多维度评价相关文章导出EndNote|Ris|Bibtex收藏本文
本文引用格式
司文洁, 吴林楠, 郭利建, 周梦蝶, 刘香利, 马猛, 赵惠贤. 小麦粒重相关基因TaCYP78A5功能标记开发及验证[J]. 作物学报, 2019, 45(12): 1905-1911. doi:10.3724/SP.J.1006.2019.91016
SI Wen-Jie, WU Lin-Nan, GUO Li-Jian, ZHOU Meng-Die, LIU Xiang-Li, MA Meng, ZHAO Hui-Xian.
小麦(Triticum aestivum L.)是世界上主要的粮食作物之一, 粒重是小麦产量的主要构成因素, 也是小麦育种中的主要选择性状[1]。粒重相关基因的功能研究和标记开发对小麦性状改良及高产育种具有重要的意义和应用价值。因此, 在小麦中许多粒重相关基因的功能研究被广泛报道。例如, Jiang等[2]克隆得到小麦TaSus2 (Triticum aestivum Sucrose synthase 2)基因, 并证明该基因与小麦产量性状相关联, 其在胚乳发育过程中表达量较高; Su等[3]通过同源克隆在小麦中获得水稻OsGW2 (Oryza sativa Grain Weight 2)同源基因TaGW2 (Triticum aestivum Grain Weight 2)基因, 证明该基因对小麦千粒重起负调控作用。Ma等[4]同源克隆得到小麦TaCwi-A1 (Triticum aestivum Grain Weight 2-A1)的全长cDNA并对其进行QTL分析, 证明其可以解释千粒重4.8%的表型差异。
功能标记(functional marker, FM)是根据功能基因的多态性将基因等位变异与表型性状相关联的一种分子标记, 且开发与特定性状相关联的分子标记是分子标记辅助育种的主要手段[5,6]。SNP-CAPS (cleaved amplified polymorphism sequences, 简称CAPS)分子标记是酶切扩增多态性序列标记技术, 能够快速检测由单碱基变异引起的酶切位点的变化, 广泛应用于植物基因分型、定位、克隆、分子鉴定等[7,8]。针对小麦的TaGW2基因开发等位基因特异性PCR (AS-PCR)标记, 并通过性状关联分析发现TaGW2-6A突变位点与籽粒的表型尤其是粒宽和千粒重紧密相关, 其中Hap-6A-A的CAPS分子标记可作为小麦千粒重的优异功能标记[3]。Zhang等[9]克隆了TaSPL20 (Triticum aestivum Squamosa-promoter binding protein-like 20)基因, 并针对TaSPL20-A开发CAPS分子标记, 其中Hap3-A能提高小麦每穂的小穗数。
细胞色素P450 (Cytochromes P450, 简称CYP)家族是最大的植物蛋白家族之一, 其中CYP78A (cytochrome P450 78A)家族一类可应用于作物改良的基因。在模式植物拟南芥(Arabidopsis thaliana)和水稻(Oryza sativa)中, 许多CYP78A家族成员被鉴定出与植物籽粒发育密切相关[10-12]。例如, 拟南芥中, KLUH/CYP78A5被证实是独立于已知母性遗传之外影响种子大小的基因, 而且其活性高低与种子大小呈正相关[10,13]。水稻中拟南芥CYP78A5的同源基因GE (GIANT EMBRYO)编码CYP78A13蛋白, 可调节胚和胚乳之间的平衡, 过表达CYP78A13会导致胚的减小和胚乳的增大, 最终导致水稻种子体积的增大[1,12,14]。在小麦中, 马猛等[15,16]首次通过同源克隆获得TaCYP78A5和TaCYP78A3基因, 并利用瞬时沉默和过表达技术揭示TaCYP78A5表达水平与小麦粒重呈正相关; 陈之忍等[17]研究表明籽粒特异性启动子pINO (Promoter of Inner No Outer)驱动的TaCYP78A5基因在小麦中过表达能够显著增加小麦粒重。以上研究表明CYP78A5基因在影响植物粒重方面具有重要功能, 但关于小麦自然群体中TaCYP78A5基因是否存在等位基因变异、TaCYP78A5的等位基因是否与产量性状关联以及TaCYP78A5基因功能标记开发等方面未见研究报道。
本研究对TaCYP78A5启动子区进行测序分析, 试图建立基于等位基因多态性位点的分子标记, 并进行等位基因分子标记的实用性验证以及分子标记与小麦千粒重的关联分析; 旨在探索TaCYP78A5基因应用于小麦高产分子育种的可能性, 挖掘与千粒重关联的优异等位基因, 并开发其功能标记, 为小麦高产、高效分子育种提供新的优异基因及功能标记。
1 材料与方法
1.1 试验材料
353份普通小麦品种中30份来自西北农林科技大学小麦试验田, 用于检测目标基因的核苷酸多态性; 黄淮麦区323份现代育成普通小麦品种由中国农业科学院景蕊莲研究员提供, 用于目标基因单倍型的检测及农艺性状的关联分析。1.2 小麦总RNA提取与cDNA合成
不同生育期小麦材料(根、茎、叶、旗叶、5 mm、10 mm幼穗、5 d、10 d、15 d、20 d籽粒)来自田间正常管理的普通小麦小偃6号, 利用北京百泰克多糖多酚植物总RNA快速提取试剂盒提取总RNA, 并利用Takara公司反转录试剂盒(Primescript RT reagent kit Perfect Real Time)进行cDNA第1链的合成。1.3 TaCYP78A5基因时空表达模式分析
以上述已获得的小偃6号不同生育期cDNA为模板, 通过实时荧光定量PCR (quantitative real-time PCR, qRT-PCR)对TaCYP78A5基因时空表达模式进行分析。上下游引物(A5-A-RT-F/R、A5-B-RT-F/R、A5-D-RT-F/R)序列见表1, PCR体系为2×TB Green Premix 12.5 μL, cDNA 50 ng, Free-water 8.5 μL, 10 μmol L-1 的上下游引物各1 μL。反应条件为95℃ 30 s; 95℃ 5 s, 66℃ 30 s, 50个循环。反应完成后绘制95℃ 10 s、65℃ 5 s、95℃ 5 s熔解曲线。所有反应均以内参基因GADPH进行归一化处理, 每样品进行3次重复。Table 1
表1
表1用于TaCYP78A5分析的引物
Table 1
| 引物名称 Primer name | 正向引物序列 Forward primer sequence (5′-3′) | 反向引物序列 Reverse primer sequence (5′-3′) |
|---|---|---|
| A5-Ap | AGCCCCTTCATCTGTCGGGTAACCC | TTAGCCGGGAGGAGGAGCAGGAG |
| A5-Bp | GAAAAGGCGAACCACGGATCATG | TAGCCGGGGGAGGAGCAGGAG |
| A5-Dp | CTTGGCGAAGCCCTGCCGAGATC | CGCCCGTGAGAGCACAGGAGT |
| GADPH | CCTTCCGTGTTCCCACTGTTG | ATGCCCTTGAGGTTTCCCTC |
| A5-A-RT | CCATTCCTCAAGTGGCTCGAT | TGGGTGCACCACCATCCTC |
| A5-B-RT | TCATTCCTCAAGTGGCTCGAC | GGGTGCAGCACCATCCTG |
| A5-D-RT | TCCTGCTCGCCGTGCTC | TTGCGCGGTGGAAGAGG |
| CAPS-A5-Ap | TACAAAGCCGGAGCGCCTTCGCGTTCTTC | GAAGCTGGCTCCTCCCGGCCTGCG |
新窗口打开|下载CSV
1.4 小麦基因组DNA提取
种子萌发后取二叶一心期新鲜叶片, 利用CTAB (Cetyltrimethylammonium Ammonium Bromide)法提取小麦基因组DNA, 以1%琼脂糖凝胶电泳检测其浓度和纯度后于-20℃保存备用。1.5 TaCYP78A5启动子序列全长扩增和序列分析
根据最新公布的中国春小麦基因组序列数据库IWGSC RefSeq v1.0 (International Wheat Genome Sequencing Consortium 2018) TaCYP78A5-2A/2B/2D (基因ID分别为TraesCS2A01G175700/TraesCS2B01G201900/TraesCS2 D01G183000)的启动子序列(简称TaCYP78A5-2Ap/2Bp/ 2Dp)多态性位点进行分析, 利用Primer Premier 5.0软件设计特异性引物序列, 以上述30份小麦品种(15份大粒品种和15份小粒品种)的基因组DNA为模板进行PCR扩增, 所用引物对分别为A5-Ap-F/R、A5-Bp-F/R 和A5-Dp-F/R (序列见表1)。PCR体系为2×KOD buffer 10 μL, 2 μmol L-1 dNTPs 2 μL, KOD 0.5 μL, 10 μmol L-1 的上下游引物各0.5 μL, DNA模板100 ng, ddH2O 5.5 μL; 反应条件为95℃ 5 min; 95℃ 30 s, 66℃ 30 s, 72℃ 2 min, 50个循环; 72℃ 10 min。PCR产物经1%的琼脂糖凝胶电泳检测后送西安擎科生物泽西生物科技有限公司测序; 并利用PlantCARE(http://bioinformatics.psb.ugent.be/webtools/plantcare/html/)在线软件预测和分析其在TaCYP78A5-2Ap/2Bp/2Dp序列的顺式作用元件进行。1.6 30份小麦品种间TaCYP78A5启动子序列测序分析及SNP位点筛选
将30份小麦品种TaCYP78A5基因启动子序列全长的PCR产物送至西安擎科生物泽西生物科技有限公司测序, 将测序结果与最新中国春小麦基因组序列数据库IWGSC中TaCYP78A5-2Ap/2Bp/2Dp序列通过DNAMAN8.0 (https://www.lynnon.com/pc/framepc.html)软件比对分析, 分别获得30份小麦品种在TaCYP78A5-2Ap/2Bp/2Dp序列区间内的SNP位点。1.7 SNP-CAPS标记的开发
