摘要/Abstract
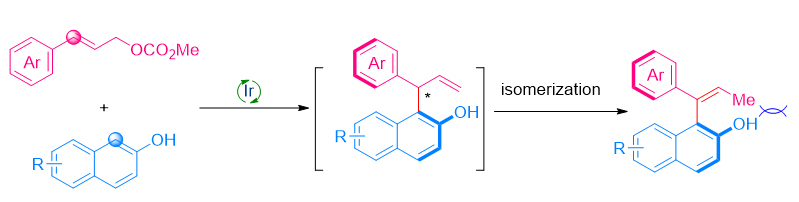
轴手性化合物是一类重要的手性化合物, 其中苯乙烯类轴手性化合物因其轴手性稳定性相对较差, 目前高效不对称合成的方法比较局限. 本工作以β-萘酚作为亲核试剂, 通过将金属铱催化不对称烯丙基取代与双键异构化串联, 实现了中心手性到轴手性的转移, 从而高效地合成了一系列β-萘酚衍生的苯乙烯类轴手性化合物.
关键词: 轴手性, 不对称催化, 铱催化, 烯丙基取代, 烯烃异构化
Axially chiral compounds represent an important class of chiral molecules. In this regard, many methods have been developed to access these compounds. However, efficient methods for the synthesis of axially chiral styrenes are limited to date, mainly due to their relative instability compared to axially chiral biaryl compounds. Iridium-catalyzed asymmetric allylic substitutions have evolved as a powerful tool in constructing C―C or C―X bonds at the allylic position. We developed an efficient sequential strategy to access a series of axially chiral styrenes by iridium-catalyzed allylic substitution and central-to-axial chirality transfer via olefin isomerization. With the iridium complex derived from [Ir(cod)Cl]2 and Alexakis ligand L1 as catalyst, and β-naphthol as nucleophiles, a broad range of axially chiral styrenes were obtained with moderate to excellent yields (28%~97% yields) and enantioselectivity (59%~98% ee). A general procedure for the asymmetric allylation of β-naphthol is described as the following: A flame-dried Schlenk tube was cooled to room temperature and filled with argon. To this flask were added [Ir(cod)Cl]2 (2.6 mg, 0.004 mmol, 2 mol%), (S,S,Sa)-L1 (4.8 mg, 0.008 mmol, 4 mol%), freshly distilled tetrahydrofuran (THF, 0.5 mL) and propylamine (0.5 mL). The mixture was stirred at 50 ℃ for 30 min and then the low-boiling solvents were removed in vacuo to give a pale yellow solid. The solid was stirred at 50 ℃ again under vacuum until it became a powder. After that, β-naphthol 1 (0.22 mmol, 1.1 equiv.), allyl carbonate 2 (0.2 mmol, 1.0 equiv.), 1,4-diazobicyclo(2.2.2)octane (DABCO) (67.3 mg, 0.6 mmol, 3.0 equiv.) and freshly distilled Et2O (2.0 mL) were added to this flask under argon atmosphere. The reaction mixture was stirred at 20 ℃ until the starting material was consumed (monitored by thin layer chromatography, TLC). The crude reaction mixture was diluted with water (5 mL), and extracted with dichloromethane (DCM, 5 mL×3). The organic layers were collected, dried over Na2SO4 and then concentrated in vacuo to afford the crude product. The residue was purified by preparative TLC to afford the product.
Key words: axial chirality, asymmetric catalysis, iridium-catalyzed, allylic substitution, olefin isomerization
PDF全文下载地址:
点我下载PDF