为了将不同品种间的SNP位点转化为SNP-CAPS分子标记, 采用二次PCR扩增。将得到的TaCYP78A5-2Ap序列利用Primer premier 5.0软件查找多态性位点, 将其转化为CAPS分子标记位点。利用Primer premier 5.0软件在候选CAPS标记位点两侧设计PCR特异性引物CAPS-A5-Ap-F/R (表1)进行扩增, DNA模板为稀释100倍的TaCYP78A5-2Ap序列全长PCR产物, PCR体系为2×KOD buffer 10 μL, 2 μmol L-1 dNTPs 2 μL, KOD 0.5 μL, 10 μmol L-1 的上下游引物各0.5 μL, DNA模板2 μL, ddH2O 4.5 μL。反应条件为95℃ 5 min; 95℃ 30 s, 72℃ 30 s, 72℃ 15 s, 45个循环; 72℃ 10 min。PCR产物经1%琼脂糖凝胶电泳检测。1.8 酶切分析及验证
根据成功转化为CAPS-5Ap标记的SNP位点, 选择合适的限制性酶进行酶切反应, 酶切反应体系为10×NEB buffer 2 μL, 限制性内切酶Hha I 0.4 μL, PCR产物18 μL。37℃酶切2.5 h, 其中Hha I购自NEB公司, 用4.5%琼脂糖凝胶电泳检测酶切产物。1.9 性状-标记关联分析
为进一步分析CAPS-5Ap标记的实用性, 以2017年收获的黄淮麦区323份现代育成普通小麦品种为材料进行扫描, 并利用Microsoft Excel软件进行千粒重数据统计和分析; 利用TASSEL 2.1软件中的普通线性模型(GLM)将开发的CAPS-5Ap分子标记与323份普通小麦品种的千粒重进行关联分析。其中, 323份普通小麦品种的千粒重表型数据, 以及单倍型与小麦千粒重关联分析由中国农科院景蕊莲研究员课题组提供。2 结果与分析
2.1 TaCYP78A5基因时空表达模式分析
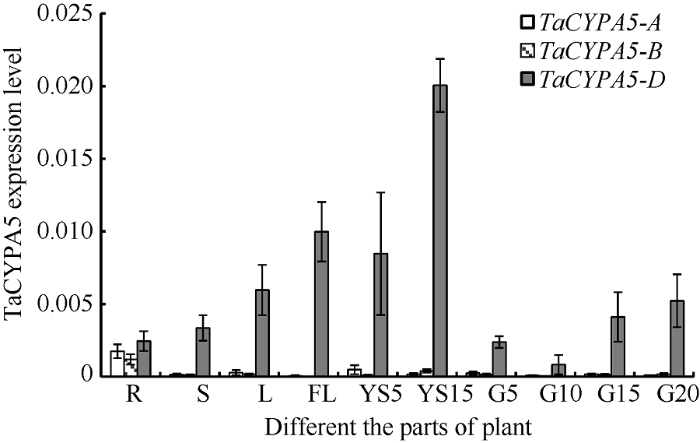
前期研究表明, 小麦中TaCYP78A5基因含有3个直系同源基因, 分别位于2A、2B和2D染色短臂, 被命名为TaCYP78A5-2A、TaCYP78A5-2B、TaCYP78A5-2D[15]。利用小偃6号不同生育期、不同部位(根、茎、叶、旗叶、5 mm和10 mm幼穗以及花后5、10、15、20 d籽粒)样品时空表达模式分析发现, TaCYP78A5基因在小麦各个部位均有表达, 且在幼穗发育阶段表达量较高; 其中TaCYP78A5-2D在各个部位表达量最高, TaCYP78A5-2A次之(图1)。图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图1TaCYP78A5-2A/2B/2D基因表达模式
R: 根; S: 茎; L: 叶; FL: 旗叶; YS5: 5 mm的幼穗; YS15: 15 mm的幼穗; GR5、GR10、GR15和GR20分别表示花后5、10、15和20 d的籽粒。
Fig. 1Gene expression pattern of TaCYP78A5-2A/2B/2D
R: root; S: stem; L: leaf; FL: flag leaf; YS5: 5 mm young panicles; YS15: 15 mm young panicles; GR5, GR10, GR15, and GR20: grain at 5 days, grain at 10 days, grain at 15 days and grain at 20 days after flowering.
2.2 TaCYP78A5启动子全长序列扩增和序列分析
为了进一步了解TaCYP78A5-2A/2B/2D不同亚基因组间差异表达的根源, 在小偃6号小麦品种中对TaCYP78A5-2A/2B/2D启动子区进行全长扩增和序列分析, 扩增获得TaCYP78A5基因启动子3个亚基因组序列信息, 其中TaCYP78A5-2Ap序列长度为1800 bp、TaCYP78A5- 2Bp序列长度为1500 bp, TaCYP78A5-2Dp序列长度为1810 bp。TaCYP78A5-2Ap/2Bp/2Dp基因序列起始密码子前1500 bp序列比对结果表明TaCYP78A5-2Ap/2Bp/2Dp序列内存在较大差异(附图1)。进一步对TaCYP78A5-2Ap/ 2Bp/2Dp序列内的作用元件分析显示, 在TaCYP78A5- 2Ap/2Bp/2Dp的-1500 bp序列内除共同含有CAAT-box和TATA-box基本顺式作用元件外, 还含有激素响应元件ABRE、GC-motif、P-box、TGA-element等和逆境响应元件; 以及TaCYP78A5-2Ap特有元件CAT-box和AT-rich sequence, TaCYP78A5-2Dp特有元件GCN4_motif和TCA- element (表2)。特别是TaCYP78A5-2Ap序列内特有的在分生组织表达的顺式作用元件CAT-box, 可能是引起TaCYP78A5-2A在根部和幼穗期表达量较高的原因。因此, 这些启动子序列和调控元件的差异可能是导致TaCYP78A5基因的3个直系同源基因间表达量巨大差异的根源。附图1
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT附图1TaCYP78A5-2Ap/2Bp/2Dp序列信息比对
Supplementary Fig. 1The sequence information alignment of TaCYP78A5-2Ap/2Bp/2Dp
Table 2
表2
表2TaCYP78A5-2Ap/2Bp/2Dp序列顺式作用元件预测
Table 2
| 顺式作用元件 cis-acting regulatory element | 生物学功能 Biological function | 出现次数Frequency | ||
|---|---|---|---|---|
| TaCYP78A5-2Ap | TaCYP78A5-2Bp | TaCYP78A5-2Dp | ||
| ABRE | 脱落酸应答元件 cis-acting element involved in the abscisic acid responsiveness | 3 | 5 | 5 |
| ARE | 厌氧感应调控元件 cis-acting regulatory element essential for the anaerobic induction | 1 | 1 | 3 |
| AT-rich sequence | 诱导终止激活因子 Element for maximal elicitor-mediated activation (2copies) | 1 | 0 | 0 |
| CAAT-box | 启动子和增强子常见的顺式作用元件 Common cis-acting element in promoter and enhancer regions | 10 | 5 | 5 |
| CAT-box | 分生组织表达的常见顺式作用元件 cis-acting regulatory element related to meristem expression | 1 | 0 | 0 |
| CCAAT-box | MYBHv1转录因子结合位点 MYBHv1 binding site | 1 | 3 | 1 |
| CGTCA-motif | 茉莉酸甲酯响应的调控元件 cis-acting regulatory element involved in the MeJA-responsiveness | 2 | 4 | 0 |
| GC-motif | 特定激素诱导增强元件 Enhancer-like element involved in anoxic specific inducibility | 1 | 2 | 0 |
| GCN4_motif | 胚乳表达相关顺式作用元件 cis-regulatory element involved in endosperm expression | 0 | 0 | 1 |
| P-box | 赤霉素响应元件 Gibberellin-responsive element | 0 | 2 | 1 |
| TATA-box | -30区编码核心启动子元件 Core promoter element around -30 of transcription start | 11 | 14 | 6 |
| TCA-element | 水杨酸响应顺式作用元件 cis-acting element involved in salicylic acid responsiveness | 0 | 0 | 1 |
| TGA-element | 激素响应元件 Auxin-responsive element | 1 | 0 | 1 |
| TGACG-motif | 茉莉酸甲酯响应的调控元件 cis-acting regulatory element involved in the MeJA-responsiveness | 1 | 4 | 0 |
新窗口打开|下载CSV
2.3 30份小麦品种间TaCYP78A5启动子SNP位点识别和筛选
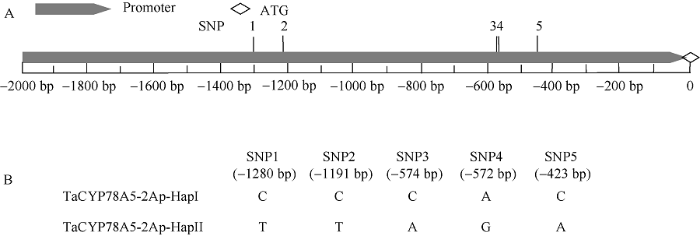
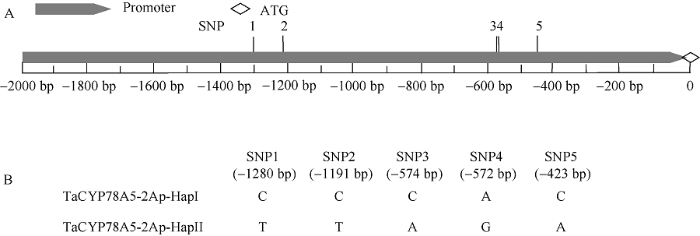
与中国春小麦基因组序列数据库IWGSC RefSeq v1.0比对显示, 在30份小麦品种间TaCYP78A5-2Ap序列共存在5个SNP位点, 分别位于-1292、-1203、-596、-594和-435 bp (图2)。而TaCYP78A5-2Bp序列区间内不存在有规律的SNP位点, TaCYP78A5-2Dp序列区间内存在2个SNP位点。因此, 后续只对不同小麦品种间等位基因TaCYP78A5- 2Ap序列进行SNP变异分析和分子标记开发。图2
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图2TaCYP78A5-2Ap序列结构和等位基因多态性分析
A: TaCYP78A5-2Ap序列结构; B: TaCYP78A5-2Ap序列多态性和等位基因变异分析。
Fig. 2Polymorphism and allelic difference analysis of TaCYP78A5-2Ap
A: structure of TaCYP78A5-2Ap; B: polymorphism and allelic variation analysis of TaCYP78A5-2Ap.
对30份小麦品种TaCYP78A5-2Ap单倍型分析发现, 5个SNP位点差异在30份品种间组成2种等位基因差异类型, 分别为单倍型TaCYP78A5-2Ap-HapI (C/C/C/A/G)和单倍型TaCYP78A5-2Ap-HapII (T/T/A/G/A)。
2.4 TaCYP78A5-Ap的 SNP-CAPS标记的开发
通过对TaCYP78A5-2A两种单倍型TaCYP78A5- 2Ap-HapI和TaCYP78A5-2Ap-HapII序列间差异分析, 发现TaCYP78A5-2Ap-HapI在-1203 bp (SNP2)存在特异性酶(Hha I)酶切位点, 而TaCYP78A5-2Ap-HapII对应位点不存在Hha I的酶切位点; 因此, 根据标记的酶切产物大小可以区分TaCYP78A5-2Ap的两种单倍型。通过在-1203 bp位点上下游设计引物CAPS-A5-Ap- F/R进行二次PCR扩增, 开发SNP-CAPS标记CAPS-5Ap, 并根据标记的酶切产物大小区分TaCYP78A5-2Ap的2种基因类型, 若酶切产物为170 bp和140 bp的2条带, 则待测小麦单倍型为TaCYP78A5-2Ap-HapI; 若酶切产物为310 bp的一条带, 则待测小麦单倍型为TaCYP78A5- 2Ap-HapII。
2.5 SNP-CAPS标记的验证
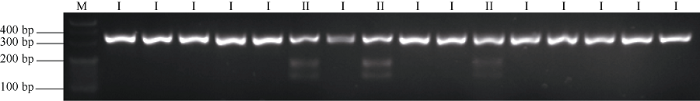
在上述30份小麦品种间对SNP-CAPS标记CAPS- 5Ap检测发现, 其中7份品种能够被Hha I酶切识别, 属于TaCYP78A5-2Ap-HapI单倍型; 其余23份品种均不能被Hha I酶切识别, 属于TaCYP78A5-2Ap-HapII单倍型(图3)。酶切结果与测序结果一致, 表明针对该位点(SNP2)开发的CAPS-5Ap标记能有效鉴别TaCYP78A5-2Ap的2种单倍型。图3
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图3TaCYP78A5-2Ap分子标记的开发
M: 20 bp DNA ladder; I/II: TaCYP78A5-2Ap的2种单倍型; I: TaCYP78A5-2Ap-HapI; II: TaCYP78A5-2Ap-HapII。
Fig. 3Molecular markers development of TaCYP78A5-2Ap
M: 20 bp DNA ladder; I/II: two genotypes of TaCYP78A5-2Ap; I: TaCYP78A5-2Ap-HapI; II: TaCYP78A5-2Ap-HapII.
为了进一步验证CAPS-5Ap标记在不同地区栽培小麦品种中的实用性, 将开发的CAPS-5Ap标记在323份现代育成小麦品种间进行扫描验证。酶切表明, 323份现代育成品种的TaCYP78A5-2Ap序列均能够被Hha I酶酶切产生与上述30品种相同的2种单倍型类型, 部分品种酶切结果如图4所示。在323份现代育成品种中共有58份品种属于TaCYP78A5-2Ap-HapI类型, 分布频率为17.96%; 265份品种属于TaCYP78A5-2Ap-HapII类型, 分布频率为82.04% (图5), 323份现代育成品种中TaCYP78A5-2Ap的单倍型类型统计情况见附表1。以上结果表明CAPS-5Ap标记可用于鉴别不同地区栽培小麦品种中TaCYP78A5-2Ap序列的2种单倍型。
图4
 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图4323份现代育成小麦品种TaCYP78A5-2Ap单倍型扫描验证
M: 20 bp DNA ladder; I/II: TaCYP78A5-2Ap的2种单倍型; I: TaCYP78A5-2Ap-HapI; II: TaCYP78A5-2Ap-HapII。
Fig. 4Scanning verification of TaCYP78A5-2Ap haploidtype in 323 modern varieties of wheat
M: 20 bp DNA ladder; I/II: two haploidtypes of TaCYP78A5-2Ap; I: TaCYP78A5-2Ap-HapI; II: TaCYP78A5-2Ap-HapII.
图5
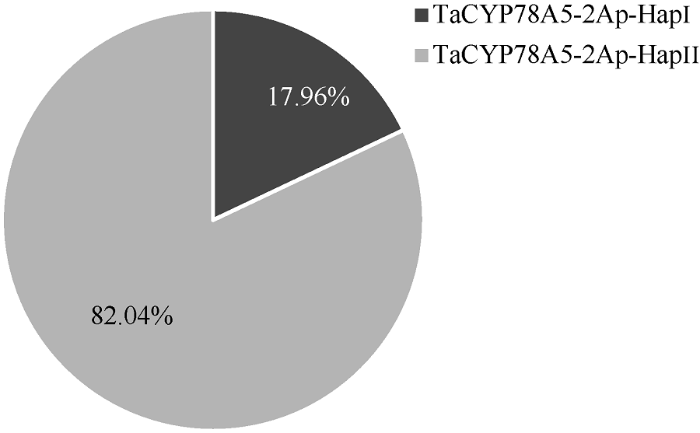 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图5323份现代育成小麦品种中TaCYP78A5-2Ap两种单倍型的分布频率
TaCYP78A5-2Ap-HapI/II: TaCYP78A5-2A启动子的2种单倍型。
Fig. 5Frequency of two TaCYP78A5-2Ap haploidtypes in 323 modern varieties of wheat
TaCYP78A5-2Ap-HapI/II: two haploidtypes for TaCYP78A5-2A promoter.
Supplementary Table 1
附表1
附表1黄淮麦区323份现代育成品种CAPS-5Ap功能标记单倍型统计
Supplementary Table 1
| 序号 Number | 名称 Name | 基因型 Haploidtype | 千粒重 1000-grain weight (g) |
|---|---|---|---|
| 1 | 大荔52 | TaCYP78A5-2Ap-HapI | 21.70 |
| 2 | 丰产1号 | TaCYP78A5-2Ap-HapI | 37.23 |
| 3 | 复壮30 | TaCYP78A5-2Ap-HapI | 24.63 |
| 4 | 衡95观26 | TaCYP78A5-2Ap-HapI | 36.53 |
| 5 | 衡观35 | TaCYP78A5-2Ap-HapI | 37.67 |
| 6 | 衡水6404 | TaCYP78A5-2Ap-HapI | 35.03 |
| 7 | 洛阳8628 | TaCYP78A5-2Ap-HapI | 39.80 |
| 8 | 石4185 | TaCYP78A5-2Ap-HapI | 37.27 |
| 9 | 石家庄8号 | TaCYP78A5-2Ap-HapI | 39.67 |
| 10 | 石麦12 | TaCYP78A5-2Ap-HapI | 42.60 |
| 11 | 石麦15 | TaCYP78A5-2Ap-HapI | 36.33 |
| 12 | 石麦18 | TaCYP78A5-2Ap-HapI | 38.50 |
| 13 | 徐州6号 | TaCYP78A5-2Ap-HapI | 37.80 |
| 14 | 豫麦48 | TaCYP78A5-2Ap-HapI | 42.87 |
| 15 | 豫麦8号 | TaCYP78A5-2Ap-HapI | 35.30 |
| 16 | 豫农949 | TaCYP78A5-2Ap-HapI | 40.47 |
| 17 | 周麦16 | TaCYP78A5-2Ap-HapI | 45.20 |
| 18 | 周麦18 | TaCYP78A5-2Ap-HapI | 40.73 |
| 19 | 石特14 | TaCYP78A5-2Ap-HapI | 28.53 |
| 20 | 西安8号 | TaCYP78A5-2Ap-HapI | 31.03 |
| 21 | 西农1018 | TaCYP78A5-2Ap-HapI | 40.13 |
| 22 | 济麦20 | TaCYP78A5-2Ap-HapI | 36.17 |
| 23 | 洛麦22 | TaCYP78A5-2Ap-HapI | 40.73 |
| 24 | 安86中17 | TaCYP78A5-2Ap-HapI | 25.50 |
| 25 | 白秃头 | TaCYP78A5-2Ap-HapI | 28.00 |
| 26 | 宝临9号 | TaCYP78A5-2Ap-HapI | 26.57 |
| 27 | 碧蚂1号 | TaCYP78A5-2Ap-HapI | 32.93 |
| 28 | 旱选10号 | TaCYP78A5-2Ap-HapI | 35.57 |
| 29 | 旱选11 | TaCYP78A5-2Ap-HapI | 36.20 |
| 30 | 旱选12 | TaCYP78A5-2Ap-HapI | 36.13 |
| 31 | 冀麦22 | TaCYP78A5-2Ap-HapI | 34.40 |
| 32 | 冀麦26 | TaCYP78A5-2Ap-HapI | 33.00 |
| 33 | 冀麦38 | TaCYP78A5-2Ap-HapI | 37.87 |
| 34 | 冀审5099 | TaCYP78A5-2Ap-HapI | 39.20 |
| 35 | 晋麦17 | TaCYP78A5-2Ap-HapI | 38.40 |
| 36 | 晋麦39 | TaCYP78A5-2Ap-HapI | 37.73 |
| 37 | 晋麦68 | TaCYP78A5-2Ap-HapI | 40.93 |
| 38 | 晋麦72 | TaCYP78A5-2Ap-HapI | 43.60 |
| 39 | 晋太182 | TaCYP78A5-2Ap-HapI | 37.27 |
| 40 | 京农80鉴107 | TaCYP78A5-2Ap-HapI | 39.47 |
| 41 | 京品11 | TaCYP78A5-2Ap-HapI | 39.33 |
| 42 | 临旱917 | TaCYP78A5-2Ap-HapI | 40.80 |
| 43 | 陇鉴294 | TaCYP78A5-2Ap-HapI | 39.53 |
| 44 | 鲁德1号 | TaCYP78A5-2Ap-HapI | 38.47 |
| 45 | 蚂蚱麦 | TaCYP78A5-2Ap-HapI | 53.27 |
| 46 | 铭贤169 | TaCYP78A5-2Ap-HapI | 27.20 |
| 47 | 农大311 | TaCYP78A5-2Ap-HapI | 36.07 |
| 48 | 农大36 | TaCYP78A5-2Ap-HapI | 32.30 |
| 49 | 庆丰1号 | TaCYP78A5-2Ap-HapI | 33.53 |
| 50 | 陕农1号 | TaCYP78A5-2Ap-HapI | 35.60 |
| 51 | 四棱红葫芦头 | TaCYP78A5-2Ap-HapI | 28.63 |
| 52 | 太原566 | TaCYP78A5-2Ap-HapI | 41.80 |
| 53 | 西峰16 | TaCYP78A5-2Ap-HapI | 31.27 |
| 54 | 小白麦 | TaCYP78A5-2Ap-HapI | 29.40 |
| 55 | 新冬22 | TaCYP78A5-2Ap-HapI | 37.58 |
| 56 | 燕大1817 | TaCYP78A5-2Ap-HapI | 26.67 |
| 57 | 中苏68 | TaCYP78A5-2Ap-HapI | 29.97 |
| 58 | 紫秆白芒先 | TaCYP78A5-2Ap-HapI | 30.27 |
| 59 | Drysdale | TaCYP78A5-2Ap-HapII | 41.87 |
| 60 | SALGEMMA | TaCYP78A5-2Ap-HapII | 26.30 |
| 61 | 百农160 | TaCYP78A5-2Ap-HapII | 38.60 |
| 62 | 博爱7023 | TaCYP78A5-2Ap-HapII | 40.90 |
| 63 | 大荔1号 | TaCYP78A5-2Ap-HapII | 28.87 |
| 64 | 泛麦8号 | TaCYP78A5-2Ap-HapII | 34.40 |
| 65 | 丰产3号 | TaCYP78A5-2Ap-HapII | 41.70 |
| 66 | 丰优5号 | TaCYP78A5-2Ap-HapII | 38.00 |
| 67 | 邯05-5092 | TaCYP78A5-2Ap-HapII | 34.00 |
| 68 | 邯6172 | TaCYP78A5-2Ap-HapII | 40.33 |
| 69 | 邯郸6050 | TaCYP78A5-2Ap-HapII | 41.93 |
| 70 | 衡216 | TaCYP78A5-2Ap-HapII | 34.63 |
| 71 | 衡4399 | TaCYP78A5-2Ap-HapII | 40.47 |
| 72 | 衡5229 | TaCYP78A5-2Ap-HapII | 32.47 |
| 73 | 衡7228 | TaCYP78A5-2Ap-HapII | 37.47 |
| 74 | 衡麦2号 | TaCYP78A5-2Ap-HapII | 36.17 |
| 75 | 衡优18 | TaCYP78A5-2Ap-HapII | 38.00 |
| 76 | 淮麦18 | TaCYP78A5-2Ap-HapII | 37.30 |
| 77 | 淮麦25 | TaCYP78A5-2Ap-HapII | 37.10 |
| 78 | 淮沭10号 | TaCYP78A5-2Ap-HapII | 37.67 |
| 79 | 兰天15 | TaCYP78A5-2Ap-HapII | 38.10 |
| 80 | 良星99 | TaCYP78A5-2Ap-HapII | 41.87 |
| 81 | 洛夫林10 | TaCYP78A5-2Ap-HapII | 40.53 |
| 82 | 洛旱11 | TaCYP78A5-2Ap-HapII | 46.53 |
| 83 | 洛旱13 | TaCYP78A5-2Ap-HapII | 45.47 |
| 84 | 洛旱2号 | TaCYP78A5-2Ap-HapII | 35.07 |
| 85 | 洛旱3号 | TaCYP78A5-2Ap-HapII | 39.60 |
| 86 | 洛旱6号 | TaCYP78A5-2Ap-HapII | 49.33 |
| 87 | 洛旱7号 | TaCYP78A5-2Ap-HapII | 49.73 |
| 88 | 洛旱8号 | TaCYP78A5-2Ap-HapII | 35.97 |
| 89 | 洛旱9号 | TaCYP78A5-2Ap-HapII | 51.67 |
| 90 | 洛麦21 | TaCYP78A5-2Ap-HapII | 41.33 |
| 91 | 洛麦23 | TaCYP78A5-2Ap-HapII | 36.33 |
| 92 | 洛农10号 | TaCYP78A5-2Ap-HapII | 34.53 |
| 93 | 漯麦8号 | TaCYP78A5-2Ap-HapII | 41.67 |
| 94 | 漯麦9号 | TaCYP78A5-2Ap-HapII | 40.53 |
| 95 | 漯优7号 | TaCYP78A5-2Ap-HapII | 41.73 |
| 96 | 青春1号 | TaCYP78A5-2Ap-HapII | 29.07 |
| 97 | 青春2号 | TaCYP78A5-2Ap-HapII | 26.33 |
| 98 | 清山843 | TaCYP78A5-2Ap-HapII | 35.73 |
| 99 | 石家庄407 | TaCYP78A5-2Ap-HapII | 36.20 |
| 100 | 石麦13 | TaCYP78A5-2Ap-HapII | 42.87 |
| 101 | 石麦19 | TaCYP78A5-2Ap-HapII | 35.00 |
| 102 | 徐州21 | TaCYP78A5-2Ap-HapII | 38.53 |
| 103 | 偃展1号 | TaCYP78A5-2Ap-HapII | 41.00 |
| 104 | 豫保1号 | TaCYP78A5-2Ap-HapII | 38.47 |
| 105 | 豫麦13 | TaCYP78A5-2Ap-HapII | 41.87 |
| 106 | 豫麦18 | TaCYP78A5-2Ap-HapII | 37.73 |
| 107 | 豫麦29 | TaCYP78A5-2Ap-HapII | 31.93 |
| 108 | 豫麦2号 | TaCYP78A5-2Ap-HapII | 34.57 |
| 109 | 豫麦38 | TaCYP78A5-2Ap-HapII | 41.93 |
| 110 | 豫麦47 | TaCYP78A5-2Ap-HapII | 39.07 |
| 111 | 豫农416 | TaCYP78A5-2Ap-HapII | 43.93 |
| 112 | 豫展4号 | TaCYP78A5-2Ap-HapII | 42.47 |
| 113 | 周麦22 | TaCYP78A5-2Ap-HapII | 43.40 |
| 114 | 周麦23 | TaCYP78A5-2Ap-HapII | 40.60 |
| 115 | 石优17 | TaCYP78A5-2Ap-HapII | 34.60 |
| 116 | 石优20 | TaCYP78A5-2Ap-HapII | 32.17 |
| 117 | 皖麦19 | TaCYP78A5-2Ap-HapII | 33.87 |
| 118 | 温麦6号 | TaCYP78A5-2Ap-HapII | 34.53 |
| 119 | 西农189 | TaCYP78A5-2Ap-HapII | 44.27 |
| 120 | 西农219 | TaCYP78A5-2Ap-HapII | 30.90 |
| 121 | 西农318 | TaCYP78A5-2Ap-HapII | 41.13 |
| 122 | 西农6028 | TaCYP78A5-2Ap-HapII | 29.83 |
| 123 | 西农688 | TaCYP78A5-2Ap-HapII | 46.80 |
| 124 | 西农797 | TaCYP78A5-2Ap-HapII | 45.20 |
| 125 | 西农9106 | TaCYP78A5-2Ap-HapII | 47.13 |
| 126 | 鑫麦296 | TaCYP78A5-2Ap-HapII | 37.33 |
| 127 | 济麦19 | TaCYP78A5-2Ap-HapII | 39.10 |
| 128 | 济麦21 | TaCYP78A5-2Ap-HapII | 40.10 |
| 129 | 济麦22 | TaCYP78A5-2Ap-HapII | 39.93 |
| 130 | 济麦4号 | TaCYP78A5-2Ap-HapII | 38.63 |
| 131 | 济南10号 | TaCYP78A5-2Ap-HapII | 41.73 |
| 132 | 济南13 | TaCYP78A5-2Ap-HapII | 37.27 |
| 133 | 济南2号 | TaCYP78A5-2Ap-HapII | 37.27 |
| 134 | 济宁3号 | TaCYP78A5-2Ap-HapII | 36.00 |
| 135 | 邯4589 | TaCYP78A5-2Ap-HapII | 37.80 |
| 136 | 衡136 | TaCYP78A5-2Ap-HapII | 34.90 |
| 137 | 济麦6号 | TaCYP78A5-2Ap-HapII | 35.20 |
| 138 | 青麦7号 | TaCYP78A5-2Ap-HapII | 29.33 |
| 139 | 西农1043 | TaCYP78A5-2Ap-HapII | 45.20 |
| 140 | 红良4号 | TaCYP78A5-2Ap-HapII | 37.20 |
| 141 | 晋麦16 | TaCYP78A5-2Ap-HapII | 40.07 |
| 142 | 晋麦25 | TaCYP78A5-2Ap-HapII | 35.80 |
| 143 | 运旱22-33 | TaCYP78A5-2Ap-HapII | 43.27 |
| 144 | 霸王鞭 | TaCYP78A5-2Ap-HapII | 37.67 |
| 145 | 白糙麦 | TaCYP78A5-2Ap-HapII | 26.57 |
| 146 | 白齐麦 | TaCYP78A5-2Ap-HapII | 28.53 |
| 147 | 宝麦5号 | TaCYP78A5-2Ap-HapII | 36.30 |
| 148 | 北京837 | TaCYP78A5-2Ap-HapII | 39.93 |
| 149 | 北京8686 | TaCYP78A5-2Ap-HapII | 36.43 |
| 150 | 北京8694 | TaCYP78A5-2Ap-HapII | 41.40 |
| 151 | 北农2号 | TaCYP78A5-2Ap-HapII | 35.60 |
| 152 | 沧麦6001 | TaCYP78A5-2Ap-HapII | 40.53 |
| 153 | 沧麦6005 | TaCYP78A5-2Ap-HapII | 41.27 |
| 154 | 沧州小麦 | TaCYP78A5-2Ap-HapII | 41.87 |
| 155 | 昌乐5号 | TaCYP78A5-2Ap-HapII | 40.27 |
| 156 | 长4640 | TaCYP78A5-2Ap-HapII | 44.67 |
| 157 | 长4738 | TaCYP78A5-2Ap-HapII | 41.60 |
| 158 | 长4853 | TaCYP78A5-2Ap-HapII | 41.93 |
| 159 | 长5259 | TaCYP78A5-2Ap-HapII | 34.70 |
| 160 | 长6154 | TaCYP78A5-2Ap-HapII | 42.87 |
| 161 | 长6359 | TaCYP78A5-2Ap-HapII | 33.80 |
| 162 | 长6452 | TaCYP78A5-2Ap-HapII | 44.33 |
| 163 | 长6794 | TaCYP78A5-2Ap-HapII | 35.83 |
| 164 | 长6878 | TaCYP78A5-2Ap-HapII | 36.13 |
| 165 | 长8744 | TaCYP78A5-2Ap-HapII | 36.67 |
| 166 | 长麦6135 | TaCYP78A5-2Ap-HapII | 39.40 |
| 167 | 长武131 | TaCYP78A5-2Ap-HapII | 39.47 |
| 168 | 长武134 | TaCYP78A5-2Ap-HapII | 43.53 |
| 169 | 长武89(1)3-4 | TaCYP78A5-2Ap-HapII | 42.13 |
| 170 | 长治516 | TaCYP78A5-2Ap-HapII | 33.97 |
| 171 | 长治620 | TaCYP78A5-2Ap-HapII | 58.13 |
| 172 | 单R8043 | TaCYP78A5-2Ap-HapII | 36.13 |
| 173 | 单R8093 | TaCYP78A5-2Ap-HapII | 37.80 |
| 174 | 单R8108 | TaCYP78A5-2Ap-HapII | 42.20 |
| 175 | 单R8194 | TaCYP78A5-2Ap-HapII | 38.07 |
| 176 | 冬协2号 | TaCYP78A5-2Ap-HapII | 35.27 |
| 177 | 丰抗13 | TaCYP78A5-2Ap-HapII | 34.10 |
| 178 | 旱选1号 | TaCYP78A5-2Ap-HapII | 39.87 |
| 179 | 旱选2号 | TaCYP78A5-2Ap-HapII | 34.60 |
| 180 | 旱选3号 | TaCYP78A5-2Ap-HapII | 30.07 |
| 181 | 黑芒麦 | TaCYP78A5-2Ap-HapII | 35.33 |
| 182 | 红和尚 | TaCYP78A5-2Ap-HapII | 35.67 |
| 183 | 葫芦头 | TaCYP78A5-2Ap-HapII | 38.33 |
| 184 | 花培6号 | TaCYP78A5-2Ap-HapII | 35.20 |
| 185 | 华北187 | TaCYP78A5-2Ap-HapII | 39.80 |
| 186 | 冀92-5203 | TaCYP78A5-2Ap-HapII | 32.53 |
| 187 | 冀麦10号 | TaCYP78A5-2Ap-HapII | 36.80 |
| 188 | 冀麦29 | TaCYP78A5-2Ap-HapII | 33.73 |
| 189 | 冀麦2号 | TaCYP78A5-2Ap-HapII | 38.60 |
| 190 | 冀麦30 | TaCYP78A5-2Ap-HapII | 30.00 |
| 191 | 冀麦32 | TaCYP78A5-2Ap-HapII | 39.93 |
| 192 | 冀麦41 | TaCYP78A5-2Ap-HapII | 30.80 |
| 193 | 冀麦6号 | TaCYP78A5-2Ap-HapII | 38.73 |
| 194 | 冀麦9号 | TaCYP78A5-2Ap-HapII | 40.07 |
| 195 | 冀麦一号 | TaCYP78A5-2Ap-HapII | 32.80 |
| 196 | 鉴26 | TaCYP78A5-2Ap-HapII | 31.73 |
| 197 | 金光 | TaCYP78A5-2Ap-HapII | 43.40 |
| 198 | 晋2148-7 | TaCYP78A5-2Ap-HapII | 41.20 |
| 199 | 晋麦13 | TaCYP78A5-2Ap-HapII | 35.73 |
| 200 | 晋麦33 | TaCYP78A5-2Ap-HapII | 39.47 |
| 201 | 晋麦44 | TaCYP78A5-2Ap-HapII | 38.60 |
| 202 | 晋麦47 | TaCYP78A5-2Ap-HapII | 39.67 |
| 203 | 晋麦50 | TaCYP78A5-2Ap-HapII | 38.13 |
| 204 | 晋麦51 | TaCYP78A5-2Ap-HapII | 38.40 |
| 205 | 晋麦53 | TaCYP78A5-2Ap-HapII | 40.93 |
| 206 | 晋麦54 | TaCYP78A5-2Ap-HapII | 42.80 |
| 207 | 晋麦57 | TaCYP78A5-2Ap-HapII | 42.67 |
| 208 | 晋麦63 | TaCYP78A5-2Ap-HapII | 44.27 |
| 209 | 晋麦79 | TaCYP78A5-2Ap-HapII | 36.87 |
| 210 | 晋麦91 | TaCYP78A5-2Ap-HapII | 40.80 |
| 211 | 晋农207 | TaCYP78A5-2Ap-HapII | 34.47 |
| 212 | 晋太102 | TaCYP78A5-2Ap-HapII | 37.80 |
| 213 | 晋太114 | TaCYP78A5-2Ap-HapII | 40.13 |
| 214 | 晋太1310 | TaCYP78A5-2Ap-HapII | 39.60 |
| 215 | 经411 | TaCYP78A5-2Ap-HapII | 39.60 |
| 216 | 京东82东307 | TaCYP78A5-2Ap-HapII | 32.53 |
| 217 | 京东83东65 | TaCYP78A5-2Ap-HapII | 35.07 |
| 218 | 京冬8号 | TaCYP78A5-2Ap-HapII | 37.20 |
| 219 | 京核8922 | TaCYP78A5-2Ap-HapII | 33.30 |
| 220 | 京花1号 | TaCYP78A5-2Ap-HapII | 34.40 |
| 221 | 京农79-15 | TaCYP78A5-2Ap-HapII | 43.80 |
| 222 | 京农84-6786 | TaCYP78A5-2Ap-HapII | 38.67 |
| 223 | 京品30 | TaCYP78A5-2Ap-HapII | 38.80 |
| 224 | 京品3号 | TaCYP78A5-2Ap-HapII | 40.87 |
| 225 | 京双16 | TaCYP78A5-2Ap-HapII | 37.50 |
| 226 | 京双2号 | TaCYP78A5-2Ap-HapII | 34.67 |
| 227 | 京选20 | TaCYP78A5-2Ap-HapII | 36.33 |
| 228 | 京选25 | TaCYP78A5-2Ap-HapII | 45.80 |
| 229 | 京延85鉴28 | TaCYP78A5-2Ap-HapII | 38.33 |
| 230 | 科农199 | TaCYP78A5-2Ap-HapII | 36.73 |
| 231 | 科遗26 | TaCYP78A5-2Ap-HapII | 35.60 |
| 232 | 科遗29 | TaCYP78A5-2Ap-HapII | 34.07 |
| 233 | 临138 | TaCYP78A5-2Ap-HapII | 44.47 |
| 234 | 临汾8050 | TaCYP78A5-2Ap-HapII | 37.27 |
| 235 | 临丰3号 | TaCYP78A5-2Ap-HapII | 41.80 |
| 236 | 临丰518 | TaCYP78A5-2Ap-HapII | 35.80 |
| 237 | 临旱5089 | TaCYP78A5-2Ap-HapII | 36.33 |
| 238 | 临旱5367 | TaCYP78A5-2Ap-HapII | 37.47 |
| 239 | 临旱6105 | TaCYP78A5-2Ap-HapII | 37.47 |
| 240 | 临旱6号 | TaCYP78A5-2Ap-HapII | 41.60 |
| 241 | 临旱935 | TaCYP78A5-2Ap-HapII | 42.20 |
| 242 | 临抗5108 | TaCYP78A5-2Ap-HapII | 38.03 |
| 243 | 陇鉴196 | TaCYP78A5-2Ap-HapII | 39.73 |
| 244 | 鲁麦14 | TaCYP78A5-2Ap-HapII | 41.87 |
| 245 | 鲁麦15 | TaCYP78A5-2Ap-HapII | 35.93 |
| 246 | 鲁麦17 | TaCYP78A5-2Ap-HapII | 44.60 |
| 247 | 鲁麦19 | TaCYP78A5-2Ap-HapII | 42.07 |
| 248 | 鲁麦23 | TaCYP78A5-2Ap-HapII | 44.87 |
| 249 | 鲁麦3号 | TaCYP78A5-2Ap-HapII | 38.27 |
| 250 | 鲁麦5号 | TaCYP78A5-2Ap-HapII | 35.73 |
| 251 | 鲁麦8号 | TaCYP78A5-2Ap-HapII | 38.07 |
| 252 | 轮抗7号 | TaCYP78A5-2Ap-HapII | 39.00 |
| 253 | 轮选987 | TaCYP78A5-2Ap-HapII | 35.53 |
| 254 | 宁冬11 | TaCYP78A5-2Ap-HapII | 41.40 |
| 255 | 农大135 | TaCYP78A5-2Ap-HapII | 43.20 |
| 256 | 农大146 | TaCYP78A5-2Ap-HapII | 40.27 |
| 257 | 农大155 | TaCYP78A5-2Ap-HapII | 28.97 |
| 258 | 农大183 | TaCYP78A5-2Ap-HapII | 30.13 |
| 259 | 农大20074 | TaCYP78A5-2Ap-HapII | 45.87 |
| 260 | 农大3159 | TaCYP78A5-2Ap-HapII | 46.33 |
| 261 | 农大33 | TaCYP78A5-2Ap-HapII | 37.87 |
| 262 | 农大81146 | TaCYP78A5-2Ap-HapII | 41.27 |
| 263 | 平凉35 | TaCYP78A5-2Ap-HapII | 34.20 |
| 264 | 平阳348 | TaCYP78A5-2Ap-HapII | 43.07 |
| 265 | 秦麦3号 | TaCYP78A5-2Ap-HapII | 36.07 |
| 266 | 秦麦7号 | TaCYP78A5-2Ap-HapII | 34.80 |
| 267 | 山农辐63 | TaCYP78A5-2Ap-HapII | 40.67 |
| 268 | 山农优麦2号 | TaCYP78A5-2Ap-HapII | 46.20 |
| 269 | 山优2号 | TaCYP78A5-2Ap-HapII | 43.73 |
| 270 | 陕225-9 | TaCYP78A5-2Ap-HapII | 37.33 |
| 271 | 陕229 | TaCYP78A5-2Ap-HapII | 42.80 |
| 272 | 陕旱8675 | TaCYP78A5-2Ap-HapII | 42.80 |
| 273 | 陕合6号 | TaCYP78A5-2Ap-HapII | 36.33 |
| 274 | 陕农2号 | TaCYP78A5-2Ap-HapII | 32.67 |
| 275 | 胜利麦 | TaCYP78A5-2Ap-HapII | 35.87 |
| 276 | 双丰收 | TaCYP78A5-2Ap-HapII | 38.67 |
| 277 | 舜麦1718 | TaCYP78A5-2Ap-HapII | 38.07 |
| 278 | 太13606 | TaCYP78A5-2Ap-HapII | 40.60 |
| 279 | 太712 | TaCYP78A5-2Ap-HapII | 42.53 |
| 280 | 太原633 | TaCYP78A5-2Ap-HapII | 38.33 |
| 281 | 泰山23 | TaCYP78A5-2Ap-HapII | 36.27 |
| 282 | 泰山24 | TaCYP78A5-2Ap-HapII | 39.13 |
| 283 | 渭麦4号 | TaCYP78A5-2Ap-HapII | 32.80 |
| 284 | 西峰20 | TaCYP78A5-2Ap-HapII | 31.13 |
| 285 | 西峰9号 | TaCYP78A5-2Ap-HapII | 31.70 |
| 286 | 小山8号 | TaCYP78A5-2Ap-HapII | 38.73 |
| 287 | 新冬20 | TaCYP78A5-2Ap-HapII | 40.27 |
| 288 | 烟农19 | TaCYP78A5-2Ap-HapII | 40.87 |
| 289 | 烟农21 | TaCYP78A5-2Ap-HapII | 37.33 |
| 290 | 延安15 | TaCYP78A5-2Ap-HapII | 37.87 |
| 291 | 原冬3号 | TaCYP78A5-2Ap-HapII | 40.00 |
| 292 | 原冬834 | TaCYP78A5-2Ap-HapII | 35.92 |
| 293 | 原冬847 | TaCYP78A5-2Ap-HapII | 33.33 |
| 294 | 原冬856 | TaCYP78A5-2Ap-HapII | 35.67 |
| 295 | 运旱102 | TaCYP78A5-2Ap-HapII | 38.87 |
| 296 | 运旱115 | TaCYP78A5-2Ap-HapII | 38.67 |
| 297 | 运旱2028 | TaCYP78A5-2Ap-HapII | 38.80 |
| 298 | 运旱20410 | TaCYP78A5-2Ap-HapII | 40.83 |
| 299 | 运旱21-30 | TaCYP78A5-2Ap-HapII | 40.13 |
| 300 | 运旱23-35 | TaCYP78A5-2Ap-HapII | 43.67 |
| 301 | 运旱618 | TaCYP78A5-2Ap-HapII | 39.60 |
| 302 | 运旱719 | TaCYP78A5-2Ap-HapII | 35.93 |
| 303 | 运旱805 | TaCYP78A5-2Ap-HapII | 38.87 |
| 304 | 早穗21 | TaCYP78A5-2Ap-HapII | 32.53 |
| 305 | 早穗65 | TaCYP78A5-2Ap-HapII | 32.40 |
| 306 | 早穗66 | TaCYP78A5-2Ap-HapII | 33.93 |
| 307 | 早洋麦 | TaCYP78A5-2Ap-HapII | 35.67 |
| 308 | 张冬29 | TaCYP78A5-2Ap-HapII | 33.00 |
| 309 | 郑丰9962 | TaCYP78A5-2Ap-HapII | 34.53 |
| 310 | 郑州24 | TaCYP78A5-2Ap-HapII | 36.42 |
| 311 | 中7902 | TaCYP78A5-2Ap-HapII | 44.60 |
| 312 | 中86I-50455 | TaCYP78A5-2Ap-HapII | 38.13 |
| 313 | 中大86-鉴2 | TaCYP78A5-2Ap-HapII | 34.87 |
| 314 | 中大91-品9 | TaCYP78A5-2Ap-HapII | 43.67 |
| 315 | 中大92-鉴49 | TaCYP78A5-2Ap-HapII | 39.53 |
| 316 | 中大92-品8 | TaCYP78A5-2Ap-HapII | 40.80 |
| 317 | 中旱110 | TaCYP78A5-2Ap-HapII | 43.47 |
| 318 | 中麦175 | TaCYP78A5-2Ap-HapII | 42.53 |
| 319 | 中麦9号 | TaCYP78A5-2Ap-HapII | 45.27 |
| 320 | 中引6号 | TaCYP78A5-2Ap-HapII | 37.07 |
| 321 | 中优9507 | TaCYP78A5-2Ap-HapII | 46.27 |
| 322 | 中作60064 | TaCYP78A5-2Ap-HapII | 36.20 |
| 323 | 中作60115 | TaCYP78A5-2Ap-HapII | 42.73 |
新窗口打开|下载CSV
2.6 籽粒性状-分子标记的关联分析
对323份小麦品种的单倍型数据与千粒重表型数据进行性状-标记的关联分析显示, TaCYP78A5-2Ap-HapI单倍型和TaCYP78A5-2Ap-HapII单倍型对应小麦品种的平均千粒重存在极显著差异(P<0.01); 其中, 单倍型TaCYP78A5-2Ap-HapII小麦品种的平均千粒重显著高于单倍型TaCYP78A5-2Ap-HapI (图6)。这表明不同单倍型TaCYP78A5-2Ap对千粒重的贡献存在极显著差异, 暗示着CAPS-5Ap标记是与粒重相关的功能标记; 单倍型TaCYP78A5-2Ap-HapII是提高千粒重的优异单倍型。图6
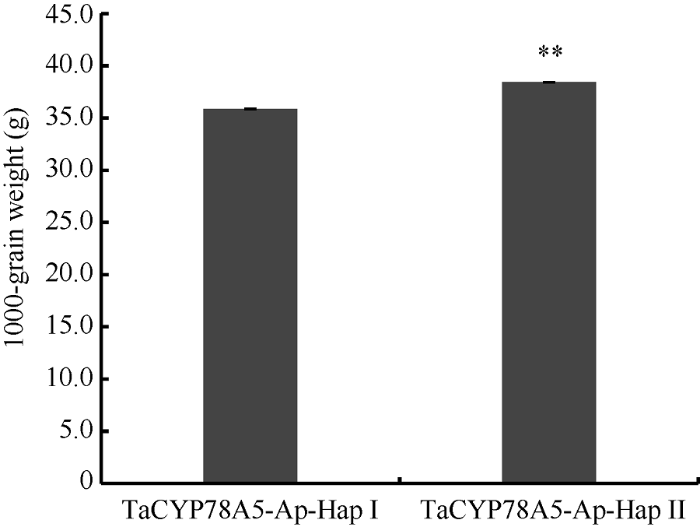 新窗口打开|下载原图ZIP|生成PPT
新窗口打开|下载原图ZIP|生成PPT图6323份现代育成小麦品种中 TaCYP78A5-2Ap两种单倍型与千粒重的方差分析
TaCYP78A5-Ap-HapI/II: TaCYP78A5-2A启动子的两种单倍型; **P<0.01; 误差显示为±SE。
Fig. 6Variance analysis of two TaCYP78A5-2Ap haploidtypes and TGW in 323 modern varieties of wheat
TaCYP78A5-Ap-HapI/II: Two haploidtypes for TaCYP78A5-A promoter; **P<0.01; Error bars denote ±SE.
3 讨论
小麦产量的提高一直是小麦育种工作不断追求的重要目标。目前, 已有不少籽粒大小相关基因被克隆并开发了其功能标记, 如TaSus2-2B[2]、TaGW2[3]、TaCwi-A1[4]、TaGS-D1[19]、TaGS1a[20]等。近年来, 植物特异的CYP78A家族的部分成员KLU/CYP78A5[10]、OsCYP78A13[12]、GmCYP78A72[14]、AtCYP78A9[21]等被证实参与植物生殖器官及种子的发育, 暗示着CYP78A家族基因可用于作物产量性状的改良。本课题组马猛等利用同源克隆技术获得TaCYP78A3和TaCYP78A5两个基因, 并证实这两个基因均可影响籽粒大小[15,16]。因此, 本研究通过检测黄淮麦区323份现代育成小麦品种TaCYP78A5的3个直系同源基因的等位基因, 并开发了其CAPS-5Ap功能标记; 该研究结果将为小麦高产、高效分子育种提供新优异基因和功能标记。分子标记辅助选择是现代分子育种的主要手段, 它通过对单倍型的直接、快速选择进行分子育种。因此, 基于已知功能基因开发其与产量性状相关的功能标记, 对未来的分子育种至关重要。Jiang等[2]针对TaSus2-2B开发功能标记Hap-H和Hap-L, 并通过关联分析方法证明Hap-H是影响千粒重的优异单倍型。Ma等[4]根据TaCwi-A1的等位变异位点开发了与粒重相关的功能标记CWI21和CWI22。相吉山等[22]利用新疆小麦品种资源进一步证实TaCwi-A1 的功能标记CWI22、CWI21 能够较好地区分小麦千粒重的大小, 可用于粒重的分子标记辅助选择。本研究首次通过对不同品种TaCYP78A5-2Ap序列多态性位点分析, 开发了TaCYP78A5等位基因功能标记CAPS-5Ap; 将CAPS-5Ap标记在黄淮麦区323份小麦品种间扫描验证表明, CAPS-5Ap标记可将323份小麦品种分为TaCYP78A5-2Ap-HapI和TaCYP78A5-2Ap-HapII两种单倍型。进一步的关联分析发现, TaCYP78A5-2Ap- HapII单倍型小麦品种的千粒重均极显著高于TaCYP78A5-2Ap-HapI, 表明TaCYP78A5-2Ap-HapII是提高千粒重的优异单倍型; TaCYP78A5-2Ap-HapII类型可用于小麦粒重的分子标记辅助选择。
本研究是基于不同小麦品种间TaCYP78A5-2Ap序列上多个SNP位点组成的单倍型为单位的结果; 后期计划进一步研究各单倍型的单一SNP位点对TaCYP78A5基因表达水平的影响, 以期了解SNP位点差异对基因表达水平, 乃至对籽粒大小和产量的影响。
附图和附表
请见网络版: 1) 本刊网站参考文献 原文顺序
文献年度倒序
文中引用次数倒序
被引期刊影响因子
DOI:10.1111/pce.2015.38.issue-4URL [本文引用: 2]
DOI:10.1007/s10142-010-0188-xURL [本文引用: 3]
DOI:10.1007/s00122-010-1437-zURL [本文引用: 3]

The OsGW2 gene is involved in rice grain development, influencing grain width and weight. Its ortholog in wheat, TaGW2, was considered as a candidate gene related to grain development. We found that TaGW2 is constitutively expressed, with three orthologs expressing simultaneously. The coding sequence (CDS) of TaGW2 is 1,275 bp encoding a protein with 424 amino acids, and has a functional domain shared with OsGW2. No divergence was detected within the CDS sequences in the same locus in ten varieties. Genome-specific primers were designed based on the sequence divergence of the promoter regions in the three orthologous genes, and TaGW2 was located in homologous group 6 chromosomes through CS nulli-tetrasomic (NT). Two SNPs were detected in the promoter region of TaGW2-6A, forming two haplotypes: Hap-6A-A (-593A and -739G) and Hap-6A-G (-593G and -769A). A cleaved amplified polymorphic sequence (CAPS) marker was developed based on the -593 A-G polymorphism to distinguish the two haplotypes in TaGW2-6A. This gene was fine mapped 0.6 cM from marker cfd80.2 near the centromere in a recombinant inbred line (RIL) population. Two hundred sixty-five Chinese wheat varieties were genotyped and association analysis revealed that Hap-6A-A was significantly associated with wider grains and higher one-thousand grain weight (TGW) in two crop seasons. qRT-PCR revealed a negative relationship between TaGW2 expression level and grain width. The Hap-6A-A frequencies in Chinese varieties released at different periods showed that it had been strongly positively selected in breeding. In landraces, Hap-6A-A is mainly distributed in southern Chinese wheat regions. Association analysis also indicated that Hap-6A-A not only increased TGW by more than 3 g, but also had earlier heading and maturity. In contrast to Chinese varieties, Hap-6A-G was the predominant haplotype in European varieties; Hap-6A-A was mainly present in varieties released in the former Yugoslavia, Italy, Bulgaria, Hungary and Portugal.
DOI:10.1007/s11032-010-9524-zURL [本文引用: 3]
DOI:10.1016/j.tplants.2003.09.010URL [本文引用: 1]
[本文引用: 1]
DOI:10.1046/j.1365-313X.1998.00123.xURL [本文引用: 1]
[本文引用: 1]
[本文引用: 1]
DOI:10.1104/pp.17.00113URL [本文引用: 1]
DOI:10.1073/pnas.0907024106URL [本文引用: 3]
DOI:10.1111/j.1365-313X.2012.04907.xURL

Seed size in higher plants is coordinately determined by the growth of the embryo, endosperm and maternal tissue, but relatively little is known about the genetic and molecular mechanisms that set final seed size. We have previously demonstrated that Arabidopsis DA1 acts maternally to control seed size, with the da1-1 mutant producing larger seeds than the wild type. Through an activation tagging screen for modifiers of da1-1, we have identified an enhancer of da1-1 (eod3-1D) in seed size. EOD3 encodes the Arabidopsis cytochrome P450/CYP78A6 and is expressed in most plant organs. Overexpression of EOD3 dramatically increases the seed size of wild-type plants, whereas eod3-ko loss-of-function mutants form small seeds. The disruption of CYP78A9, the most closely related family member, synergistically enhances the seed size phenotype of eod3-ko mutants, indicating that EOD3 functions redundantly with CYP78A9 to affect seed growth. Reciprocal cross experiments show that EOD3 acts maternally to promote seed growth. eod3-ko cyp78a9-ko double mutants have smaller cells in the maternal integuments of developing seeds, whereas eod3-1D forms more and larger cells in the integuments. Genetic analyses suggest that EOD3 functions independently of maternal factors DA1 and TTG2 to influence seed growth. Collectively, our findings identify EOD3 as a factor of seed size control, and give insight into how plants control their seed size.
DOI:10.1111/tpj.12223URL [本文引用: 3]

Among angiosperms there is a high degree of variation in embryo/endosperm size in mature seeds. However, little is known about the molecular mechanism underlying size control between these neighboring tissues. Here we report the rice GIANT EMBRYO (GE) gene that is essential for controlling the size balance. The function of GE in each tissue is distinct, controlling cell size in the embryo and cell death in the endosperm. GE, which encodes CYP78A13, is predominantly expressed in the interfacing tissues of the both embryo and endosperm. GE expression is under negative feedback regulation; endogenous GE expression is upregulated in ge mutants. In contrast to the loss-of-function mutant with large embryo and small endosperm, GE overexpression causes a small embryo and enlarged endosperm. A complementation analysis coupled with heterofertilization showed that complementation of ge mutation in either embryo or endosperm failed to restore the wild-type embryo/endosperm ratio. Thus, embryo and endosperm interact in determining embryo/endosperm size balance. Among genes associated with embryo/endosperm size, REDUCED EMBRYO genes, whose loss-of-function causes a phenotype opposite to ge, are revealed to regulate endosperm size upstream of GE. To fully understand the embryo-endosperm size control, the genetic network of the related genes should be elucidated.
DOI:10.1016/j.cub.2010.01.039URL [本文引用: 1]

Summary
Growth control in animals and plants involves mobile signals [ [1] and [2]]. Depending on their range of action, these signals coordinate the growth of cells within an organ or the growth of different organs in a larger, functionally integrated structure [ [3], [4], [5], [6] and [7]]. In plants, flowers are such integrated structures, yet it remains poorly understood how growth of the constituent organs is coordinated to ensure their correct relative sizes. The cytochrome P450 KLUH/CYP78A5 and its homolog CYP78A7 promote organ growth via a non-cell-autonomous signal [ [8], [9] and [10]]; however, the range of this signal and thus its developmental function are unknown. Here we use a system for the predictable generation of chimeric plants to determine the range of the KLUH-dependent signal. In contrast with the largely autonomous behavior of another tested growth-control gene, we find that KLUH activity extends beyond individual organs and flowers. Its overall activity is integrated across an inflorescence to determine final organ size, which is largely independent of the genotype of the individual organs. Thus, the KLUH-dependent signal appears to move beyond individual organs in a flower, providing a mechanism for coordinating their growth and ensuring floral symmetry as an important determinant of a plant's attractiveness to pollinators [11].Highlights
? The KLUH-dependent growth signal is active beyond individual floral organs ? Its total activity is integrated across an inflorescence to determine organ size ? The long range of action suggests a mechanism for coordinating growth in flowersDOI:10.1093/mp/sst107URL [本文引用: 2]

The GIANT EMBRYO (GE) gene encodes a CYP78A subfamily P450 monooxygenase, which controls rice embryo development and coordinates embryo and endosperm growth. GE also plays a pivotal role in shoot apical meristem (SAM) maintenance and grain yield improvement.Angiosperm seeds usually consist of two major parts: the embryo and the endosperm. However, the molecular mechanism(s) underlying embryo and endosperm development remains largely unknown, particularly in rice, the model cereal. Here, we report the identification and functional characterization of the rice GIANT EMBRYO (GE) gene. Mutation of GE resulted in a large embryo in the seed, which was caused by excessive expansion of scutellum cells. Post-embryonic growth of ge seedling was severely inhibited due to defective shoot apical meristem (SAM) maintenance. Map-based cloning revealed that GE encodes a CYP78A subfamily P450 monooxygenase that is localized to the endoplasmic reticulum. GE is expressed predominantly in the scutellar epithelium, the interface region between embryo and endosperm. Overexpression of GE promoted cell proliferation and enhanced rice plant growth and grain yield, but reduced embryo size, suggesting that GE is critical for coordinating rice embryo and endosperm development. Moreover, transgenic Arabidopsis plants overexpressing AtCYP78A10, a GE homolog, also produced bigger seeds, implying a conserved role for the CYP78A subfamily of P450s in regulating seed development. Taken together, our results indicate that GE plays critical roles in regulating embryo development and SAM maintenance.
DOI:10.1093/jxb/erv542URL [本文引用: 3]
DOI:10.1111/tpj.12896URL [本文引用: 2]
[本文引用: 1]
[本文引用: 1]
DOI:10.1007/s11032-014-0102-7URL [本文引用: 1]
DOI:10.1016/j.fcr.2013.07.012URL [本文引用: 1]
DOI:10.1104/pp.113.218214URL [本文引用: 1]

Synchronized communication between gametophytic and sporophytic tissue is crucial for successful reproduction, and hormones seem to have a prominent role in it. Here, we studied the role of the Arabidopsis (Arabidopsis thaliana) cytochrome P450 CYP78A9 enzyme during reproductive development. First, controlled pollination experiments indicate that CYP78A9 responds to fertilization. Second, while CYP78A9 overexpression can uncouple fruit development from fertilization, the cyp78a8 cyp78a9 loss-of-function mutant has reduced seed set due to outer ovule integument development arrest, leading to female sterility. Moreover, CYP78A9 has a specific expression pattern in inner integuments in early steps of ovule development as well as in the funiculus, embryo, and integuments of developing seeds. CYP78A9 overexpression did not change the response to the known hormones involved in flower development and fruit set, and it did not seem to have much effect on the major known hormonal pathways. Furthermore, according to previous predictions, perturbations in the flavonol biosynthesis pathway were detected in cyp78a9, cyp78a8 cyp78a9, and empty siliques (es1-D) mutants. However, it appeared that they do not cause the observed phenotypes. In summary, these results add new insights into the role of CYP78A9 in plant reproduction and present, to our knowledge, the first characterization of metabolite differences between mutants in this gene family.
DOI:10.3864/j.issn.0578-1752.2014.13.019URL [本文引用: 1]

【目的】验证已开发的TaCwi-A1功能标记CWI22、CWI21检测小麦千粒重的可靠性,为分子标记辅助选择提供参考信息。同时用该标记检测新疆小麦品种资源,探讨TaCwi-A1等位变异类型及分布频率。【方法】首先以110份新疆冬小麦品种资源为材料,用CWI22、CWI21检测TaCwi-A1基因型,并利用SKCS测定千粒重,比较TaCwi-A1a、TaCwi-A1b基因型品种间千粒重的差异。再以1 241份新疆小麦品种资源为材料,对TaCwi-A1基因型进行分子标记检测。【结果】在110份新疆冬小麦品种资源中,有46份材料能够用CWI22扩增出402 bp的目的片段,说明含有TaCwi-A1a;有64份材料能够用CWI21扩增出404 bp的目的片段,说明含有TaCwi-A1b;并且TaCwi-A1a基因型品种(系)的千粒重(43.5 g)显著高于TaCwi-A1b(40.9 g)(P<0.05)。1 241份新疆小麦品种资源中,TaCwi-A1a的分布频率为62.6%,TaCwi-A1b为37.4%。其中,冬小麦中TaCwi-A1a的分布频率为63.0%,TaCwi-A1b为37.0%;春小麦中TaCwi-A1a的分布频率为61.7%,TaCwi-A1b为38.3%,并且TaCwi-A1a在不同类型冬、春小麦品种资源中的分布频率大小顺序均为国外品种(系)>国内品种(系)>自育品系>审定品种>地方品种;新疆小麦审定品种中,冬小麦TaCwi-A1a的分布频率为40.0%,TaCwi-A1b为60.0%,春小麦TaCwi-A1a的分布频率为68.6%,TaCwi-A1b为31.4%。在1990年以前、1991—2000年、2001年以后3个阶段的审定品种中,TaCwi-A1a和TaCwi-A1b的分布频率分别为11.1%和88.9%、50.0%和50.0%、69.2%和30.8%。【结论】TaCwi-A1的分子标记CWI22、CWI21能够较好地区分小麦千粒重的大小,可用于粒重的分子标记辅助选择。在新疆小麦品种资源中,TaCwi-A1a有较高的分布频率。其中,冬小麦品种资源中的分布频率略高于春小麦品种资源,引进品种(系)高于自育品种(系),自育品种(系)高于地方品种。在地方品种和自育品种(系)中,TaCwi-A1a在春小麦中的分布频率明显高于冬小麦,说明新疆冬、春小麦育种对粒重的选择存在一定的差异;但总体都有较强的选择压力,使TaCwi-A1a在审定品种中的分布频率逐渐提高。
DOI:10.3864/j.issn.0578-1752.2014.13.019URL [本文引用: 1]

【目的】验证已开发的TaCwi-A1功能标记CWI22、CWI21检测小麦千粒重的可靠性,为分子标记辅助选择提供参考信息。同时用该标记检测新疆小麦品种资源,探讨TaCwi-A1等位变异类型及分布频率。【方法】首先以110份新疆冬小麦品种资源为材料,用CWI22、CWI21检测TaCwi-A1基因型,并利用SKCS测定千粒重,比较TaCwi-A1a、TaCwi-A1b基因型品种间千粒重的差异。再以1 241份新疆小麦品种资源为材料,对TaCwi-A1基因型进行分子标记检测。【结果】在110份新疆冬小麦品种资源中,有46份材料能够用CWI22扩增出402 bp的目的片段,说明含有TaCwi-A1a;有64份材料能够用CWI21扩增出404 bp的目的片段,说明含有TaCwi-A1b;并且TaCwi-A1a基因型品种(系)的千粒重(43.5 g)显著高于TaCwi-A1b(40.9 g)(P<0.05)。1 241份新疆小麦品种资源中,TaCwi-A1a的分布频率为62.6%,TaCwi-A1b为37.4%。其中,冬小麦中TaCwi-A1a的分布频率为63.0%,TaCwi-A1b为37.0%;春小麦中TaCwi-A1a的分布频率为61.7%,TaCwi-A1b为38.3%,并且TaCwi-A1a在不同类型冬、春小麦品种资源中的分布频率大小顺序均为国外品种(系)>国内品种(系)>自育品系>审定品种>地方品种;新疆小麦审定品种中,冬小麦TaCwi-A1a的分布频率为40.0%,TaCwi-A1b为60.0%,春小麦TaCwi-A1a的分布频率为68.6%,TaCwi-A1b为31.4%。在1990年以前、1991—2000年、2001年以后3个阶段的审定品种中,TaCwi-A1a和TaCwi-A1b的分布频率分别为11.1%和88.9%、50.0%和50.0%、69.2%和30.8%。【结论】TaCwi-A1的分子标记CWI22、CWI21能够较好地区分小麦千粒重的大小,可用于粒重的分子标记辅助选择。在新疆小麦品种资源中,TaCwi-A1a有较高的分布频率。其中,冬小麦品种资源中的分布频率略高于春小麦品种资源,引进品种(系)高于自育品种(系),自育品种(系)高于地方品种。在地方品种和自育品种(系)中,TaCwi-A1a在春小麦中的分布频率明显高于冬小麦,说明新疆冬、春小麦育种对粒重的选择存在一定的差异;但总体都有较强的选择压力,使TaCwi-A1a在审定品种中的分布频率逐渐提高。
